Abstract
Objectives: Gene–environment interaction is an emerging hypothesis to expound not only the autism pathogenesis but also the increased incidence of neurodevelopmental disorders (such as autistic spectrum disorder, attention-deficit, hyperactivity disorder). Among xenobiotics, mycotoxins are worldwide contaminants of food that provoke toxicological effects, crucially resembling several symptoms associated with autism such as oxidative stress, intestinal permeability, and inflammation. Here, we focused on a group of mycotoxins to test their role in the manifestation of autism, try to explain their mechanism of action, and discuss possible preventive and therapeutic interventions.
Methods: Autistic children (n = 52) and healthy children [n = 58 (31 siblings and 27 unrelated subjects)] were recruited and body fluids and clinical data collected. The diagnosis of autism was made according to DSM V criteria, then with GMDS 0-2, WPPSI, and ADOS. Ochratoxin A (OTA), gliotoxin, zearalenone, and sphingosine/sphinganine ratio were determined by LC analysis in sera and urines. Statistical analysis was performed by the Wilcoxon Rank Sum (Mann–Whitney) test and Spearman test.
Results: By comparing the results of autistic patients with those of unrelated controls, a significant association was found for OTA levels in urines (P = 0.0002) and sera (P = 0.0017), and also comparing patients with siblings and unrelated controls together (P = 0.0081).
Discussion: Our results are the first describing a possible role of OTA in the pathobiology of autism. Recalling the male prevalence of ASD (male/female = 4–5/1), it is noted that, in animal models, OTA exerts its neurotoxicity especially in males. Moreover, in vitro, OTA increases microRNA-132 that is dysregulated in autistic patients and involved in reciprocal regulation of the autism-related genes MeCP2 and PTEN. A personalized diet coupled with probiotic administration, especially OTA adsorbing Lactobacillus, could ameliorate autistic symptoms in OTA-positive patients.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, characterized by a set of impairments in communication and social interactions, behavioural stereotypes, and a range of cognitive deficits. Although ASD is a complex multifactorial disorder with genetic heterogeneity that involves hundreds of genes, about 70% of cases lack a causative genetic factor.Citation1 Patients with autism often suffer from comorbidities such as genetic syndromes (i.e. X-fragile and Rett syndrome) or genetic metabolic disorders (i.e. phenylketonuria (PKU))Citation2 as well as epilepsy and gastrointestinal (GI) disorders. Interestingly, GI disorders that include constipation, diarrhoea, intestinal permeability, and dysbiosis are associated with the severity of ASD.Citation3
In the last decades, the prevalence of autism increased dramatically and, in 2012, 1/68 children have been diagnosed with autism.Citation4 Since the increase in rates could be only partially attributed to better diagnosis and ascertainment, the possible role of environmental factors triggering the disorder has been proposed. Recent studies agree that the genetic susceptibility to various polluted environmental factors, such as water, air, and food, to which we are unavoidably exposed, might be implicated in the increased risk of neurodevelopmental disordersCitation5 because of the presence of toxicants. Moreover, since ASD affects much more males than females (male:female = 4–5:1), male-specific deleterious factors increasing males’ risk or female-specific protective elements preserving females from ASD have been hypothesized.Citation6 Heavy metals, pesticides, and other xenobiotics found in food are recognized risk factors for ASD and it has been hypothesized that they may contribute to the severity of the disorder.Citation7 Interestingly, among xenobiotics, mycotoxins exert toxicological effects that may be crucially associated with the manifestation of the autistic disorders, being able to alter the immune and neurological systems, generate oxidative stress, and provoke damages to the intestinal barrier.Citation8,Citation9
Mycotoxins are a group of chemical contaminants produced by ubiquitous fungi (mainly of Aspergillus, Fusarium, and Penicillium genera) on a wide variety of foods under specific conditions of temperature and humidity, both in the field and during storage. Their toxicity is of human and animal health concern, since the exposure arises not only from food and feed ingestion but also from inhalation in environmental contaminated areas. In early nineties, FAO estimated that moulds affect approximately 25% of the crops worldwide, enlightening a global safe food problem and an economical issue.Citation10 Epidemiological correlations between several mycotoxins and diverse health problems, including neurological and developmental effects, have already been established in both animals and humans.Citation11–13
