1. Introduction
Bones are the third most affected organs by metastasis (Du et al. 2010). The qualitative effect of cancer types on metastatic bone has been largely described. Bone metastases weaken bones (Coleman Citation1997) by creating lytic, blastic or mixt (i.e. lytic and blastic) lesions. The prediction of fracture risk is clinically assessed by evaluating several parameters such as the size and location of the lesion or the Mirels’ score. However, it seems these predictions overestimate the risk of fracture and are not specific enough to produce a reliable prediction (Van Der Linden et al. Citation2004).
A quantification of the effect of the different cancer types on mechanical properties of the bone should improve clinical evaluation of the fracture risk. As a first step, the goal of our study was to develop a better assessment of the bone fracture risk by evaluating the mechanical properties of mice tibia affected by three different human cancer cell lines.
2. Methods
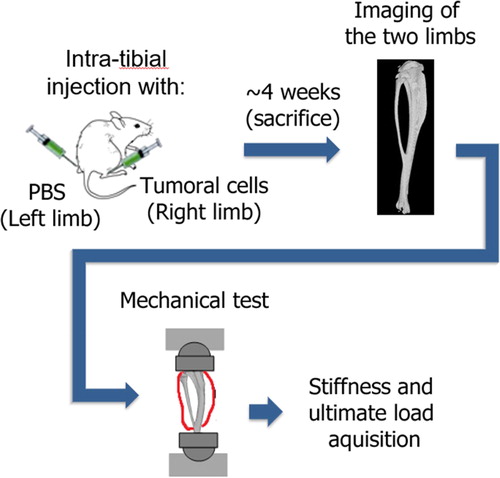
Six-weeks-old BALB/c nude mice (n = 39) were intra-tibially injected with three different tumor cells lines in their right limb and PBS (Phosphate Buffered Saline) in the left limb (sham limb). Sixteen mice received 100,000 human B02 tumor cells (breast cancer, lytic), 12 mice had 800,000 CMET cells (lung cancer, mixt) and 11 mice had 100,000 KRAS cells (lung cancer, lytic). Previously, the number of cells was chosen to approximately obtain the same metastasis development (i.e. same size) in 30 days. Control mice (n = 8) were injected with PBS in both limbs to evaluate the bone resistance without metastasis.
After 30 days, mice were sacrificed, and both tibias were subjected to a compressive test (Bose Access 5500). The distal part was embedded in resin and a mold was made on the proximal part to homogeneously distribute the load. Each tibia went through a pre-cycling (30 cycles between −0.5 N and −2 N at 0.5 Hz) immediately followed by a quasi-static test (speed 0.03 mm/s) until failure (Delpuech et al. Citation2019). Axial load (0.03 N accuracy) and displacement (3 µm accuracy) were sampled during the test at 60 Hz. The stiffness and ultimate load of each limb were extracted from these tests (). A Kruskal-Wallis and Mann-Whitney U tests were then computed in order to evaluate the differences.
3. Results and discussion
After Kruskal-Wallis test, Mann-Whitney U tests showed significant differences between the resulting ultimate load of the control group with each of the three other groups (p < 0.001) while there were no differences between the B02, CMET and KRAS cell lines (p ≫ 0.05) (, ). The same result was found for the stiffness (, ).
Figure 2. A: Ultimate load of mice tibias for each group. B: Stiffness of mice tibias for each group. *** p-value < 0.001 for Mann-Whitney U test with control group (n: number of samples).
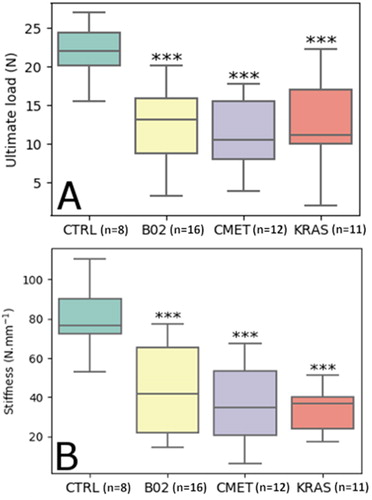
Table 1. Mean (SD) of each group for ultimate load and stiffness (n: number of samples).
The three tested cell lines reduced the bone strength and stiffness in the same order of magnitude.
The cancer type (osteolytic or mixt) did not influence the bone mechanical properties with the current protocol (same metastasis development but different cells number).
These results are to be taken cautiously as several uncertainties could not be perfectly controlled such as the tibia orientation which could variate around a roughly estimated ± 10° along the sagittal and frontal axis, due to the opacity of the soft tissues that could not be removed as it would probably imply a degradation of the metastasis area. Another source of uncertainty appears in the evaluation of the stiffness and ultimate load which were quantified over the machine displacement and load data. Machine displacement is not strictly equivalent to the bone displacement as soft tissues and the embedding material could impact the behavior of the embedding material/bone system (Maquer et al. Citation2014).
4. Conclusion
The three types of cancer cells considered in the current study reduced the stiffness and ultimate load of the mice tibia. There was no significant difference among the three types of cancer cell for both parameters with an identical lesion size.
Based on these experimental data, the next step will use Finite Elements Analysis on the already scanned tibias to assess bone failure strength.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partly funded by LabEx Primes (ANR-11-LABX-0063) and MSDAVENIR Research Grant.
References
- Coleman, R. E. (1997). Skeletal complications of malignancy. Cancer 80:1588–1594.
- Delpuech B, Nicolle S, Confavreux C, Bouazza l, Clezardin P, Mitton D, Follet H. 2019. Failure prediction of metastatic bone with osteolytic lesion in mice. In: Proceedings of the 23rd congress of the European Society of Biomechanics. Presented at the 25th congress of the European Society of Biomechanics, Vienne, Autriche, p. 2 (oral presentation).
- Du Z-Y, Zang J, Tang X-D, Guo W, Chinese Orthopaedic Association Bone Oncology Group. 2010. Experts' agreement on therapy for bone metastases. Orthop Surg. 2(4):241–253.
- Maquer G, Schwiedrzik J, Zysset PK. 2014. Embedding of human vertebral bodies leads to higher ultimate load and altered damage localisation under axial compression. Comput Methods Biomech Biomed Eng. 17(12):1311–1322.
- Van der Linden YM, Dijkstra PDS, Kroon HM, Lok JJ, Noordijk EM, Leer JWH, Marijnen CAM. 2004. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 86(4):566–573.