Abstract
In this paper, a model for the radiation treatment of cancer which includes the effects of the cell cycle is derived from first principles. A malignant cell population is divided into two compartments based on radiation sensitivities. The active compartment includes the four phases of the cell cycle, while the quiescent compartment consists of the G 0 state. Analysis of this active-quiescent radiation model confirms the classical interpretation of the linear quadratic (LQ) model, which is that a larger α/β ratio corresponds to a fast cell cycle, while a smaller ratio corresponds to a slow cell cycle. Additionally, we find that a large α/β ratio indicates the existence of a significant quiescent phase. The active-quiescent model is extended as a nonlinear birth–death process in order to derive an explicit time dependent expression for the tumour control probability (TCP). This work extends the TCP formula from Zaider and Minerbo and it enables the TCP to be calculated for general time dependent treatment schedules.
Keywords:
1. Introduction
Cancer is a disease that affects millions of people worldwide. According to the World Health Organization, 12.5% of total deaths every year worldwide are caused by cancer [Citation14]. Also, in 30% of these cases, the patients could have been cured had they received early diagnosis and effective treatment. Time and cost are two factors that limit the number of patients eligible to receive early treatment. For these reasons, mathematical modelling is a helpful tool in not only predicting tumour growth and cancer spread, but also in determining the effectiveness of a specific treatment.
One of the common therapies used to treat cancer is external beam radiotherapy. This treatment works by transferring energy to a cell, which causes structural damage that affects cell viability. Based on the interaction of radiation with individual cells, we derive radiation dependent death rates for tumour cells. The main objective in this work is to derive a mathematical model for the radiation treatment of cancer, which includes cell cycle dynamics, that can be used explicitly to compute the tumour control probability (TCP). The TCP can be used to predict the outcome of a given treatment schedule.
A widely used mathematical model of the radiation treatment of cancer is the linear quadratic (LQ) model, which predicts the surviving fraction of clonogens after a treatment of dose D is applied to a tumour. This model, in its basic form, assumes that the tumour cell population is uniform and that the effect of the treatment is independent of the cell cycle. This assumption leads to an oversimplification which prevents complete understanding of the system dynamics. In this paper, we divide the cell population into two compartments: active cells, representing cells in the G 1, S, G 2 and M phases and quiescent cells, representing cells in the G 0 phase. We use this simple, two compartment setup to explain the derivation of a TCP formula. We believe that the method used can be extended to describe a system with more compartments; however, the computations would become very tedious. In this work, we consider only a two-compartment model and leave the inclusion of more compartments for future studies. Let u(t) represent the active cells and q(t) the quiescent cells. The basic cell cycle radiation model of this paper is then
The assumption that new daughter cells enter directly into the G 0 phase is supported by many experimental observations and has been used in Swierniak [Citation22].
Formerly, as in Ref. [Citation17], the G
0-phase was not distinguished from the G
1-phase. Pioneering cellular biologists believed there to be only four cell cycle phases: G
1, S, G
2 and M. However, the G
1 phase was recognized as being highly variable in length and in cellular activity. This phase variability was due to the fact that many cells were in a period of inactivity, now known as the G
0 phase. Our model is equally valid for a model for a u-compartment consisting of S, G
2 and M phases and a q compartment consisting of the G
0 and G
1 phases. The model's ability to describe the basic biological system demonstrates one of the strengths of mathematical modelling, which is that we can generalize and derive basic properties that are valid for a wide range of applications. In model (Equation1), the time dependent parameters Γ
u
(t) and Γ
q
(t) denote the radiation damage to the two cell compartments, respectively.
To derive radiation induced death rates Γ
u
and Γ
q
, we begin modelling on the subcellular scale. A critical event is DNA double strand breaks (DSB) that occur by the interaction of two radiation energy depositions, referred to as single-hit events. We make the transition to the cellular level and derive probabilities of cell death. Based on these probabilities, we define macroscopic death rates, Γ
u
and Γ
q
to make the transition to a fully macroscopic model (Equation1). In this procedure, we follow the scaling hierarchy from microscopic to macroscopic that was also suggested in Bellomo and Maini [Citation3].
