1. Introduction
Microfluidics has become an essential tool for cell manipulation, research and diagnosis. In microfluidics, dynamic self-organization with long-range order has often been observed, for emulsions or suspensions of particles (Beatus et al. Citation2012). Such self-organizations, when poorly understood, often perturb the designing process of microfluidic systems. On the contrary, understanding how and why particles organize enables to turn self-organizations into efficient ways of achieving particle sorting, sizing, or ordering, for instance, in specific applications. Red blood cells (RBCs) are known to reach organized structures in small tubes (for instance Tomaiuolo et al. Citation2012). We report here such a self-organization for RBCs flowing in channels having a height comparable to the size of the RBCs and a width 3 to 7 times larger. We are not aware of any study focusing on the flow of deformable particles in such configurations. RBCs are shown to form trains in such configurations, and even lines at specific distances from the walls in some cases. Experiments in microfluidic channels demonstrate the existence of such a phenomenon and provide statistics about train formation (Iss et al. Citation2019). Numerical simulations allow dedicated test cases supporting the analysis of the flows, to identify the physical mechanisms at the origin of these specific organizations.
2. Methods
2.1. Microfluidic experiments
A microfluidic chip made of polydimethylsiloxane (PDMS) (Sylgard 184, Dowsil) is used for the experiments. The part of interest consists in a 6 mm long channel of height 9 ± 1 microns (normal to the field of view) and a width of 30 or 60 microns. A pressure controller generates a flow with typical velocities of the order of 1 mm/s. Blood samples consisting of a droplet of 30 µL obtained via pinprick are immediately processed: RBCs are extracted by washing the blood sample 3 times with Dulbecco’s phosphate buffer saline without calcium and magnesium (Gibco), adjusted with glucose to 295 mOsm and finally resuspended. All details about the experimental method can be found in Iss et al. (Citation2019).
2.2. Numerical simulations
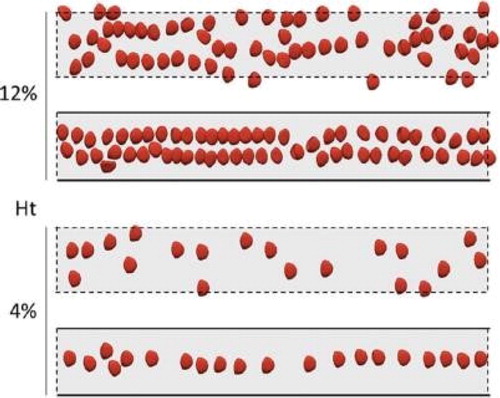
Numerical simulations are performed with the YALES2BIO (http://imag.umontpellier.fr/∼yales2bio) solver, dedicated to the simulation of blood flows, using the immersed boundary method (Sigüenza et al. Citation2016). The red blood cell membrane is modelled as an infinitely thin elastic surface resisting shear, area changes and bending (Mendez and Abkarian Citation2018). The internal viscosity is assumed to be 5 times larger than the suspending viscosity. Simulations are performed in a periodic channel, the organization of the suspension emerging over time instead of space in the experiment. Spherical capsules simulations have also been performed to test the effect of deformability on the behaviour of the suspensions. The volume fraction (haematocrit, Ht) has been varied from 2 to 20%.
3. Results and discussion
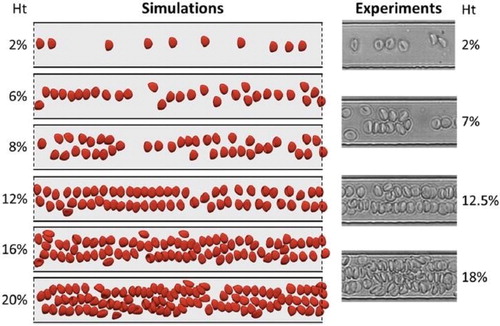
displays results for the self-organization of RBCs in a confined channel. In the simulations, the channel width and height are 30 and 8 microns, respectively. In a dilute suspension, the RBCs leave the near-wall region due to the action of a lift force associated with the presence of the wall and the curvature of the velocity profile (Losserand et al. Citation2019). They form a unique line of cells at the mid-width of the channel. When Ht increases, the one-line configuration is not stable. If the number of RBCs is locally too high, they organize in two lines or even three when Ht is close to 20%. The distance between the RBCs may be short compared to the size of the RBCs. The number of lines is not uniform in the field of view and depends on the bulk haematocrit, but more specifically on the local number of RBCs per unit channel length. also shows the excellent qualitative agreement between the experiments and the simulations, both in terms of RBCs shape and organization in the channel. In larger channels, the near-wall region is also devoid of RBCs, but the organization in the bulk of the channel is less structured, with the emergence of local trains of several RBCs (Iss et al. Citation2019) instead of lines.
Figure 1. Simulations of RBC flow. Left: long-time RBC flows in a periodic 30 micron-wide channel with increasing Ht. The solid and dashed lines refer to wall and periodic boundary conditions, respectively.
Right: Typical images from the experiments.
elucidates the role of lateral confinement by replacing lateral walls (in the width direction) by periodic conditions: in the latter case, RBCs do not organize in lines, but may form trains if the local density is sufficient. Simulations with variable deformability have also demonstrated its importance in the speed at which self-organization is reached, from a random initial situation (Iss et al. Citation2019).
4. Conclusions
Experiments and simulations demonstrate the existence of self-organization in small channels of different aspect ratios. We showed that when the local density of cells is sufficient, confined flows in the height direction promote the formation of trains, which can be understood from the action of quadrupolar interactions between particles (Janssen et al. Citation2012), which tend to realign and particles and make them reach a preferred inter-particle distance. On the contrary, the formation of lines completely depends on the presence of lateral walls, which make RBCs lift away (Losserand et al. Citation2019). This migration away from the wall has two effects: it increases the local density of RBCs, thus favouring interactions, but also creates preferred positions for RBCs travelling in the channel, depending on the number of RBCs travelling side by side. Deformability has also been shown to be an indispensable ingredient to allow such a self-organization. Finally, this phenomenon has the potential to be used to focus and order deformable cells and particles in microfluidic applications.
Additional information
Funding
References
- Beatus T, Bar-Ziv RH, Tlusty T. 2012. The physics of 2D microfluidic droplet ensembles. Phys Rep. 516(3):103–145.
- Iss C, Midou D, Moreau A, Held D, Charrier A, Mendez S, Viallat A, Helfer E. 2019. Selforganization of red blood cell suspensions under confined 2D flows. Soft Matt. 15(14):2971–2980.
- Janssen PJA, Baron MD, Anderson PD, Blawzdziewicz J, Loewenberg M, Wajnryb E. 2012. Collective dynamics of confined rigid spheres and deformable drops. Soft Matt. 8(28):7495–7506.
- Losserand S, Coupier G, Podgorski T. 2019. Migration velocity of red blood cells in microchannels. Microvasc Res. 124:30–36.
- Mendez S, Abkarian M. 2018. In-plane elasticity controls the full dynamics of red blood cells in shear flow. Phys Rev Fluids. 3:101101(R).
- Sigüenza J, Mendez S, Ambard D, Dubois F, Jourdan F, Mozul R, Nicoud F. 2016. Validation of an immersed thick boundary method for simulating fluid–structure interactions of deformable membranes. J Comput Phys. 322:723–746.
- Tomaiuolo G, Lanotte L, Ghigliotti G, Misbah C, Guido S. 2012. Red blood cell clustering in Poiseuille microcapillary flow. Phys Fluids. 24(5):051903.