1. Introduction
Skin is a complex multi-layered tissue, consisting of three main parts: the epidermis, the dermis and the hypodermis. The dermis is responsible for most of the complex mechanical properties of skin, such as viscoelasticity, non-linearity and anisotropy. At the microscopic level the dermis consists for the greater part of extracellular matrix, compounded mainly of collagen fibers forming a disordered network. The mechanical properties of skin have been studied in the past, but their exact link with the microscopic organization is still an open question.
Recent experimental developments (Goulam Houssen et al. Citation2011; Bancelin et al. Citation2015; Lynch et al. Citation2017) have allowed observing the microstructure of skin (or other similar, collagen-rich tissues) during mechanical assays. They have shown that the classical interpretation of the stress/stretch relationship through the microstructure cannot be used to explain the observed evolution of the microstructure, emphasizing the role of the non-reversible deformation of the tissue in the evolution of the microstructure.
2. Methods
We investigated the mechanical properties of skin while observing the collagen fibres microstructure. To do so, we performed an in situ mechanical assay under a multiphoton microscope with Second Harmonic Generation (SHG) detection. SHG allows imaging specifically, without staining and in 3D, the collagen fibres of the tissue.
We quantified the orientation of the collagen fibers as well as local stretch, at the same region of the same sample, at different stretch levels.
This was done for young WT, genetically-modified (Bancelin et al. Citation2015) and aged mice (Lynch et al. Citation2017), enabling comparing the consequences of modifications of skin microstructure
3. Results and discussion
SHG images give access to optical cross-section of mice skin at a depth of few micrometres below the surface. From these images, it was possible to extract the local collagen orientation (Bancelin et al. Citation2015).
In parallel, we measure the strain field at the size of full image by tracking the hair shafts (see ). Local measurement of the stain showed that, at the scale of the field of view, the sample has the same stretch than the macroscopic one, and that there is no shear.
Figure 1. Collagen fibers network image, obtain by SHG imaging of a mice skin under traction (λ = 1.1). The typical size of the image is 400x400µm. Collagen fibers appear in green. Black holes are the hair shafts.
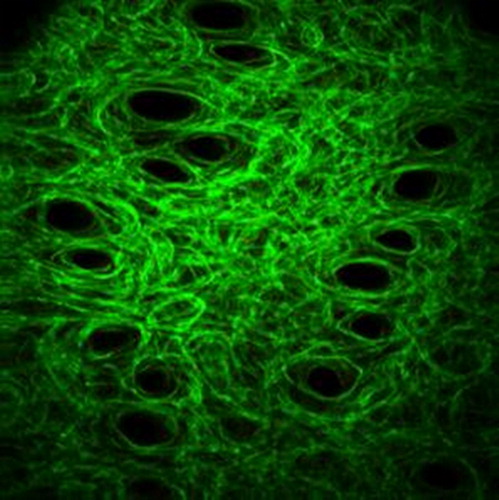
From the local orientation, we extract a histogram of orientations, from which we extract either the entropy or the orientation index (related to the fraction of collagen fibres aligned in the direction of traction).
We observe a perfect superposition of the nominal stress/stretch curve and of the Orientation Index versus stretch curve, for all types of mice, but with different scalings (see ), depending on the mutation.
Figure 2. Orientation index and nominal stress versus stretch for mice skin. The orientation index quantifies the fraction of fibers aligned in the direction of traction. Figure from Bancelin et al. (Citation2015).
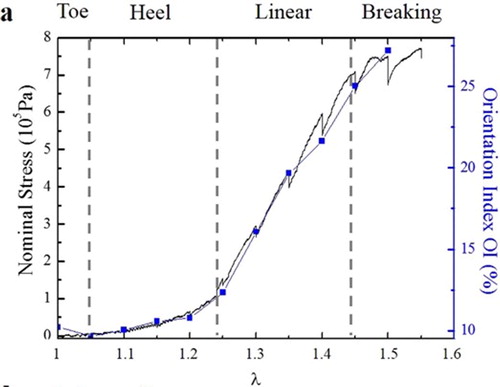
This implies that the network almost doesn’t evolve in the toe/hell regions, and collapses to the direction of traction during the linear region, contrary to the classical description of the microscopic role of collagen (Veronda & Westmann Citation1970; Brown 2006).
It is also possible to use our results to test the affine assumption for the motion of the collagen. Under this assumption, the kinematic of the fibres is the one of the surrounding volume. As we measure the strain locally, we can compute the evolution of the initial histogram of orientation, and compare it with the measured one. Qualitatively, our results agree with the affine assumption that is used in general in microstructural models of skin (Jayyosi et al. Citation2017). However, quantitatively, we observed that affine assumption overestimates the fibre alignment.
The results obtained for genetically-modified or aged mice are similar to the wild-type ones. However, the different parameters does not evolve the same way. We are relating the effects of the different alterations on the collagen fibers or on the non-fibrillar matrix to the evolutions of these parameters. Using these data, we propose a scenario in which the network is first stabilized by the non-fibrillar matrix, before collapsing at larger strain.
4. Conclusions
We developed a multiscale experimental approach to quantitatively measure the evolution of the collagen microstructure during a mechanical assay. Our results showed a double regime: first the collagen network almost doesn’t evolve while the stress is low; second it becomes more and more aligned while the stress increases.
Our observations are true for different genetically- modified mice or aged mice. However, the mechanical and microstructural responses are different. Based on these observations, we propose a scenario to explain the double regime of the collagen fibres rearrangement during a mechanical assay.
Acknowledgements
We thank Mr V. de Greef for his help in the experiments.
Additional information
Funding
References
- Bancelin S, Lynch B, Bonod-Bidaud C, Ducourthial G, Psilodimitrakopoulos S, Dokládal P, Allain J- M, Schanne-Klein M-C, Ruggiero F. 2015. Ex vivo multiscale quantitation of skin biomechanics in wild-type and genetically-modified mice using multiphoton microscopy. Sci Rep. 5(1):17635.
- Brown IA. 2006. Scanning electron-microscope study of effects of uniaxial tension on human skin. Br J Dermatol. 89(4):383–393.
- Goulam Houssen Y, Gusachenko I, Schanne-Klein M-C, Allain J-M. 2011. Monitoring micrometer- scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J Biomech. 44(11):2047–2052.
- Jayyosi C, Affagard J-S, Ducourthial G, Bonod-Bidaud C, Lynch B, Bancelin S, Ruggiero F, Schanne-Klein M-C, Allain J-M, Bruyère-Garnier K, et al. 2017. Affine kinematics in planar fibrous connective tissues: an experimental investigation. Biomech Model Mechanobiol. 16(4):1459–1473.
- Lynch B, Bonod-Bidaud C, Ducourthial G, Affagard J-S, Bancelin S, Psilodimitrakopoulos S, Ruggiero F, Allain J-M, Schanne-Klein M-C. 2017. How aging impacts skin biomechanics: a multiscale study in mice. Sci Rep. 7(1):13750.
- Veronda DR, Westmann RA. 1970. Mechanical characterization of skin—finite deformations. J Biomech. 3(1):111–124.
