 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
The numerical simulation of Growth and Remodelling (G&R) of soft tissues has attracted increasing attention over the last few years, especially in the case of predicting the progression of mechanobiological diseases such as hypertension or aneurysm. For instance, Ascending Thoracic Aortic Aneurysm (ATAA) is a lethal cardiovascular emergency whose computational modelling using Finite Element Methods (FEM), and, in particular case of patient-specific models, is considered challenging due to the existence of complex boundary conditions and geometries in conjunction with layer-specific G&R responses (Mousavi et al. Citation2019). This challenge has been considerably addressed in different literatures. For example, remodeling of a damaged tissue is studied by (Comellas et al. Citation2016) by proposing an original constitutive model. Their model did not consider the evolving pre-stretch of each constituent.
A homogenized Constrained Mixture Model (CMM) was introduce by Braeu et al. (Citation2017) using an informal temporal averaging approach. In contrast to non-homogenized CMM which involves myriads of evolving configurations, the homogenized CMM is designed on the basis of a single time-independent reference configuration per material species. In this case, each point will evolve with a time-dependent inelastic local deformation of G&R. (Latorre and Humphrey Citation2018) recently introduced a new rate-based CMM formulation aimed at tackling G&R problems through a static approach. In this case, they could study mechanobiological equilibrium and stability of soft tissues exposed to transient or sustained changes.
In spite of significant role of CMM methodology in enhancing the insight into arterial wall G&R, it has mostly been employed in the case of canonical problems (Cyron et al. Citation2016). Complementary of those work, (Mousavi et al. Citation2019) pioneered on proposing a nonlinear FEM-based solution on the homogenized CMT to simulate G&R in patient-specific ATAA. In this work, active smooth muscle tension is additionally considered to that presented model in order to investigate its effects on the progression of the G&R on a patient-specific ATAA geometry.
2. Methods and material models
The homogenized Constrained Mixture Model (CMM) is employed in this work in order to model a multi-layer (media and adventitia) arterial wall comprised of different constituents such as elastin collagen fiber families
and Smooth Muscle Cells (SMCs)
Each constituent possess a deposition stretch of
with
with respect to the reference homeostatic configuration leading to an individual deformation gradient per constituent. Therefore, differential mass increment of the
th constituent deposited at time, the total deformation gradient will read
This can be re-expressed by a multiplicative decomposition of the total deformation gradient into an elastic,
and inelastic parts,
leading to
Note that all the mixture constituents deform in the stress field together under a total deformation gradient
The mechanobiological constitutive model is implemented as a UMAT in ABAQUS on the basis of the concept of deposition stretch (Mousavi and Avril Citation2017). In this case, a nearly incompressible neo-Hookean strain energy density for the multi-layered model (media and adventitia) is employed for elastin constituent while Fung-type passive strain energy is utilized for four collagen fiber families and SMCs (Braeu et al. Citation2017, Mousavi et al. Citation2019).
with
and
denotes mass fraction of the corresponding constituent,
and
are stress-like material parameters,
stands for bulk modulus,
and
are dimensionless material parameters and
denotes the corresponding fiber direction in reference configuration. Regarding the active part of the SMCs energy functional which is the main objective of the current study,
is the active stretch in the fiber direction which is comprised of the ratio
Here,
is the total stretch in fiber direction and
is the total stretch in the fiber direction at hemostasis state. This implies that
when we are in mechanical equilibrium.
denotes the maximal active Cauchy stress and
and
are the active stretches at maximum and zero active stresses.
Finally, the model is applied onto the geometry of a real human ATAA in response to a localized elastin loss in order to investigate the process of G&R.
3. Results and discussion
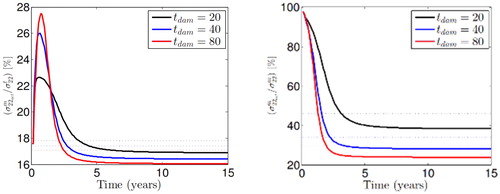
The contribution of the active SMCs stresses into the SMCs and total stress field is initially studied on the basis of the utilized strain energy functional (see ). The results show that depending on the elastin degradation damage time, the contribution of the active SMCs stresses into the total SMCs and total stress fields in circumferential direction are approximately %20 and %40, respectively. This, in fact, demonstrates the significant role of the active SMCs contribution into the stress field.
Figure 1. Active SMCs contribution into SMCs and total stress in circumferntial direction for different elastin degradation damage time.
shows the normalized collagen mass density and maximum principal stress distribution in a two-layer patient-specific human ATAA. The elastin loss results in continuous adaptation of stress regime due to collagen deposition in media. In this case, the active stress of the SMCs, behaving on the basis of its hill-functionality, increases the amount of stress which results in a transfer of stress from media to the adventitia layer. The results show that the faster rate of collagen deposition in media leads to less contractility of the SMCs. Indeed, the increase in collagen deposition results in a thicker media layer and, therefore, more stiff arterial wall. In addition, it is observed that the adaptation of SMCs to elastin degradation depends on their active stresses corresponding to their maximum active stretches.
Figure 2. Normalized collagen mass density (left) and maximum principal stress distribution (right) in a two-layer patient-specific human ATAA.
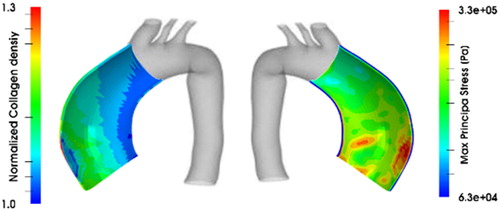
In conclusion, the SMCs contractility has a considerable contribution (as observed around 20%) in the stress fields and that, the stress transfers from media to adventitia layer. In addition, the adaptation of stress regime due to elastin degradation could dramatically depend on the collagen deposition and SMCs’ contractility. The role of the latter decrease as the rate of collagen deposition increases.
References
- Braeu FA, Seitz A, Aydin RC, Cyron RC. 2017. Homogenized constrained mixture models for anisotropic volumetric growth and remodeling. Biomech Model Mechanobiol. 16(3):889–906.
- Comellas E, Gasser TC, Bellomo FJ, Oller S. 2016. A homeostatic driven turnover remodelling constitutive model for healing in soft tissues. J R Soc Interface. 13(116):12.
- Cyron CJ, Aydin RC, Humphrey JD. 2016. A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech Model Mechanobiol. 15(6):1389–1403.
- Ghavamian A, Mousavi SJ, Avril S. 2020. Computational Study of Growth and Remodeling in Aortic Aneurysms Considering the Length-Tension Relationship of Smooth Muscle Cells. Frontiers in Bioengineering and Biotechnology. Under reviwe.
- Latorre M, Humphrey JD. 2018. Critical roles of time-scales in soft tissue growth and remodeling. APL Bioeng. 2(2):026108
- Mousavi SJ, Farzaneh S, Avril S. 2019. Patient-specific predictions of aneurysm growth and remodeling in the ascending thoracic aorta using the homogenized constrained mixture model. Biomech Model Mechanobiol. 18(6):1895–1913.