To finally derive a formula for the TCP, we go back to the cellular scale and consider a nonlinear birth–death process describing the cell kinetics, where we assume f(u) = μu. The main result of this paper is the extension of the active-quiescent model (Equation1) to a nonlinear birth–death process (see equations (Equation32
) and (Equation33
)) which is explicitly solved to obtain the dynamic equation for the TCP, which is given by
We use the remainder of the introduction to present a brief overview of the cell cycle in section 1.1, and of radiation treatment in section 1.2. In section 1.3, we review existing TCP models, in particular the work of Zaider and Minerbo [Citation25]. In section 2, we give a detailed, physically based derivation of the radiation cell cycle model (Equation1). We use singular perturbation arguments in section 3 to investigate the effects of the model parameters μ and γ on the model dynamics. This analysis allows us to compare model (Equation1
) to the LQ-model. We relate the standard α/β ratio to our model parameters and we find that a large α/β ratio indicates the presence of a significant quiescent compartment. In section 4, we extend the active-quiescent model (Equation1
) to a nonlinear birth–death process such that the mean field equations are given by (Equation1
). We derive a system of hyperbolic partial differential equations for the corresponding moment generating functions and we explicitly solve this hyperbolic system. This procedure leads to the explicit TCP formula in equation (Equation2
). Lastly, in section 5, we summarise and discuss our results. The TCP formula (Equation2
) is used in Yurtseven et al. [Citation24] to investigate various treatment schedules.
1.1 The cell cycle
Here, we review some basic facts about the cell cycle from Refs. [Citation1,Citation7,Citation13]. The cell cycle can be split into four phases, where the culmination occurs when the cell divides and two daughter cells are formed. Although not considered part of the cell cycle, it is also important to recognize that there is a resting phase that cells may enter. This
phase is very important when modelling radiation treatment of cancer because the cell is less sensitive to radiation when in this phase.
The first phase is known as the gap 1 phase . It occurs just after the cell has split, but must not be mistaken for the resting phase
. During this phase, the cell begins to manufacture more proteins in preparation for division. It also experiences other growth: metabolism increases, RNA synthesis is elevated, and organelles duplicate. This phase lasts about 12 h. The second phase is known as the stationary phase, although a cell in the S phase is biologically active. During the S phase, the DNA is copied so that when the cell divides, both cells will have a copy of this genetic information. This phase lasts about 6–10 h. The S phase is followed by the gap 2 phase
. The gap 2 phase occurs just before the cell begins to divide into two cells and is a preparation stage for its chromosome duplication. This phase lasts about 2–6 h. The cell can then enter the M phase where the cell division occurs and two new cells are formed. The chromosomes are first lined up and pulled to either end of the cell, the membrane is pinched together and two daughter cells are formed. This phase lasts about 30 min. After cell division occurs, the cell may enter a resting state, referred to as the
phase. During this phase of inactivity, cells have not begun to divide. This period of inactivity can last anywhere from a few hours to a lifetime and is the phase with the most variable time frame. Once the cell is signalled to reproduce, it then moves into the
phase. Most stem cells found in normal tissues are in the
phase and are not committed to division. Also, tumor spheroids show an inner necrotic core surrounded by a ring of resting cells due to lack of nutrients [Citation4].
1.2 Radiation treatment of cancer
Radiation therapy works to treat cancer by attacking reproducing cancer cells, but also inadvertently affects the normal, healthy cells that are proliferating. This damage to normal cells is the cause of side effects. Every time radiotherapy is given there must be a balance between attacking cancer cells and avoiding destruction of healthy cells.
Radiation can be simply described as energy that is carried by waves or streams of particles. The key aspect in using this energy to treat cancer is that radiation has the capability of altering genetic material in a cell. As seen in the cell cycle description, this genetic code controls cell growth and proliferation. A cell's sensitivity to radiation, referred to as radiosensitivity, depends on its current position in the cell cycle. Radiation is more effective on cells that are active and dividing quickly, while it is much less effective on cells that are in the resting phase, as well as on cells that divide slowly [Citation15].
In radiation therapy, ionizing radiation is used to destroy or shrink tumours. Ionizing radiation causes ionization, which is the loss of an electron in atoms or molecules. When an electron is lost, energy is transferred and this energy disrupts chemical bonds, resulting in ionization. These ionizations, when induced by radiation, can act directly on molecules forming cellular components, or indirectly on water molecules. When acting on water, the ionizations cause water-derived radicals (highly reactive molecules that can bind to and destroy cellular components). These radicals quickly react with molecules near them and this results in chemical bond breakage or oxidation of the affected molecules. In cells, there is a variety of possible radiation induced lesions, although the most harmful to the cell are the lesions which effect the DNA structure.
DNA occurs in pairs of complementary strands and radiation can induce single strand breaks, or DSB. Single strand breaks are the more common lesion of the two and can usually be repaired by the cell (undamaged strand serves as template for production of complementary strand). DSB are caused by either a single event, which severs both DNA strands, or by two independent single strand break events close in time and space. DSB are considered more harmful than single strand breaks, as they are difficult to repair. Even when repair is attempted, broken ends may be joined together, leading to misrepairs. These misrepairs cause mutations, aberrations (of chromosomes), or cell death.
