1. Introduction
Controlled bio-fabrication of biological tissues requires the consideration of complex mechanobiochemical mechanisms involved in cell self-organization. Despite the progress in understanding the principles that underlie morphogenesis at the organ level, we have yet to understand it at the tissue ‘building block’ level: the cells. We aim to develop a computational framework to study tissue morphogenesis through a large spectrum of cell-cell and cell-extracellular matrix (ECM) interactions in two or three dimensions, with a micrometer resolution. To verify our models, simulations are designed to be compared with time-lapse acquisitions of bio-printed cells and ECM. With the conjunction of computational models and bio-printing techniques, we think we can facilitate the investigation of the effects of numerous parameters (cell and ECM initial patterns, ECM remodelling, maturation medium, ECM stiffness).
2. Methods
The software SimCells (Ballet Citation2018), optimized to run on GPU with highly parallelized execution, was enhanced to mix its agents system with a mass-spring system (MSS) representing the ECM. The framework integrates the biochemical and biomechanical cell activities in a spatially and temporally discrete environment. Simulated cells have a three-dimensional deformable membrane, can migrate, divide, die and impact and sense their local environment. The fibrous ECM is represented by a MSS that exhibits non-linear properties and viscoplastic behaviour under cells remodelling mechanisms ().
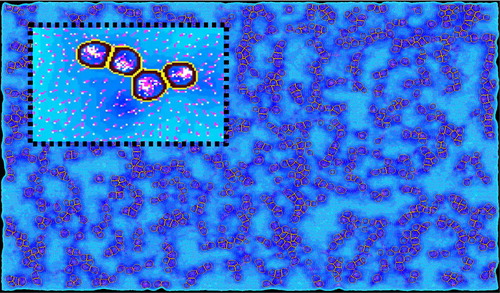
Figure 1. 2D visualisation of a simulation where virtual cells react to and deform the gel (dots), causing mechanical constraints (heatmap) and fiber reorientation (vectors). Scales are arbitrary here.
Laser (Guillotin et al. Citation2010) and extrusion bioprinting are used to deposit fibroblasts with a micrometer resolution on collagen gels with fluorescent microbeads. High spatial and temporal resolution data are generated with time-lapse microscopy. Particle image velocimetry analysis and beads tracking are performed to study local ECM deformations.
3. Results and discussion
The first experimental data helped us to build a two-dimensional prototype of simulation based on qualitative analysis (). In this first simulation, we are essentially interested in the virtual cell competitive adhesion between their cell cluster and the ECM. Preliminary results show promising results to predict tissue deformations between several bio-printed cell clusters. This model will evolve in precision and complexity in conjunction with the image acquisitions and analysis we will be able to perform. We currently work on new AI-based algorithms to obtain other data from those images (cell tracking, ECM fibers analysis). The final scales at which we want to predict 3 D ECM remodelling and deformable cell self-organization are hundreds of thousands of cells inside ∼1 cm3 environments. Software’s and frameworks to create agent-based models allow us to simulate large systems with high numbers of agents (Swat et al. Citation2012; Kang et al. Citation2014), but present several limitations to study the mechanisms at the scales we are interested in. Although ECM fiber-based models have been shown to be closer to reality than homogeneous non-linear models (Rudnicki et al. Citation2013), such precise representation is not compatible with our goals. We want to keep the computation time low to have fast feedback during the modeling process, hence our choice to implement a MSS. MSS models have been shown to successfully predict large ECM deformations (Hughes et al. Citation2018), and we think it is possible to link this kind of model to cell-scale mechanisms.
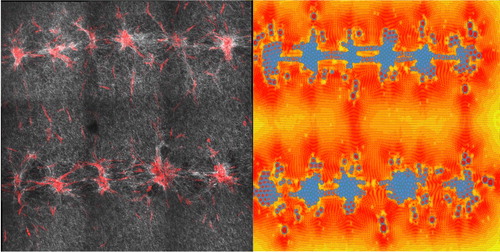
Figure 2. On the left, confocal microscopy and SHG image after 72 h of maturation. On the right, a 2D prototype simulation let us explore the impact of initial cell patterns on ECM deformation (scale bar = 300 µm).
Thus, we present a framework able to include the relevant chemical and mechanical mechanisms needed to explore new insights in mechanobiology at cell and tissue levels.
References
- Ballet P. 2018. SimCells, an advanced software for multicellular modeling: application to tumoral and blood vessel co-development. Avalaible from: https://hal.archives-ouvertes.fr/hal-01853293.
- Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Rémy M, Bordenave L, Amédée J, et al. 2010. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 31(28):7250–7256.
- Hughes AJ, Miyazaki H, Coyle MC, Zhang J, Laurie MT, Chu D, Vavrušová Z, Schneider RA, Klein OD, Gartner ZJ. 2018. Engineered tissue folding by mechanical compaction of the mesenchyme. Development Cell. 44(2):165–178.
- Kang S, Kahan S, McDermott J, Flann N, Shmulevich I. 2014. Biocellion: accelerating computer simulation of multicellular biological system models. Bioinformatics. 30(21):3101–3108.
- Rudnicki MS, Cirka HA, Aghvami M, Sander EA, Wen Q, Billiar KL. 2013. Nonlinear strain stiffening is not sufficient to explain how far cells can feel on fibrous protein gels. Biophys J. 105(1):11–20.
- Swat MH, Thomas GL, Belmonte JM, Shirinifard A, Hmeljak D, Glazier JA. 2012. Multi-scale modeling of tissues using CompuCell3D. Methods Cell Biol. 110:325–366.
