1. Introduction
In the human skeleton, two types of tissue can be found: soft and hard tissue. While the first one encompasses parts such as skin, tendons or internal organs, the second one concerns tissues which are mineralised, in other words, bones of the skeleton. Its mechanical behaviour is investigated for several decades but still remains a challenge nowadays. However, the complexity of the multi-scale architecture makes the cortical bone tough to be easily understood. Numerous studies aims at comparing and correlating the architecture with the macroscopic mechanical behaviour of the bone (Bala et al. Citation2016; Mirzaali et al. Citation2016). A dynamic process, called bone remodelling, occurs and is activated by the osteocytes which detect mechanical stimuli. Then, new bone matrix and architecture are created by a creationresorption process named Bone Multicellular Unit (BMU). Obviously, the activity of the tissue is gender, age, lifestyle and disease dependant. All of these factors make correlating macroscopic mechanical behaviour with microscopic architecture and external elements a major issue to solve. Yet, the majority of architectural studies is limited to a 2D description that is insufficient to understand all phenomena which occur during a mechanical loading. The aim of this paper is to combine a 3D architectural study with macroscopic tensile test of the cortical bone located in two different types of bone: a weight-bearing (Femur) and a non-weight-bearing (Humerus) bone. The novelty of this paper is the use of an innovative method to quantify volumetric features of vascular canals and connectivity within the bone that was reported in a recent study (Roothaer et al. Citation2018), with mechanical testing performed at the same time.
2. Methods
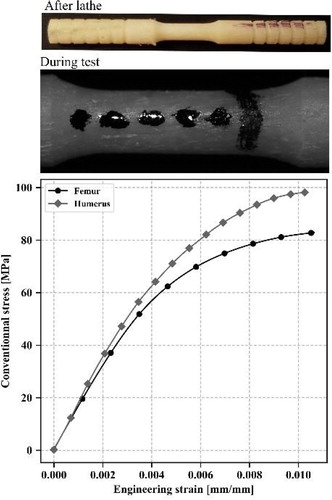
The study consists in the harvesting of dog-bone shaped cortical bone samples from 4 male cadaveric subjects (Age = 80.2 ± 7.6 years old). Hence, 25 samples (14 femur, 11 humerus) were successfully performed. A desktop lathe (Optimum TU 2004 V) was used so as to machine a cylindrical dumbbell sample from mid-shaft of paired humeri and femurs. Each sample has a length of 50 mm, with a 6 mm gauge length on the middle. The diameter of the reduced section is 2.5 mm. Gauge length is fully scanned at a 3 µm isotropic voxel size using a SkyScan 1172 high resolution micro-CT scanner (80 kV, 100 µA, rotation step: 0.25°). This resolution is enough to clearly identify vascular canals within the human cortical bone. Contours of all slices are obtained by applying a global Otsu threshold. Contours are considered as vascular canal contours. Then 17 geometrical features are computed (Roothaer et al. Citation2018). The lacunarcanalicular network isn’t investigated. Subsequently, the cortical sample is tensile tested. A quasi-static loading generating low strain rate (10−4 s−1) is applied using the ElectroPuls E3000 electro-magnetic device (INSTRON). A 3 kN dynamic load capacity is employed and samples are gripped using pneumatic grips so as to keep a constant gripping pressure throughout the test. Strains are acquired with an optical camera (Manta G-895, 1 Hz) associated with black marks drawn on the useful length. Then, displacements are measured from Digital Image Correlation and tracking Matlab toolbox. Finally, engineering strains are computed. Engineering stresses are obtained by dividing measured load by minimal bone matrix area computed from tomographic data. All the data are handled with Python 3 and associate libraries. To preserve bone from drying, specimens were stored in distilled water at 4 °C and tested a maximum of two weeks after the body harvesting.