Deletion of DNA segments is the most common form of radiation damage in cells that survive radiation treatment. The deletion may be caused by the misrepair of two separate DSB in a DNA molecule by joining of the two outer ends and loss of the section between the breaks. However, it may also be a result of the cleaning process, where enzymes digest nucleotides (component molecules of DNA) of the broken ends prior to rejoining them to repair a single double strand break [Citation12].
The LQ model is the most common cell survival formalism used when modelling radiation treatment of tumours. The LQ expression was originally used by Sinclair in 1966 [Citation16], who found that an expression of this form fit the cell survival data he analysed, although he did not biologically justify this discovery. The model determines the surviving fraction of cells after radiation of a specified dose, where a cell survives the dose application if it is able to act as a progenitor for significant line of offspring. (The operational definition commonly used for cell survival is that a cell survives if it produces at least 50 offspring.) The LQ formula is written as
The ratio of the two adjustable parameters in the LQ model, α and β, has been found to correlate with the cell cycle length. Tissues which have a slow cell cycle are composed of cells which proliferate slowly. These slow cycling tissues correspond with a smaller ratio. Brain tissue and the spinal cord are examples of slow cycling tissues, both of which have an
ratio of about 3 Gy [Citation5]. Tissues which have a fast cell cycle are composed of cells that proliferate quickly and are associated with a larger
ratio. Most tumours are composed of cells which cycle quickly, and have an
ratio of approximately 10 Gy. It is important to note that in radiotherapy, it is usually the case that the slow cycling tissues are those which we are trying to protect.
1.3 The tumour control probability
The probability that no malignant cells are left in a specified location after irradiation is known as the TCP. This probability is used to determine an optimal treatment strategy where the dose to the tumour is increased without increasing the damaging effects of radiation of healthy tissues.
1.3.1 Poisson statistics
The most common and simplest expression for the TCP is one which relies on Poisson statistics.
If the number of cells present before treatment, n, is large, and if cell survival after treatment is a rare event, the probability that k cells survive is given by
Expression (Equation4) is only valid when the cell survival probability is small and the number of cells surviving irradiation is much less than the initial number of tumour cells, which are the usual conditions in radiotherapy treatments.
1.3.2 Binomial statistics
Let p denote the survival probability of an individual cell. If we assume that all cells are identical and independent, then we obtain from a binomial distribution
1.3.3 Zaider and Minerbo TCP formulation
Zaider and Minerbo [Citation25] were the first who found a method to express the TCP as a time-dependent dynamical expression that could be applied to all treatment protocols. In their formulation, Zaider and Minerbo apply a birth–death model (see Kendall [Citation9]) to the radiation treatment of cancer. This birth–death process has been solved explicitly to obtain an expression for the TCP, which satisfies where
is the probability that i clonogens are alive at time t. In their model, the cell birth rate is denoted by b and the cell death rate is denoted by δ. The death rate δ is composed of death due to radiation,
, and radiation-independent death, d. That is,
. The radiation death term
is known as the hazard rate, which for the LQ survival probability (Equation3
), is
.
The master equation for the birth–death process of cancer growth and radiation treatment is given by an infinite system of ordinary differential equations (ODEs) for the ,
The mean field equation of (Equation6) for the expectation
is given by
The solution of the mean field equation (Equation10
) is the mean cell number of the cancer cells. Hence
denotes the surviving fraction of tumour cells. Note that this interpretation of
is not used in the original formula (Equation8
) of Zaider and Minerbo. There the term
denotes the survival probability for a given hazard function
and it should not be mixed up with the surviving fraction of the cells
.
Note that for no birth, b = 0, we obtain the binomial model (Equation5).
The active-quiescent model presented in this paper extends Zaider and Minerbos model by including the effects of varying sensitivities within a tumour. The detailed derivation of equation (Equation2) is given in section 2. Before we do this, we give a detailed derivation of the active-quiescent radiation model (Equation1
).