Overall, some mycotoxins such as ochratoxin A (OTA), fumonisins (FBs), zearalenone (ZEA), gliotoxin (GLIO), and aflatoxins (AFs) provoke immunosuppressive effects and oxidative stress. Specifically, OTA produced by some species of Aspergillus spp. and Penicillium spp. is among the most abundant food and feed contaminants. OTA is nephrotoxic, hepatotoxic, and carcinogenic and increases intestinal permeability provoking macromolecule trafficking through intestinal wall strongly impairing immune system and altering the so-called ‘gut–brain’ axis. This axis is a complex bidirectional cross-talk between the enteric and central nervous system that acts through neuroendocrine and neuroimmune signals. Alterations of the ‘gut–brain’ axis impair neuropsychological functions and are involved in some psychiatric conditions including autism.Citation14 FBs’ intake is a potential risk factor in impairing human neural tube development through disruption of sphingolipid metabolism and folate transport.Citation15 ZEA and its metabolites (α and β-zearalenols, α and β-ZEL) were detected in foetuses of rats administered with this compound during pregnancy, confirming that mycotoxins are present and transmissible in foetal–maternal biological fluids. AFs, which correlate with acute toxicosis and liver cancerCitation9 and have been associated with stunting, were detected in serum, urine, and amniotic fluid of pregnant women; prenatal exposure to 1.2 mg/kg body weight over 4 days of aflatoxin B1 (a mutagenic and genotoxic contaminant classified in group I by the International Agency for Research on Cancer, IARC) produced a delay of early response development, impaired loco-motor coordination, and impaired learning ability in the offspring of rats exposed to this mycotoxin during the middle of gestation.Citation9
Thus, since many neurological, inflammatory, and GI symptoms, induced by mycotoxin exposure, are similar to those that are often associated with ASD as comorbidities, it was postulated that ASD comorbidities (assessed by some clinical parameters such as intestinal permeability, oxidative, and inflammation markers) could be worsen by the toxic action of these contaminants. Mycotoxins are ubiquitously present in the environment and children are exposed via food or polluted environments. In this scenario, a causative relation between mycotoxins and the deterioration of autism manifestation has been explored and results are compared with those of a study, which found results contrary to ours.Citation11
In this study, the levels of mycotoxins (GLIO, OTA, ZEA, α-ZEL, and β-ZEL) and sphinganine and sphingosine ratio (Sa/So) in serum and urine of patients and controls were tested, their possible association with the disorder and/or some clinical parameters, as well as their mode of action and possible preventive and therapeutic interventions, are discussed and proposed.
Methods
Study design
Here, we present an observational pilot study that tested the association between mycotoxins’ exposure and ASD onset, as well as among mycotoxins and some other clinical symptoms and parameters associated with autism: cognitive, social and behavioural profiles, intestinal permeability, food and mould IgG antibody, and oxidative stress indicators. Mycotoxins were determined by chromatographic analysis in the body fluids from Italian ASD patients (n = 52) and healthy controls (n = 58). The mycotoxin content was statistically analysed between the two groups as well as within the group of autistic patients, in relation with symptoms and clinical data.
Criteria for selecting the sample and recruitment to the study
The group of autistic children as well of healthy controls were recruited from the IRCCS E. Medea (Italian Research Hospital, Istituto di Ricovero e Cura a Carattere Scientifico, Bosisio Parini-Lecco). In this institute, patients diagnosed with ASD were routinely subjected to genetic testing by array-CGH analysis. Since our purpose was to investigate the gene–environment interaction in autism pathogenesis, children with genetic syndromes were not included in the study. The patient and control groups included in the study consisted of: n = 52 clinic group (43 boys and 9 girls, age 2–9 years) and n = 58 healthy children as control group (30 boys and 28 girls, age 2–11 years). The control group included pure controls (i.e. not related with autistic group, n = 27, 16 boys and 11 girls) and siblings (n = 31, 14 boys and 17 girls) that are related with autistics being a group of brothers or sisters of one individual of the clinic group.
