?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Keywords:
1. Introduction
The cornea is the outermost transparent tissue of the eye composed of five distinct layers. The keratoconus disease is described as a progressive and non-inflammatory corneal ectasia caused by decrease in the biomechanical strength of the tissue. As the cornea becomes thinner and softer, it cannot support the inner eye pressure anymore and assumes a conical shape which leads to increasingly blurred vision (Ferrari and Rama Citation2020).
A quantity of prospective, retrospective, histologic, proteomic and genomic studies have been conducted on Keratoconus but the exact pathogenesis is still considered an enigma. This observation may favorize a mechanical origin of the disease. Indeed, many ophthalmologists recommend their patients to stop rubbing their eyes to avoid a further development of the ectasia. However, the link between eye rubbing and Keratoconus physiopathology needs clarification (Naderan et al. Citation2015).
This paper uses numerical simulation to investigate the mechanical degradation that could arises from eye rubbing and the possible link with the development of the disease. Added to this, we successfully modelled the state of a keratoconic cornea and performed a parametric analysis on the mechanical support of the different layers according to their rigidity and fibers organization.
2. Methods
A biomechanical model is used to describe the cornea mechanical behavior, taking into account 3D fiber dispersion. We modelled a geometry composed of the 5 layers of the cornea inserted into a piece of deformable sclera to allow realistic boundary conditions. The border of the sclera is then defined as fully blocked.
We modelled preferential directions for fibers in the stroma of the cornea, with 2 orthogonal families of fibers in posterior parts and a more random 3D directions in the anterior parts of the stroma (Cheng et al. Citation2015; Meek and Boote Citation2009; Montanino et al. Citation2018).
The internal surfacic pressure applied inside the cornea represent the fluidic intra ocular pressure set to 17.1 mmHg = 2280 Pa.
2.1. Constitutive equation
The Holzapfel-Gasser-Ogden constitutive law is chosen to account for strain-hardening hyperelasticity and anisotropy of the tissue with several families of fibers having different directions and distributions. This mechanistic material model is versatile and has been applied already to a lot of different soft tissues and to the analysis of the human cornea in several studies (Montanino et al. Citation2018).
The local deformation is decomposed into volume-changing (volumetric) and volume-preserving (isochoric or deviatoric) parts. The strain energy (W) is related to the strain invariants (I) expressed with stretch ratio.
The solution is computed with an implicit scheme.
3. Results and discussion
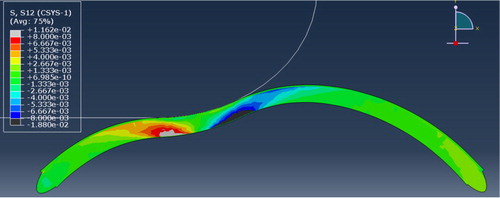
We performed a FEA of a virtual rigid finger rubbing the cornea with a frictionless contact to reproduce the presence of the eyelid. The simulation pointed out that collagen lamellae in posterior areas are most stressed and most likely to endure damage, ply delamination and slip than anterior areas during simulation of eye rubbing ().
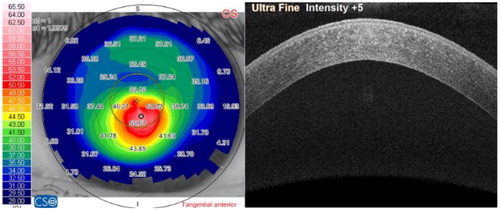
It may signify that a habit of eye rubbing can cause damage to posterior parts of the tissue. This hypothesis is supported by early OCT imaging of keratoconic corneas that do not show anterior layers degradation ().
Added to this, histological studies showed that anterior parts of the cornea are also particularly degraded in late stage of the disease (Fernandes et al. Citation2008; Ferrari and Rama Citation2020), meaning that degradative processes occur as well in anterior layers and participate to the disease progression.
In order to figure out how anterior and posterior microstructural degradation can lead to tissue thinning and ectasia, we ran several parametric analysis and highlighted the influence of each layers degradation in the disease. We saw that tissue remodeling from pathological origin can induce conic deformation only under particular conditions ().
Figure 3. Section view of corneal deformation against intra ocular pressure with assigned pathological mechanical properties.
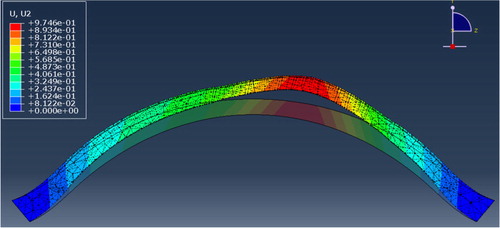
In comparison to the softening of the tissue by a factor 10 that we applied, the most important result we got was the predominant influence of collagen lamellae direction in the posterior corneal layers to induce the conic deformation. From this result we assumed that tissue remodeling with lamellae slippage away from the cone apex may play a major role in the pathogenesis like hypothetized earlier by Meek and Boote (Citation2009).
4. Conclusions
Our results show that both anterior and posterior layers degradation have influence in the pathogenesis of Keratoconus, with a possible early posterior degradation. By changing the orientation of the fibres from parallel to the surface to ectatic, we saw that the conical shape is recreated without altering the anterior layers of the cornea. This result differs from the current theory regarding the pathogenesis of keratoconus stating the involvement of epithelial cells and degradation of anterior layers in the disease process (Fernandes et al. Citation2008; Meek and Boote Citation2009; Ferrari and Rama Citation2020).
The hypothesis ready for discussion is that the mechanical supporting role of the posterior parts is also affected in the disease. Since the majority of studies have focused on the damage to anterior layers, they have neglected the importance of the posterior stroma in the biomechanics of the cornea.
In order to address the precise relationship between eye rubbing and Keratoconus, the hypothesis of mechanical damage caused by eye rubbing and consecutive lamellae slippage will be discussed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This work is supported by a grant from Region Grand Est, France.
References
- Cheng X, Petsche SJ, Pinsky PM. 2015. A structural model for the in vivo human cornea including collagen-swelling interaction. J R Soc Interface. 12(109):20150241
- Fernandes BF, Logan P, Zajdenweber ME, Santos LN, Cheema DP, Burnier MN. 2008. Histopathological study of 49 cases of keratoconus. Pathology. 40(6):623–626.
- Ferrari G, Rama P. 2020. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul Surf. 18(3):363–373.
- Meek KM, Boote C. 2009. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res. 28(5):369–392.
- Montanino A, Gizzi A, Vasta M, Angelillo M, Pandolfi A. 2018. Modeling the biomechanics of the human cornea accounting for local variations of the collagen fibril architecture. Z Angew Math Mech. 98(12):2122–2134.
- Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M. 2015. Characteristics and associations of keratoconus patients. Cont Lens Anterior Eye. 38(3):199–205.