1.3.4 The normal tissue complication probability
During the radiation treatment of cancer, healthy tissue is inadvertently irradiated. For treatment to be useful, it is important that the damage of healthy tissue is kept to a minimum. The normal tissue complication probability (NTCP) is used as a measure for the sensitivity of normal tissue for a given radiation treatment schedule. NTCP models are based on the damage probability of so called “functional subunits” (FSU) [Citation8,Citation18]. Of course, it is desirable to achieve a TCP close to 1, however, at the same time the NTCP must be at an acceptably low level. Hence, for real treatments it is important to look at both the TCP and the NTCP simultaneously. In Stavreva et al. [Citation20], the above mentioned Poissonian TCP formula and related NTCP formulas are computed for various model assumptions. The model of Zaider and Minerbo [Citation25] has been compared to NTCP models in Weldon [Citation19]. For the TCP formula derived in this paper, there is no corresponding NTCP model available. We plan in future research to extend existing NTCP models to NTCP models of comparable detail to the TCP model studied here.
2. Derivation of an active-quiescent radiation model from first principles
In this section, a model for the radiation treatment of cancer cells that includes active and quiescent cell phases is derived. The derivation begins from first principles, at the microscopic level, by considering target sites inside a cell that can potentially be damaged by radiation. A target site is damaged when an energy deposition via radiation occurs at that site. These energy depositions are modelled as stochastic events referred to as hits. The model includes a quiescent state, , and an active state, which combines the
and M-phases.
Target theories for radiation damage on living tissues have been discussed since the work of Lea presented in 1955 in Ref. [Citation10]. We give a complete new derivation here to include time dependent treatment schedules, rather than considering only the total dose.
The model derivation is divided into five steps. In Step 1, a single cell is considered and the probabilities that this cell is damaged by radiation are defined. In Step 2, a group of cells is considered. In Step 3, using radiation physics, the expressions for the damage probabilities are derived. In Step 4, the radiation induced death rates for active and quiescent cell compartments are derived. Lastly, in Step 5, the death rates are used to formulate our active-quiescent radiation model (Equation1).
Step 1: one cell
We consider a single cell, which contains specific sites, called active sites, that are susceptible to radiation damage. We assume that a single cell has n active sites, labelled , where
need two-hits to be damaged and
need only one hit to be damaged.
We assume radiation has energy E and we define as the probability that radiation with energy E causes the site
to experience a single-hit event in a given unit of time
. Similarly, we define
as the probability that radiation with energy E causes the site
to experience a two-hit event in a given unit of time
. A two-hit event consists of two separate single-hit events, close in both time and space, so it is expected that these two probabilities are related (see Step 3).
The probability that at least one site experiences a single-hit event can be computed. Any of the n sites susceptible to radiation damage can experience a single-hit event:
Similarly, the probability that at least one site experiences a two-hit event can be computed, where only the sites susceptible to two-hit damage need to be considered:
Step 2: many cells
Consider a group of cells , where each cell has an associated number of active sites susceptible to single-hit damage and two-hit damage, respectively. In this step, the probabilities that the k-th cell dies after a single-hit, or a two-hit event are defined, where a cell is considered dead if it produces less than 50 offspring.
The probability that a cell dies after experiencing single-hit event is denoted by
, and the probability that a cell dies after experiencing a two-hit event is defined as
. The numbers of single-hit and two-hit active sites for cell
are denoted by
and
, respectively. Recall that hit events are stochastically independent and that two single-hit events close enough in time and space comprise a two-hit event. Then the probability that a cell
dies is
Using expressions (Equation12) and (13) we obtain
In order to simplify the above probability, it is assumed that all cells are identical. Later, we will split cells into active and quiescent compartments and then we assume that all active cells are identical and all quiescent cells are identical. Here we assume for all that
As a result of this assumption, probability (Equation14) reduces to
Step 3: radiation physics
In this step, expressions for the single-hit and two-hit event probabilities previously defined as and
are derived. The probability that an active site
is hit by radiation in the time interval
is proportional to the energy E of the radiation beam as well as to the time interval
, hence
. Let
denote the dose accumulated at time t and so
is the radiation dose rate. This dose rate is proportional to the energy E of the radiation beam. Hence we obtain
In order to compute , the probability that two stochastically independent one-hit events occur close in time and space must be calculated. The assumption is made that two single-hits must occur in a time interval of length ω>0 in order to produce a two-hit event.
Suppose that a single-hit event occurred in the time interval . In order to have a two-hit event at time t, another single-hit must have occurred in the interval
. From above, the probability density of a single-hit event is
. Now define
Step 4: radiation induced death rates
If P(death) denotes the probability of death in a time unit , then
At this point, it is necessary to discern between active and quiescent cells. The cells are separated into these two respective compartments, where the active compartment u includes the cell cycle phases , S,
, M and the quiescent compartment q includes only the
phase. Hence, we obtain corresponding radiation death rates as
In the sequel, we assume that two-hit events, that include DSB, play a minor role for the quiescent cells and that when these cells do receive a DSB they have a good chance of repairing this lesion. Hence, in general, we expect . In parts of the analysis and numerical simulations, we will simply assume
.