The inclusion criteria were done upon the age range (from 2 to 12 years old), diagnosis of autistic spectrum disorder, and the exclusion criteria were the presence of other genetic syndromes (e.g. X fragile, karyotype alterations), epilepsy, or other medical disorders or neurological disturbances.
Experienced and specialized neuropsychiatric doctors and psychologists examined all the children according to national and international guidelines. The diagnosis of ASD was made in accordance with the criteria of the DSM-V (Diagnostic and Statistical Manual of Mental Disorders V) of the American Psychiatric Association. The cognitive profile was assessed with Griffiths Mental Development Scales, Revised (GMDS 0–2) in children up to 2 years old and with Wechsler Preschool and Primary Scale of Intelligence (WPPSI) in more competent children, over 2 years old. The cognitive scale was clustered as follows: above the average, in the norm, mild mental retardation, moderate mental retardation, severe mental retardation, profound mental retardation, and not otherwise specified.
The probands were positive to Autism Diagnostic Observation Schedule (ADOS) and Diagnostic Interview, Revised (ADI-R). Furthermore, following ADOS, trained mental health clinician assessed social and behavioural deficits such as language impairment and communication, hyperactivity, social interaction, stereotyped behaviours.
Under the approval and jurisdiction of its Ethical Committee and with informed consent by a parent or guardian, the IRCCS E. Medea hospital operators collected whole blood (n = 110), serum (n = 110), and urine (n = 110) samples, from both autistic and control groups whose identity remained anonymous as encoded by an Identification-Number (ID).
Genetic test
Informed consent for genetic test was obtained from all the children diagnosed with ASD and genetic analysis by array-CGH was performed in IRCCS E. Medea.
Briefly, DNA was isolated from the peripheral blood using standard procedures and array-CGH analysis was performed using the Agilent array 180 K (Agilent Technologies) according to the manufacturer’s protocol. Data analysis was carried on by Agilent Cytogenomics Ed 2.5.8.1. Oligo positions are referred to the UCSC Genome Browser (Feb 2009 assembly, hg19).
Clinical parameters
Analyses of ceruloplasmin (CER), transferrin (TRF), eosinophilic cationic protein (ECP), malondialdehyde (MAL) and free glutathione (free GSH), measurement of food and mould-specific IgGs and intestinal permeability () were performed in IRCCS E. Medea.
Table 1 List of clinical trial parameters
CER and TRF on serum were assayed by means of immunonephelometry BN-Prospec (Dade Behring, Siemens, Los Angeles, CA, USA) system and quantified using a certified standard (N-protein standard SL human, REF OQIM). ECP was measured with a solid-phase two-site chemiluminescent immunometric assay kit by a fully automated platform Immulite 2000 XPi (Siemens, Los Angeles, CA, USA). MAL was tested in serum after protein precipitation and derivatization of the residue; the analysis was performed by high performance liquid chromatography (HPLC) with a fluorimetric detection (excitation wavelength of 515 nm and an emission wavelength of 553 nm; Shimadzu Italia, Milan, Italy) using certified standards (Biopure and Sigma Aldrich). Free GSH was analyzed in whole blood in EDTA samples by a HPLC kit (Chromsystems, Munchen, Germany) and fluorescence detection (excitation wavelength 385 nm and emission wavelength 515 nm; Shimadzu Italia, Milan, Italy). Internal standard (IS) procedure was applied for quantification, adding the IS kit (Chromsystems Diagnostics) in samples before extraction.
As for the intestinal permeability test, after collection of a basal urine sample, the fasting subjects drank a solution containing 5 g of lactulose (LAC) and 2 g of mannitol (MAN) in 50 ml of water, at a dose of 2 ml/kg body weight, 3 hours before bedtime. Urine was collected during the next 12 hours (overnight) in the presence of 2 ml sodium azide (15 g/l) as a preservative. Two hours after the test was started, children were encouraged to drink water. The total volume of urine was measured, and a 10 ml portion was stored at –20°C.