3. Results and discussion
25 tensile tests were successfully performed up to sample failure. displays the mean stress-strain curves obtained for each bone. This plot reveals that humerus tends to be stiffer than the femur one. This observation is confirmed by comparing elastic modulus of each bone: 16.9 ± 1.9 and 19.1 ± 3.1 GPa are measured for femurs and humeri, respectively. Displacements and load measurements uncertainties are considered and reveal a 7.4 ± 1.9% elastic modulus uncertainty. By individually comparing, same trend is found for each subject. These findings are reinforced by the global and individual values of stress at failure:
82.9 ± 13.8 MPa and 98.4 ± 12.8 MPa for femur and humerus, respectively.
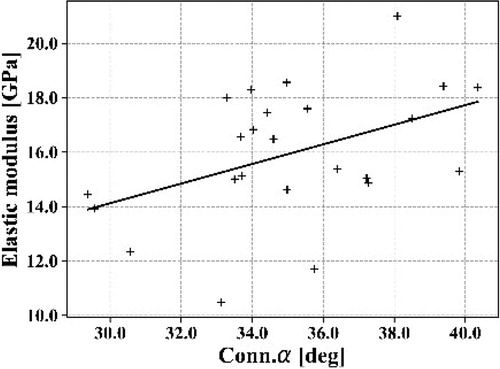
Mechanical data are consistent with similar study (Bry Citation2015; Mirzaali et al. Citation2016). These results show how important it is to consider the type of bone for further mechanical models. Considering the volume porosity, also named BV/TV, non-weight-bearing bone seems to be more porous (8.13 ± 7.46%) than weight-bearing bone (4.75 ± 1.81%). Canals are wider (diameter: 80 ± 36 µm versus 63 ± 14 µm) but shorter (length: 234 ± 70 µm versus 261 ± 25 µm) in humeral shafts. Statistical values highlight that data are more dispersive for humeri than femurs. This could be explained by upper limbs are highly bone remodelling sensitive and thus can strongly differ from one individual to another. Yet, subjects’ background is unknown. The disparity found between left and right arms could be initiated by asymmetric immobilisation (post-surgical consequences), or side preference, for example. A correlation study was assessed and some trends can be highlighted such as bone elastic modulus with volume porosity (spearman value = -0.62) or with the mean connectivity angle (see ). It reveals that connectivity angle has an impact on the mechanical behaviour. A wider connectivity angle implies a stiffer elastic behaviour. This would suggest that narrow connectivity generate local stress concentrations that makes bone softer. Second, it also reveals that the 3D architectural assessment is necessary to understand the behaviour of the bone. Indeed, this angle can only be computed from 3D architectural assessment. However, R2 is weak (0.19) due to highly dispersive values. Therefore, this study needs more samples in order to be more meaningful and to reduce the impact of outliers.
4. Conclusions
The current study consists in the assessment of four male subjects by comparing architectural and mechanical data for a weight bearing and a nonweight-bearing bones. The novelty of the study is the 3 D assessment of vascular cortical porosity associated with quasi-static tensile tests. Humeral samples were stiffer and more porous than femoral ones. Some correlations are found but need to be investigated with more human subjects.
Additional information
Funding
References
- Bala Y, Lefèvre E, Roux J.-P, Baron C, Lasaygues P, Pithioux M, Kaftandjian V, Follet H. 2016. Pore network microarchitecture influences human cortical bone elasticity during growth and aging. J Mech Behav Biomed Mater. 63:164–173.
- Bry R. 2015. Contribution à l’étude de la variabilité des propriétés mécaniques de l’os cortical diaphysaire d’un os porteur (fémur) et non-porteur (humérus). Valenciennes.
- Mirzaali M.J, Schwiedrzik J.J, Thaiwichai S, Best J.P, Michler J, Zysset P.K, Wolfram U. 2016. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone. 93 (Suppl C):196–211.
- Roothaer X, Delille R, Morvan H, Bennani B, Markiewicz E, Fontaine C. 2018. A threedimensional geometric quantification of human cortical canals using an innovative method with micro-computed tomographic data. Micron. 114:62–71.