 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Organs-on-chip is one of the most promising tools for producing more realistic in vitro models of human organs and tissues. One limitation of current organ-on-chip systems is the difficulty of developing the associated vasculature, which explains the growing interest in microvessel-on-chip systems (Pradhan et al. Citation2020).
Two fabrication approaches are used to create the microvessels-on-chip: self-assembly and guided growth. In the first case, cells are seeded in a hydrogel matrix and allowed to auto-organize to form microvessel structures by vasculogenesis (Campisi et al. Citation2018), while in the second case, cell growth is guided using pre-fabricated vessel structures (Polacheck et al. Citation2019). Typically, these systems are mainly used for drug testing and for developing biological models of microvascular diseases.
An additional interest in microvessel-on-chip systems stems from the fact that the mechanical environment can be finely tuned, which promises to enhance our understanding of vascular mechanobiology and to enable controlled mechanistic studies of microvascular pathologies. Therefore, the aim of this project is to develop a simple microvessel-on-chip where the effects of individual mechanical factors can be explored.
2. Methods
2.1. Microvessel fabrication
We use the guided growth method to fabricate the microvessel. The chip consists of a pre-fabricated PDMS (poly-di-methyl-siloxane) scaffold, where a 120 µm-diameter needle is inserted into an open and empty chamber. Liquid collagen is poured around the needle and allowed to polymerize ().
Figure 1. HUVEC-lined microvessel-on-chip. (a) Microchip schema. (b) HUVECs of the wall of the vessel. Stained with phalloidin green (actin) and DAPi (nucleus). (c) V-cadherin in red (adherent junctions). (d) Dextran inside the microvessel lumen. Fluorescence imaging with 70 kDa TRITC-dextran. (e) Time series of dextran diffusion into the hydrogel.
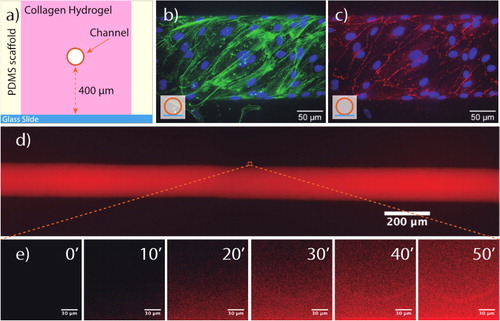
After collagen hydrogel formation, the needle is removed, leaving an open channel that constitutes the microvessel lumen. Human umbilical vein endothelial cells (HUVECs) are then flown into the lumen and allowed to sediment for 10 min, with the excess cells removed after that time by a small flow. The cells are cultured under constant low flow perfusion (shear stress of ∼0.1 Pa) for a period of 72 h to ensure the maturation of cell-cell junctions () and the formation of a confluent monolayer.
2.2. Permeability quantification
An important function of endothelial cells is to regulate vascular permeability (Linville et al. Citation2020). We have therefore focused on the permeability of our microvessel-on-chip to molecules of different sizes both in the presence and absence of endothelial cells and under both static and flow conditions. To quantify permeability, we have measured the transport of fluorescently labeled dextran of different molecular weights (3 to 70 kDa) from the microvessel lumen into the collagen hydrogel within which the channel is formed.
To measure the permeability under the different conditions, we used fluorescence video-microscopy to acquire images at seven different positions in the chip every 15 sec. The intensity profiles were extracted with ImageJ. shows HUVEC-lined microvessel lumen full of dextran diffusing into the hydrogel. As can be observed in , dextran diffuses from the channel into the hydrogel progressively over time. The rate of change of the recorded intensity within the hydrogel decreases with increasing dextran molecular weight.
As shown elsewhere (Huxley et al. Citation1987), the permeability () can be computed as follows:
where
is the rate of change of fluorescence intensity within the hydrogel,
is the increase in the intensity until luminal filling and
is the microvessel radius. All post-processing of the intensity data is performed in Matlab.
3. Results and discussion
After 72 h of low flow (shear stress of ∼0.1 Pa), HUVECs reach a confluent monolayer, as can be seen from the images of the lumen wall ().
Characterization of dextran transport across the microvessel wall shows that lining the channel walls with endothelial cells significantly reduces microvessel permeability, confirming that this cellular layer acts as a barrier. shows that for 70 kDa dextran, when no cells are present, dextran diffuses rapidly from the lumen into the hydrogel. In contrast, when HUVECs are present, dextran accumulation outside the microvessel wall (dashed lines) is much slower with a significant dextran concentration difference between the lumen (solid line) and the wall throughout the recording period, indicating that the vessel acts as an effective transport barrier.
Figure 2. Time evolution of the fluorescence intensity of 70 kDa dextran. Green: microvessel with no cells; blue: microvessel lined with HUVECs. The solid line indicates the change in the lumen, while the dashed and dash-dot lines, respectively show the change in regions of the hydrogel wall slightly above and below the channel. The arrow denotes the point at which dextran is introduced, and regions I, II and III, respectively correspond to the time required for dextran to reach the channel, completely fill the lumen, and diffuse from the lumen into the vessel wall.
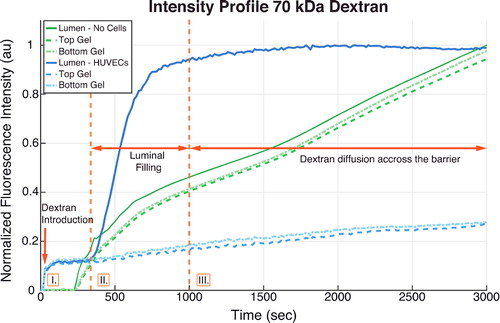
For lower molecular weight dextran, channel filling occurs much more rapidly. The computed permeability is generally in the range of 10−7 to 10−6 cm/s, denoting that the HUVEC-lined microvessel wall acts as a selective barrier for different molecular sizes. As expected, the permeability decreases significantly with increasing dextran molecular weight.
4. Conclusions
We have developed a microvessel-on-chip whose endothelial lining acts as an effective permeability barrier. Current efforts are focused on further quantifying the permeability measurements, evaluating the effect of flow on permeability, and elucidating if permeability regulation is affected by the presence of other mural cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 2018. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 180:117–129.
- Huxley VH, Curry FE, Adamson RH. 1987. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol Heart Circul Physiol. 252(1):H188–H197.
- Linville RM, DeStefano JG, Sklar MB, Chu C, Walczak P, Searson PC. 2020. Modeling hyperosmotic blood-brain barrier opening within human tissue-engineered in vitro brain microvessels. J Cereb Blood Flow Metab. 40(7):1517–1532.
- Polacheck WJ, Kutys ML, Tefft JB, Chen CS. 2019. Microfabricated blood vessels for modeling the vascular transport barrier. Nat Protoc. 14(5):1425–1145.
- Pradhan S, Banda OA, Farino CJ, Sperduto JL, Keller KA, Taitano R, Slater JH. 2020. Biofabrication strategies and engineered in vitro systems for vascular mechanobiology. Adv Healthcare Mater. 9(8):1901255.