Analyses were carried out with a P/ACE MDQ Capillary Electrophoresis System in the reverse polarity mode (Beckman Coulter) with a DAD detector (254 nm). The applied procedure was performed according to Paroni et al.,Citation16 slightly modified. Standard solutions of all sugars were prepared in water at different concentrations (LAC: 0.01–0.5 mg/ml; MAN: 0.05–2 mg/ml) and rhamnose was used as the IS, 10 mg/ml (Standard WHO 1st IRP 67/86).
All urine samples were pre-cleaned with OASIS HLB columns. After activation of the HLB column, 1 ml of urine sample added with IS was passed through the HLB column. The eluant was collected in a tube containing 0.5 g of Amberlite HCl resin, vortexed and, after centrifugation, the supernatant was analysed.
Mould- and food-specific IgG antibodies (against Alternaria tenuis, Aspergillus clavatus, Asp. flavus, Asp. fumigatus, Asp. nidulans, Asp. niger, Asp. oryzae, Asp. terreus, Candida albicans, Cladosporium herbarum, Fusarium moniliforme, Pennicillium brevicompactum and P. notatum, carrot, celery, cod fish, egg white, gluten, honey, α-lactalbumin, β-lattoglobulin, orange, peanut, wheat, yolk) were assessed by the Immulite 2000 XPi automated system with an ‘allergen-Specific IgG’ test on a solid-phase, enzyme-labelled chemiluminescent immunometric assay. Chemiluminescent signal was measured as proportioned to the bound enzyme (Standard WHO 1st IRP 67/86).
Mycotoxin analysis
Analysis of GLIO, OTA, ZEA, and its metabolites α and β zearalenol (α and β-ZEL), Sa, and So were performed at ‘GMO and Mycotoxin Unit’, Istituto Superiore di Sanità, Rome, Italy. All laboratory health and safety procedures were implemented for the experimental work, as the laboratory works under a quality control system have been accredited since 2006. Analytical methods were internally validated and performances of precision and trueness (in terms of relative standard deviation of repeatability and recoveries, see ) were in compliance with requirements set for mycotoxins.Citation17
Table 2 Validation parameters for GLIO, OTA, Sa, So, and ZEA. LoQ, relative standard of repeatability (RSDr), and recovery are shown
GLIO, OTA, ZEA, α-ZEL, β-ZEL, Sa, and So were extracted and detected by ultra performance liquid chromatography (UPLC) system from Waters Corporation system equipped with UV, fluorimetric, and triple quadrupole (MS/MS) as detectors (Acquity UPLC, ESI Quattro Premier mass spectrometer of Waters Corporation, Milford, MA, USA). For all the analyses, an Acquity BEH column (BEH UPLC C18 1.7 mm 2.1 × 100 mm) was used in combination with an Acquity UPLC column in-line filter unit from Waters Corporation. Mycotoxins were quantified with standards purchased from Biopure and Sigma-Aldrich diluted in appropriate working solutions. The principles of the methods for the mycotoxin analyses in serum and urine are briefly reported below.
Analysis of GLIO and OTA
Extraction from serum and urine
The extraction of GLIO and OTA was performed adding ethyl acetate (0.5 ml/0.8 ml urine). The extraction step was carried out twice, followed by an evaporation step until dryness under gentle stream of nitrogen. The residue was re-dissolved in the mobile phase (acidulated aqueous solution 2% acetic acid in methanol, MeOH/H2O, 50/50, v/v) until analysis.
Detection
The detection was performed in UPLC with fluorimetric and UV detectors. The flow rate of the mobile phase was set at 0.4 ml/min and the column oven temperature was 40°C. The UPLC system was coupled to an Acquity UPLC tunable UV detector with the absorbance set at 260 nm for GLIO and to an Acquity tunable fluorimeter detector with λex set to 333 and λem set to 460 for OTA.
Analysis of ZEA and α- and β-zearalenols
Extraction from serum
The extraction of ZEA and its metabolites from serum samples was performed according to the protocol of Beom et al.Citation18 with some minor modifications: a portion of serum was extracted by diethylether (2 ml) on a vortex mixer (Eppendorf Thermomixer Comfort) and then centrifuged (ALC PK131, Cool Working System). The upper organic layer was transferred and evaporated on a heating block at 40°C under gentle stream of nitrogen.
