1. Introduction
Lung biomechanics has been extensively studied by physiologists, experimentally as well as theoretically, laying the ground for our current fundamental understanding of the relationship between function and mechanical behavior. However, many questions remain, notably in the intricate coupling between the multiple constituents. These fundamental questions represent real clinical challenges, as pulmonary diseases are an important health burden. Interstitial lung diseases, for instance, affect several million people globally. Idiopathic Pulmonary Fibrosis (IPF), notably, a progressive form of interstitial lung diseases where some alveolar septa get thicker and stiffer while others get completely damaged, remains poorly understood, poorly diagnosed, and poorly treated (Nunes et al. Citation2015).
2. Methods
2.1. Lung poromechanical model
We recently developed a model of the lungs at the breathing time scale and the organ space scale (Patte et al. Citation2019), based on a general poromechanical formulation compatible with large strains and thermodynamics (Chapelle and Moireau Citation2014), where the “solid” phase is composed of both tissue and blood while the fluid phase is the air. Several pulmonary-specific hypotheses have been formulated, assuming that the transformation is quasi-static and that the fluid pressure inside the alveoli is homogeneous and given. The problem is then simplified and becomes a coupled problem between the two unknowns, the displacement U and the porosity φ.
In our model, specific boundary conditions are imposed on the lungs themselves, modeling the effect of diaphragm-induced loading and rib cage: a pressure applied on lung surface representing pleural pressure, and a frictionless contact with the moving thorax (Patte et al. Citation2019). Moreover, the constitutive behavior of our model allows to reproduce the volumetric response of lungs to a change of pressure as observed in experimental data, as well as the quasi-incompressibility of the solid phase (Patte et al. Citation2019).
2.2. Model personalization
The proposed model can be personalized using clinical data (Patte et al. Citation2019). Two 3 D CT-scans I0 and I1, acquired at end-exhalation and at end-inhalation respectively, are used (1) to generate (after segmentation (Fetita et al. Citation2019)) a patient-specific geometry (see ), (2) to get a personalized porosity field, and (3) to personalize parts of the boundary conditions after processing (Genet et al. Citation2018). In principle, pleural pressure could be measured in patients and used in the model. However, in this work, normal values were used since the data was not available. As a consequence, the results obtained are relative to these values.
The data allows also to personalize parts of the material model. Regional mechanical parameters are estimated by minimizing the error between data and model. We investigated two types of parametrization of the problem, where the estimated material parameters can be the absolute (i.e., independent from porosity, in which case the effective parameters depend linearly on the porosity) or the effective (i.e., those characterizing the mixture) parameters. We also investigated two different cost functions, differing by the nature of the data considered. Either a FEMU strategy is used, considering lung volume displacement computed by image registration (Genet et al. Citation2018), or an integrated image correlation approach is used, based on the 3DCT images directly.
Since the images match with a loaded configuration, we also focused on the inverse problem of finding the unloaded configuration associated with the loaded configuration observed in vivo, and corresponding issues in the poromechanical setting. Indeed, special care should be taken to ensure a positive porosity for the unloaded configuration. We proposed then two methods to that purpose, using either an additional energy describing the collapse of the mixture pores or a contact-like formulation.
3. Results
Using images from patient suffering from pulmonary diseases, regional mechanical parameters can be estimated in diseased lungs. The personalization was applied on a first patient with Idiopathic Pulmonary Fibrosis (IPF).
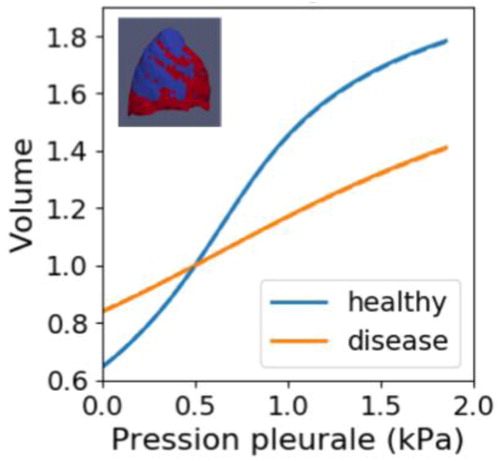
We first showed that the model becomes closer to the data in the case where two material regions are defined, corresponding to the healthy and the diseased regions of the lung, as observed on the images. The same trend appears when the problem is parametrized using absolute parameters. Then the diseased region results in a stiffer material than the healthy region, see . It is consistent with the fact that IPF leads to tissue stiffening.
4. Conclusions
Our results illustrate how our pulmonary poromechanical model can be used as a diagnosis tool in the clinic when it is personalized to a patient using clinical data acquired in routine.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chapelle D, Moireau P. 2014. General coupling of porous flows and hyperelastic formulations—from thermodynamics principles to energy balance and compatible time schemes. Eur J Mech Part B: Fluids. 46:82–96.
- Fetita C, Kim Y-W, Tarando SR, Brillet P-Y. 2019. Image biomarkers for quantitative analysis of idiopathic interstitial pneumonia. Medical Imaging 2019: Computer-Aided Diagnosis.
- Genet M, Stoeck CT, von Deuster C, Lee LC, Kozerke S. 2018. Equilibrated warping: finite element image registration with finite strain equilibrium gap regularization. Med Image Anal. 50:1–22.
- Nunes H, Schubel K, Piver D, Magois E, Feuillet S, Uzunhan Y, Carton Z, Tazi A, Levy P, Brillet P-Y, et al. 2015. Nonspecific interstitial pneumonia: survival is influenced by the underlying cause. Eur Respir J. 45(3):746–755.
- Patte C, Genet M, Fetita C, Brillet P-Y, Chapelle D. 2019. Mécanique pulmonaire personnalisée: Modélisation et estimation—Application à la fibrose pulmonaire. 15ème Colloque National en Calcul de Structures (CSMA 2019). Presqu’île de Giens, Var, France. https://hal.archives-ouvertes.fr/hal-02068920.