Step 5: ODE model
In order to use the above radiation induced death rates and
in an ODE model, first consider a cell cycle model for active and quiescent cells without the effects of radiation. Let
denote the density of active cells and
denote the density of quiescent cells. Further, let
denote the reproduction of active cells, where it is assumed that the two daughter cells enter the quiescent compartment at birth. Moreover, γ>0 denotes the rate at which a quiescent cell becomes active. Then a simple cell cycle model is given as (see Swierniak [Citation22])
3. Singular perturbation analysis for a time dependent dose rate
In this section, we consider a time dependent dose rate and study scaling limits for the model parameters to compare the model (Equation1
) to the standard LQ model. To do this, we assume that
is linear, that
and that
. The model is then given by
3.1 Slow transition between active and quiescent compartments, and slow proliferation
In the first case when both γ and μ are small, let and
, where ε is small. These values are substituted into the active-quiescent model (Equation19
), which yields
(LQ-assumption): The total dose D is given in a time interval
that is smaller than the mean two-hit interaction time:
. Moreover, we observe the result after treatment is completed for some T with
.Under assumption (LQ-assumption) the remaining integral term in equation (Equation20
) is zero and we obtain an LQ-model
The linear dependence for large D is dominated by the term and the quadratic term is given by
. Hence for the LQ model, we find qualitatively
and
. In particular, if the initial condition of the resting cells is
, then we obtain the LQ model with
.
3.2 A transformation with radiation
To investigate the behaviour of equation (Equation19) if the parameters γ and μ are of different sizes, we transform to a set of new variables
and
. The new variable Z is equal to the total number of cells and W corresponds to the total number of cells making a transition between the compartments. This transformation is applied to the cell cycle model (Equation19
) to obtain the transformed system:
3.2.1 The case of γ large and 
Now consider the case where the transition rate from the quiescent to the active cell compartment is large compared to the proliferation rate. This assumption implies that and the system (Equation21
) becomes
3.2.2 The case of μ large and 
Now consider the case where the proliferation rate is large relative to the transition rate. This implies that and so the system (Equation21
) becomes
3.3 Model comparison
Using the results from the perturbation analysis and the additional assumption (LQ-assumption), a relationship between the parameters in the active-quiescent model, μ and γ, and the parameters in the LQ Model, α and β, can be established. Typically, a small ratio corresponds with a slow cell cycle, whereas a larger parameter ratio indicates a fast cell cycle. The parameter relationships are summarized in table .
Table 1. Comparison between the parameters in the active-quiescent model, μ and γ and the parameters in the LQ Model, α and β.
Case 1
In this case, both μ and γ are small, which corresponds with a slow cell cycle and a significant quiescent phase. The ratio is equal to the active-quiescent model ratio
. In the classical interpretation, a slow cell cycle corresponds with a small
ratio. The ratio
is relatively small, compared with the cases 2 and 3 below.
Case 2
In this case, μ is large and γ is small, which corresponds with a fast cell cycle. The ratio is obtained for the active-quiescent model, which confirms the classical interpretation, in which the ratio is assumed to be large.
Case 3
In this third case, μ is small and γ is large. This corresponds with slow reproduction and effectively no quiescent compartment. The active-quiescent ratio in this case is
. In the classical interpretation, the
ratio should be relatively small.
In addition to the classical understanding of the ratio, we derive the following biologically relevant conclusion from our model:
Biotheorem:
Based on our model, we conclude that a large
ratio indicates the presence of a significant quiescent compartment.
4. Tumour control probability
In this section, an expression for the TCP of system (Equation1) is derived. The TCP is the probability that no malignant cells are left in an affected region. This is a useful tool in predetermining the success of radiotherapy treatment over time for a cancer patient.
Here, we do not make use of the specific radiation death terms as given in equation (Equation17). The TCP derivation given here is valid for general, time dependent radiation death rates
and
(that are integrable and bounded).
The active-quiescent model (Equation19) is a deterministic ODE-model for cancer growth and radiation treatment. As such it is valid for relatively large cell densities where stochastic effects are negligible. When we talk about tumour control, we aim to reduce the tumour population to a level close to extinction. Hence, we are interested in small cell levels, where stochastic effects play an important role. To address this appropriately, we follow the ideas of Zaider and Minerbo [Citation25] and extend the ODE-model (Equation19
) into a nonlinear birth–death process. A birth–death process is a Markov process, where birth and death events are modelled as stochastic events. In this context, it makes sense to talk about TCP as the extinction probability of cancer cells (see also [Citation9]). To be consistent with the ODE model (Equation1
) derived earlier, we show that the birth–death process leads to equation (Equation19
) in a mean field approximation, i.e. the mean values of the cell numbers satisfy equation (Equation19
).