Extraction from urine
Urine samples were spiked with the IS and filtered. After mixing with acetate buffer solution 1 M (pH = 2), all samples were incubated with β-glucuronidase/arylsulphatase. Samples were then loaded onto EASI-EXTRACT® ZEARALENONE column (R-Biopharm Rhône LTD, Glasgow, Scotland). Analytes were eluted, evaporated to dryness under nitrogen stream, and reconstituted in the mobile phase.
Detection
The dissolved samples were automatically injected into UPLC-MS/MS (Acquity UPLC system from Waters Corporation). Serum and urine samples were analysed in ESI– in a Quattro Premier mass spectrometer of Waters Corporation. In , the MS/MS parameters are reported.
Table 3 Precursor, most abundant fragments and operating conditions for ZEA, ZEA metabolites, Sa, and So
Analysis of Sa and So
Sa and So extraction from serum and urine
The extraction of Sa and So was performed according to Bielawski et al.,Citation19 with minor modifications a reported: serum samples (homogenized in RPMI serum-free media) and urine pellet obtained after centrifugation were used. Afterwards, 10% phosphate buffer saline, 0.1 ml of formic acid, and 3 ml of extraction solvent (ethyl acetate/isopropanol, 85:15, v/v) were added in each sample. After mixing, the samples were centrifuged and the upper phase was evaporated to dryness under nitrogen stream. Dry residues were reconstituted in 250 μl of the mobile phase (1.5 mM ammonium acetate in MeOH containing 0.2% formic acid) and a portion of 20 μl was injected in the UPLC-MS/MS (Acquity UPLC system from Waters Corporation).
Detection
Samples were automatically injected into UPLC-MS/MS and analysed in ESI+ in a Quattro Premier mass spectrometer of Waters Corporation. Same MS/MS conditions for serum and urine samples were applied. In , the MS/MS parameters for the two compounds are listed.
Statistical analysis
A descriptive analysis was performed to examine the variables and elucidate notable aspects of the dataset. Those samples with mycotoxin levels below the limit of quantification (LoQ, see for values) were assigned with a corresponding value of LoQ/2 (medium bound approach).
Since the Shapiro–Wilk test revealed a non-normal distribution of the variables, all statistical correlations were performed with non-parametrical analysis, which does not imply any distribution assumption.
To investigate possible patterns between variables, Spearman test coefficient (ρ) was assessed as a robust measure. As a reference, the coefficient varies in the range +1 and −1, where zero indicates no correlation; −1 indicates a negative correlation (increasing one variable the other decreases proportionally), while +1 indicates a positive correlation (increasing one variable the other increases proportionally).
To compare the levels of mycotoxins within each group (autistic and controls), the Wilcoxon Rank Sum (Mann–Whitney) test (MWW) was applied. The same test was used to compare levels between sub-groups (SGs) defined for a specific comorbidity. Statistical significance was set at P < 0.05. Analyses were performed using STATA (Stata/IC 14.0, Copyright 1985–2015 StataCorp LP, College Station, TX, USA).
Results
Genetic test
Only patients negative for syndromic genetic variants were included in the study.
Diagnosis and clinical parameters
The diagnosis of ASD was made in accordance with DSM V criteria.
As for MDA, CER, TRF, free GSH, ECP, and intestinal permeability, the percentage of subjects out of the range, average values, and range of obtained values for each group of children are summarized in . All thresholds and reference range values reported are intended as for adults. It is noteworthy that percentages of subjects out of range (below or above threshold) are present only in the autistic group and the comparison of average values among groups did not produce statistically significant differences.
Table 4 Values of clinical parameters obtained from autistic and control group
The results obtained by specific fungal IgG analyses are summarized in . Percentages, indicating those children with values higher than 20 mg/l (chosen as threshold), show that the autistic group presented values out of the range for Aspergillus Flavus, Candida albicans, and Fusarium moniliforme. It is noteworthy that the highest values are found in the autistic group and the comparison of average values among did not produce statistically significant differences.
