1. Introduction
Our group aims at proposing a protocol for evaluating the subject-specific risk for sacral pressure ulcer (PU). The idea is to model the way soft tissue will be deformed under the external pressure and to compute internal strains. It has been indeed showed that high strains can create deep tissue injuries (Ceelen et al. Citation2008). Because of the clinical constraints, we propose to use Ultrasound (US) to build the subject-specific biomechanical model of the sacral soft tissue as an alternative to 3D MR imaging (because of cost, time and accessibility). This paper focuses on the way such a subject-specific model will be designed and more specifically on the methodology we propose for estimating the constitutive parameters of the internal sacral soft tissue, namely the adipose tissue and the muscle. The values of these parameters have indeed a strong influence on the computed internal strains.
2. Methods
2.1. Experimental set up and the numerical model
One subject participated in the experiment (male, 33 y.o., BMI = 26.1 kg/m2). Data for the subdermal tissue were acquired for the participant laying in the prone position. An ultrasound acquisition of the subdermal tissue in the region above the superior process of median sacral crest was performed using a commercial device (Aixplorer, SuperSonic Imagine, France) with a linear ultrasound (US) probe of 8 MHz central frequency (SuperLinear SL 15-4). Custom made load sensor was attached to the ultrasound probe (Fougeron et al. Citation2020). Several cycles of the indentation with the probe were performed and the one with the clearest video frames in the beginning and the end of the indentation cycle was chosen as a basis to build a 2D finite element (FE) model in Ansys 19.2 software. The model geometry was obtained by extracting the coordinates of the splines delimiting the skin, fat, muscle and bone via Matlab R2019a and by converting the units from pixels to millimeters. For this purpose, the first frame of the indentation cycle was used ().
Several assumptions were made: since the model was built in 2D, the plane strain formulation was used; and the US probe was assumed to be in contact with the skin throughout 2 cm in width (relevant for the recalculation of the force applied by the probe from 3D to 2D). The load cycle was divided into 4 load steps for the consecutive comparison between the frames of the US data with the results of the numerical model.
All displacements were constrained on the lower wall of the 2D model, below the bone, and the bonded contact was used for the nodes of the different tissue types. The load was applied to the top of the US probe, with only Y direction displacement of the probe being allowed. The no-separation contact was used between the probe and the skin. And plane183 element type was used for the mesh.
2.2. Constitutive model
For the bone, the linear elastic material model was used with Young’s modulus of 13.4 GPa, Poisson’s ratio (ν) of 0.3 based on the values reported in the literature (Rho et al. Citation1997). First-order Ogden hyperelastic material model was chosen to represent the soft tissues behavior. The values for the skin were fixed to 20 kPa for the shear modulus (µ1) and 5 for the exponent (α) based on the values reported in the literature (Luboz et al. Citation2014). The same value of α = 5 was set up for both muscle and adipose tissues. The calculation of d (incompressibility parameter) was based on the literature (Macron et al. Citation2018).
2.3. Calibration of the material properties
The Matlab algorithm using the least square method was utilized for the inverse evaluation of the soft tissues’ (adipose tissue and muscle) material parameters. Two cases were compared: (1) optimization based on the variation in only the total thickness of soft tissues above the sacrum (2) optimization based on a combination of the two error vectors (for the variation in the total thickness and separately for the variation in muscle thickness). A sensitivity analysis of the shear moduli values to the change in values of exponents (α) and Poisson’s ratios (ν) was performed: first, only the shear moduli values for both analyzed tissues were optimized, while α and ν were fixed. And after, this optimization was recalculated changing the fixed values of α and ν by 1%. Change in Poisson's ratios showed to have a noticeable effect (up to 30%) on the values of the µ1.
Therefore, four parameters were calculated in the end of optimization process: Poisson’s ratios and shear moduli for adipose and muscle tissues.
3. Results and discussion
The values of the material properties of the analyzed soft tissues obtained after the optimization process are provided in .
Table 1. Resultant shear modulus and Poisson’s ratio parameters of the two cases of optimization for muscle (_m) and fat (_f) tissues.
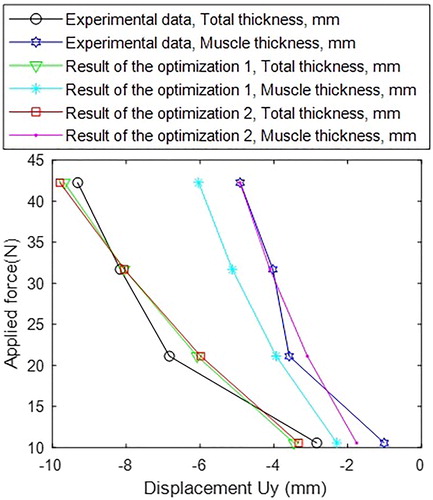
shows the comparison between the change in the total and muscle thicknesses in the numerical model using the optimal µ1 and ν parameters from two optimizations and the values from the US images.
While the results of two optimizations were close for the total thickness of the soft tissues, they vary for the evaluation of the muscle thickness. This suggests that the minor improvement in one parameter (total thickness) could have a major effect on the separate tissue types if they are not considered during the optimization.
The proposed FE model also has some limitations: due to acquisitions being performed manually, slight change of the probe angle is allowed, which could have an effect on the tissue’s geometry during the indentation cycle. Future work will include the development of the protocol to reduce this bias. Another limitation is the 2D modality of US, effects of that will be assessed in the following work.
4. Conclusions
Presented work shows the possibility of using the US data for the assessment of the soft tissues’ material properties. This is clinically relevant, since US allows to obtain a subject-specific anatomical data, while being much more accessible and less costly than MRI. Results also imply the importance of using several parameters representing different types of soft tissues for the inverse optimization of the material properties.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 811965.
References
- Ceelen KK, Stekelenburg A, Loerakker S, Strijkers GJ, Bader DL, Nicolay K, Baaijens FPT, Oomens CWJ. 2008. Compression-induced damage and internal tissue strains are related. J Biomech. 41(16):3399–3404.
- Luboz V, Petrizelli M, Bucki M, Diot B, Vuillerme N, Payan Y. 2014. Biomechanical modeling to prevent ischial pressure ulcers. J Biomech. 47(10):2231–2236.
- Rho JY, Tsui TY, Pharr GM. 1997. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials. 18(20):1325–1330.
- Macron A, Pillet H, Doridam J, Verney A, Rohan PY. 2018. Development and evaluation of a new methodology for the fast generation of patient-specific finite element models of the buttock for sitting-acquired deep tissue injury prevention. J Biomech. 79:173–180.
- Fougeron N, Rohan P-Y, Haering D, Rose J-L, Bonnet X, Pillet H. 2020. Combining freehand ultrasound-based indentation and inverse finite element modelling for the identification of hyperelastic material properties of thigh soft tissues. J Biomech Eng c. 142(9):22.