 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Keywords:
1. Introduction
Cell migration is essential for many biological processes such as tissue morphogenesis, wound healing or metastatic invasion in cancer. It is a complex and highly regulated phenomenon closely guided and fine-tuned by both chemical and mechanical cues. Whereas chemoattraction has been extensively studied, the mechanical influence remains to be fully elucidated. Although cell sensitivity to the substrate rigidity is known under the term durotaxis (Marzban et al. Citation2018) and substrate anisotropy is known to influence cellular organization (Checa et al. Citation2015) much less is known about cell sensitivity to environmental stresses and strains. This paper proposes to specifically focus on the cell sensitivity to substrate deformations during migration. Those are assumed to play a role in long-range cell-cell interactions (Han et al. Citation2018) by which a cell deforms the substrate (Tanimoto and Sano Citation2014) and influences the orientation of migration of other cells in its neighbourhood. This form of mechanotaxis (to which we will refer as strain mechanosensing) could in particular explain how cells migrate towards each other to form vascular loops during angiogenesis when chemotaxis is ruled out.
2. Methods
1.1. Generalities
Our model is based on a hybrid discrete-continuum description of cells migrating on a substrate. An agent-based model for cell migration is developed, in which cells are modelled as hexagonal agents moving on a two-dimensional hexagonal lattice. Each cell of the lattice contains at most one agent. At each time step, a moving agent goes to one of its six neighbouring positions on the lattice (see ) with probabilities that are influenced by external cues within the microenvironment. A moving agent exerts traction forces and consequently deforms the substrate. The latter is modelled as an elastic flat domain whose quasi-static deformations are calculated under the plane-stress assumptions using the finite element method (FEM).
Figure 1. Central: example of probability distribution p(α) for the ready-to-move agent to select angle α for next move. The peak of the Von Mises distribution is centered at 5π/6 and corresponds here to an arbitrarily fixed angle of the substrate’s principal deformation. Insert: ready-to-move agent (red) and its six neighboring potential destinations on the lattice - shaded cell (#3) represents the selected destination.
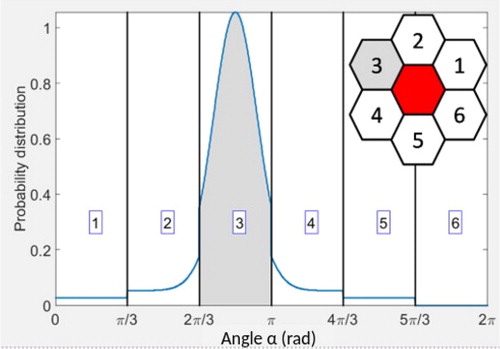
Our model is therefore a coupled system where cues of chemical nature are neglected in order to focus on the effects of mechanical cues. We assume in particular that cells are sensitive to deformations within the substrate and preferentially follow the direction of highest strain with probability to define.
1.2. Model of strain mechanosensing
Without any mechanical cues, an agent is assumed to randomly select its direction of movement, i.e., to go with equal probability Pi = 1/6 to one of its six neighbouring positions on the lattice (see ).
When substrate deformations occur, cell reaction to these deformations is modelled by adding a continuous Von Mises probability distribution centered at the angle of substrate’s principal deformation (the width of the distribution representing cell sensitivity to the deformation). In order to ensure the preferred orientation to be the one of highest strain, this probability distribution is weighted by average deformations over each of the six neighbouring positions (see ).
This final distribution p(α) is therefore a combination of randomness and strain mechanosensing with contributions respectively weighted by parameter ξ ∈ [0,1] and 1-ξ (ξ = 0: movement guided by strain mechanosensing only; ξ = 1: fully random movement). This distribution density is finally integrated to obtain the six discrete probabilities of movement
towards the neighbour i on the lattice (see ).
3. Results and discussion
In order to illustrate the potential impact of strain mechanosensing on cell migration, two sets of numerical simulations are performed. A hexagonal 20x20 lattice is used for the agent-based model whereas a 400x400 regular square grid is used for the FEM simulations with clamping conditions.
In numerical experiment (1), a single agent freely moves in the domain and exerts traction forces on the substrate when moving; the corresponding deformation field is therefore self-generated and of dynamical nature. Numerical experiment (2) includes a fixed distribution of traction forces at the center of the domain, which mimics a virtual non motile agent and provides an additional static contribution to the deformation field (see ). Modelling sensitivity to strain mechanosensing with comparison to random migration is achieved in both experiments by using two different values of parameter ξ.
Figure 2. Central: ratio T2/T1 of time spent by the moving agent at distance N cells from the central static deformation for ξ = 0.25 and ξ = 0.5. Insert: moving agent (red) (and its self-generated deformation field) located at the N = 5 cell distance from the static deformation field at the center of the domain.
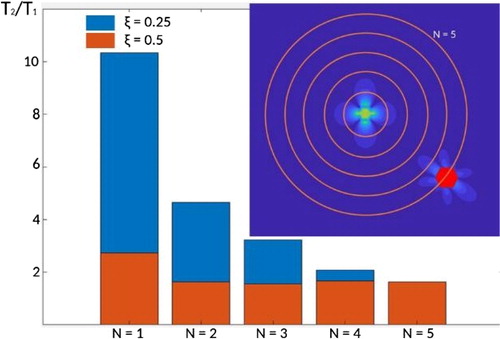
Quantification of the influence of long-range substrate deformations on cell migration is achieved by looking at the amount of time spent close to the static deformation - experiment (2) - and compared to experiment (1). Specifically, if T denotes the time spent by the moving agent at a distance less or equal to N cells from the middle of the domain, the ratio T2/T1 (where the subscript corresponds to the experiment) is presented on for varying N.
The first observation is that the ratio T2/T1 is a decreasing function of N: a moving agent spends more time closer to the static deformation (N close to one) than further away (larger values of N). The second observation is that the higher the sensitivity (smaller values of ξ), the larger the time spent close to the static deformation, with as much as an unexpected 10 times ratio. This makes the strain mechanosensing a very efficient process to allow cell to migrate toward deformed regions of the substrate.
4. Conclusion
A phenomenological model is developed to describe cell sensitivity to substrate deformations, a process referred to as strain mechanosensing. Our simulations show that this long-range interaction process can be very efficient to allow cells to move toward deformed regions. Consequently, as moving cells exert traction forces and generate their own deformation field, stress mechanosensing is a very good candidate to explain how moving cells could attract each other without any cue of chemical nature.
Additional information
Funding
References
- Checa S, Rausch MK, Petersen A, Kuhl E, Duda GN. 2015. The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech Model Mechanobiol. 14(1):1–13.
- Han Y, Ronceray P, Xu G, Malandrino A, Kamm RD, Lenz M, Broedersz CP, Guo M. 2018. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc Natl Acad Sci Usa. 115(16):4075–4080.
- Marzban B, Yi X, Yuan H. 2018. A minimal mechanics model for mechanosensing of substrate rigidity gradient in durotaxis. Biomech Model Mechanobiol. 17(3):915–922.
- Tanimoto H, Sano M. 2014. A simple force-motion relation for migrating cells revealed by multipole analysis of traction stress. Biophys J. 106(1):16–25.