4.1 The corresponding birth–death process
We define as the probability that i active cells are alive at time t and similarly,
as the probability that j resting cells are alive at time t. If
, then we use the convention that
and
are equal to zero. Initially, at time equal to zero, the density of active cells is defined to be
, which implies that
. At time zero, the density of resting cells is defined as
, which implies that
. The probability that no malignant cells are left in the affected region, i.e. the TCP, is given by
.
In order to determine equations for both and
, the biological process that the system intends to describe is recalled. First, we consider a single active cell. This cell must have entered the active compartment from the resting cell compartment. Once a cell becomes active, it can: leave the active cell compartment by replicating; undergo cell death due to radiation; or it can remain in the active cell compartment.
Also, consider a single quiescent cell. This cell must have entered the quiescent compartment from the active compartment. It is important to note, however, that when a cell leaves the active compartment, it replicates and two cells must enter the quiescent compartment. This implies that it is never the case that the number of resting cells increases by only one. Once a cell has become quiescent, it can: leave the quiescent cell compartment by becoming active; undergo cell death due to radiation; or it can remain in the quiescent cell compartment.
In order to mathematically describe the birth–death process, we consider events that can occur in a very small time interval, .
For ,
For ,
For , equations (Equation30
) and (Equation31
) are rewritten in differential form to obtain:
The expected values of and
are given by the respective densities of the active and quiescent cells, earlier defined as
and
, so
In order to solve the birth–death process given by the system of equations (Equation32) and (Equation33
), define the moment generating functions
and
as:
Using the method of characteristics for , we find the solutions as
Finally, using the solutions for and
, we obtain an explicit expression for the TCP from
Note that in order to compute the TCP from equation (Equation19), the solutions
and
of the mean field equation (Equation19
) are needed. For this reason, in numerical studies we first solve the ODE-model (Equation19
) and then substitute these solutions into the TCP.
4.2 An example of constant irradiation
In Yurtseven et al. [Citation24], a detailed sensitivity analysis of the parameters of the TCP model is given and various treatment schedules are simulated and compared. Here, we only show the TCP curve for constant radiation and we assume that the two-hit interaction interval length ω>0 is small compared to the observation period. Then the radiation death rates (Equation17
) take the form
In order to do this, realistic parameter values must be determined. For an initial number of tumour cells, an estimate given in Wyatt et al. [Citation23] is used, which is 108 cells at diagnosis. This estimate for the initial number of tumour cells must be divided into two compartments: active and quiescent. Since there is no readily available data on the states of tumour cells, it is assumed that half of the cells are in the active state and half are in the quiescent state. The parameter that is the most difficult to determine is γ, the transition rate from the quiescent to the active cell compartment. Prior to the recognition of this resting state, was included in the
phase, which accounted for a highly variable length in the cell cycle. For the parameter γ, an estimate for the transition rate from the
phase to the S phase is used.
For the parameters and
, estimates for the α parameter in the LQ model are used, since in both cases the parameters represent damage due to single-hit events. For
, the parameter for the active cell single-hit damage, a value of α for radiosensitive prostate tumour cells is used. For
, the single-hit damage parameter for the resting cell compartment, an α estimate for radioresistant prostate tumour cells is used. Similarly, for B, the term which represents damage due to two-hit events, an estimate for the β parameter in the LQ model for radiosensitive prostate tumour cells is used. These α and β estimates were taken from Leith et al. [Citation11]. Lastly, the estimate for the length of time in which two single-hits must occur to cause a two-hit event, defined as ω, is obtained from Carlson et al. [Citation6].
The values of the parameters used are given in table .
Table 2. Parameter estimates for the active-quiescent TCP.
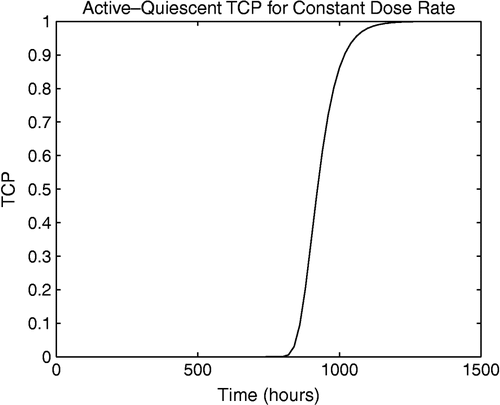
Assume the dose rate is a constant value of 2.75 Gy day− 1. Figure depicts a plot of the TCP as a function of time for this treatment schedule. The plot shows that at approximately 750 h, or 1 month, the TCP begins to increase, which suggests this is when the treatment begins to have a positive effect. By 1200 h, or 50 days, the TCP has reached its maximum value, which indicates a high probability of tumour eradication. The TCP for specific treatment schedules that are used to treat cancer is considered in the paper by Yurtseven et al. [Citation24].
5. Discussion
In this paper, the radiation treatment of cancer cells, beginning at the cellular level, is mathematically described. Using this mathematical description, which consists of a system of two ODEs (Equation1), an expression for the TCP (Equation2
), which is useful in determining the outcome of a specific treatment schedule, is subsequently derived.
The effects of radiotherapy on cancer cells have been studied extensively for many years. The first widely recognized mathematical model of this process came in 1966, when the LQ model was first developed. Many later mathematical models of the radiation treatment of cancer have considered an inhomogeneous cell population of varying radiosensitivities, without classifying the mechanism behind these variations. It is only recently that the quiescent, or , phase was recognized as a cellular state which the cell can enter from the classical four phase cell cycle. It has been shown that cells in this quiescent state are less sensitive to radiation than cells which are proliferating. For this reason, it is important to consider this resting state when developing a model of this process. In the active-quiescent model presented here, the cell population is divided into two compartments: a quiescent compartment
and an active compartment, which includes the
, S,
and M phases of the cell cycle. The derivation of the active-quiescent radiation model began from first principles, by considering how radiation affects a single cell. Once cell specific target sites that could be damaged via energy deposits were established, the model was extended to groups of cells in the two respective compartments. The result of this derivation was a system of two ODEs, which includes the effects of a time-dependent radiation dose rate and incorporates the dynamics of the active-quiescent cell cycle (see equation (Equation1
)).
Once the active-quiescent radiation model was established, the effects of the two parameters which governed a cell's transition through the quiescent and active phases were considered. The first parameter, μ, describes the proliferation rate and the second parameter, γ, describes the transition rate from the quiescent to the active cell compartment. Perturbation analysis showed that the relative sizes of these parameters have a significant effect on the solutions of the active-quiescent system. When μ and γ are both small, a modified linear-quadratic model is obtained. When γ is large and μ is small, the one-hit event damage on resting cells has no effect, while when μ is large and γ is small, the effect of radiation on the active cells does not contribute to the leading order system behaviour. The comparison to the LQ-model leads to the confirmation that a large ratio corresponds to a fast cell cycle and a smaller
ratio corresponds with a slow cell cycle. Also, this comparison led to the hypothesis that a large
ratio indicates a significant quiescent compartment.
Following the model analysis, the results of Zaider and Minerbo [Citation25] were extended by deriving an expression for the active-quiescent TCP. To do this, a method similar to that used in Ref. [Citation25] was employed. The first step was to extend the active-quiescent model to a nonlinear birth–death process (see equations (Equation32) and (Equation33
)). The resulting system of infinitely many differential equations was then solved using generating functions. Once the solution was obtained, an explicit expression for the active-quiescent TCP, or the probability that there are zero tumour cells at time t, could be calculated. This expression can be used to analyze a variety of treatment plans of varying dose rates, number of fractions, and overall treatment time (see [Citation24]).
Acknowledgements
We thank G. de Vries, O. Yurtseven and the members of the Mathematical Physiology Group at University of Alberta for helpful remarks and discussions. We are grateful to have continued support through NSERC and through the MITACS project on Mathematical Modeling in Pharmaceutical Development under the leadership of Jack Tuszynski.
Additional information
Notes on contributors
A. Dawson
† †Supported by MITACS.T. Hillen
‡ ‡ [email protected]. Supported by NSERC and MITACS.Notes
†Supported by MITACS.
‡ [email protected]. Supported by NSERC and MITACS.
References
- Baserga , R. 1985 . The Biology of Cell Reproduction , Cambridge : Harvard University Press .
- Basse , B. , Baguley , B.C. , Marshall , E.S. , Joseph , W.R. , van Brunt , B. , Wake , G. and Wall , D.J.N. 2002 . A mathematical model for analysis of the cell cycle in human tumour . Journal of Mathematical Biology , 47 : 295 – 312 .
- Bellomo , N. and Maini , P. 2005 . Preface . Mathematical Models and Methods in Applied Sciences , 15 : iii – vii .
- Byrne , H.M. 2003 . “ Modelling avascular tumour growth ” . In Cancer Modelling and Simulation , Edited by: Preziosi , L. 75 – 120 . London : Chapman and Hall .
- Carlone, M., 2004, Determining the α/β ratio for prostate cancer using clinically measured dose response data, PhD thesis, Carleton University, Canada.
- Carlson , D. , Stewart , R. , Li , X. , Jennings , K. , Wang , J. and Guerrero , M. 2004 . Comparison of in vitro and in vivo α/β ratios for prostate cancer . Physics in Medicine and Biology , 49 : 4477 – 4491 .
- Heath , J.K. 2001 . Principles of Cell Proliferation , Oxford : Blackwell Science Ltd. .
- Jackson , A. , Kutcher , G.J. and Yorke , E.D. 1993 . Probability of radiation-induced complications for normal tissues with parallel architecture subject to non-uniform irradiation . Medical Physics , 20 : 613 – 625 .
- Kendall , D.G. 1948 . On the generalized “birth-and-death” process . Annals of Mathematical Statistics , 19 : 1 – 15 .
- Lea , D.E. 1955 . Actions of Radiations on Living Cells , New York : Cambridge University Press .
- Leith , J.T. , Quaranto , L. , Padfield , G. , Michelson , S. and Hercbergs , A. 1993 . Radiobiological studies of PC-3 and DU-145 human prostate cancer cells: X-ray sensitivity in vitro and hypoxic fractions of xenografted tumors in vivo . International Journal of Radiation Oncology, Biology and Physics , 25 : 283 – 287 .
- Mendelsohn , J. , Howley , P.M. , Israel , M.A. and Liotta , L.A. 2001 . The Molecular Basis of Cancer , Philadelphia : W.B. Saunders Company .
- Murray , A. and Hunt , T. 1993 . The Cell Cycle: An Introduction , New York : W.H. Freeman and Company .
- World Health Organization, 2006-03-11. http://www.who.int/cancer/en/.
- Prescott , D.M. and Flexer , A.S. 1982 . Cancer: The Misguided Cell , Massachusetts : Sinauer Associates Inc. .
- Sinclair , W.K. 1966 . The shape of radiation survival curves of mammalian cells cultured in vitro . Biophysical Aspects of Radiation Quality, International Atomic Energy Agency, Technical Reports Series , 58 : 21 – 43 .
- Smith , J.A. and Martin , L. 1973 . Do cells cycle? . Proceedings of the National Academy of Science of the United States of America , 70 : 1263 – 1267 .
- Stavrev , P. , Stavreva , N. , Niemierko , A. and Goitein , M. 2001 . Generalization of a model of tissue response to radiation based on the ideas of functional subunits and binomial statistics . Physics in Medicine and Biology , 46 : 1501 – 1518 .
- Stavrev , P. , Weldon , M. , Warkentin , B. , Stavreva , N. and Fallone , B.G. 2005 . Radiation damage, repopulation and cell recovery analysis of in vitro tumour cell megacolony culture data using non-poissonian cell repopulation tcp model . Physics in Medicine and Biology , 50 : 3053 – 3061 .
- Stavreva , N. , Stavrev , P. , Warkentin , B. and Fallone , B.G. 2002 . Derivation of the expressions for γ50 and D50 for different individual tcp and ntcp models . Physics in Medicine and Biology , 47 : 3591 – 3604 .
- Swanson , K.R. , True , L.D. , Lin , D.W. , Buhler , K.R. , Vessella , R. and Murray , J.D. 2001 . A quantitative model for the dynamics of serum prostate-specific antigen as a marker for cancerous growth . American Journal of Pathology , 158 : 2195 – 2199 .
- Swierniak , A. , Polanski , A. and Kimmel , M. 1996 . Optimal control problems arising in cell-cycle-specific cancer chemotherapy . Cell Proliferation , 29 : 117 – 139 .
- Wyatt , R.M. , Beddoe , A.H. and Dale , R.G. 2003 . The effects of delays in radiotherapy treatment on tumour control . Physics in Medicine and Biology , 48 : 139 – 155 .
- Yurtseven, O., de Vries, G. and Hillen, T., 2006, A sensitivity analysis of tumor control probability, in preparation.
- Zaider , M. and Minerbo , G.N. 2000 . Tumour control probability: a formulation applicable to any temporal protocol of dose delivery . Physics in Medicine and Biology , 45 : 279 – 293 .