Table 5 Results for mould and food IgG antibody test per patient group
Results obtained for specific food antibody IgG found on serum of both clinic and control groups are reported in . Carrot, celery, codfish, and peanut presented a very small number of subjects with values above the threshold (20 mg/l) and only in the autistic group; for the other IgGs different trends are shown. That the higher values for gluten and wheat occurred in the autistic group in comparison with controls should be highlighted.
Content of mycotoxins in the body fluids
Method parameters obtained during in-house validation are summarized in .
Mycotoxins mean concentration values, percentages of values above the LoQ, median and concentration range are shown in , for each group in urine and serum. ZEA and its metabolites resulted all <LoQ, in serum and urines. Higher percentages of OTA were found in serum than in urine, while GLIO and Sa/So showed higher percentages in urine when compared with serum. The biomonitoring scenario showed OTA as the most abundant mycotoxin with higher values in serum, even if comparable with those found in other studies.Citation20 GLIO has been monitored for the first time in children specimen. As for Sa/So ratio, values are reported in . Since in other studies this ratio reference values are not available, also in this case it was difficult to derive any conclusion.Citation21
Table 6 Results for GLIO, OTA, and Sa/So per patient group
Statistical tests
Comparing mycotoxins profile and quantitative variables among the groups and SGs
Comparing by Wilcoxon Sum Rank test levels of mycotoxin in serum and urine of autistic group with those of controls (siblings and pure controls together), significant difference was highlighted for OTA in urine (P = 0.0081); the same analysis was performed comparing autistic children and sibling and pure control groups, separately. Significant differences were highlighted between autistic group and not paired control group for OTA in serum and urine (P = 0.0002 in urine and P = 0.0017 in serum), and for GLIO in urine (P = 0.0401). shows the bar graphs representing average values of mycotoxin for these comparisons that were statistically relevant (P < 0.05).
Figure 1 Bar graphics representing comparisons of average values of mycotoxin levels in serum and urine. P-values are reported.
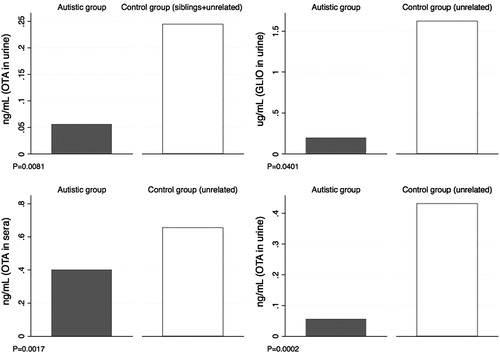
No significant differences between ASD subjects and their siblings were found.
The presence of OTA depicts a scenario of exposure through contaminated food and it is highlighted that despite sharing comparable food habits and diets, as it came out from the collected food questionnaires (data not shown), the three groups show statistically different average values. Going deeply into the values, those of the autistic group are lower in comparison with those of the controls, assuming that the toxin might escape from the regular route of excretion and metabolism, OTA biotransformation could follow other pathways and be modulated by other factors driven by genetic factors.
In order to explore the association more in detail, the trend of the association between mycotoxins and variables was explored within the patient group. Thus, the autistic group was divided into SGs. Two SGs showing specific comorbidity were identified: (i) a SG with IgG values above the threshold (>20 mg/l, SG1+), (ii) a SG with intestinal permeability values above the threshold (>0.03, SG2+); these two were compared with the corresponding SGs without comorbidity (SG1− and SG2−). The SGs are described in . A MWW test was conducted and the following significant associations were found: patients SG1+ with IgG values above the threshold (>20 mg/l) for yolk and honey displayed levels of Sa/So in urine statistically different when compared with levels of SG1− group (P = 0.0218 and P = 0.0087).
Table 7 SGs of autistic patients with and without a specific comorbidity
No differences were found between the other SGs.
Performing statistical tests on IgG values within the autistic SG with or without intestinal permeability issue, significant differences were outlined for IgG against Candida Albicans (P = 0.0244).
Correlations between mycotoxins and quantitative variables in the autistic group
Assessing correlations by the Spearman test among mycotoxins levels in urine or serum in the whole dataset, OTA in urine was associated with OTA in serum (ρ = 0.3132, P = 0.0037), and GLIO in serum (ρ = –0.3002, P = 0.0189).
Assessing correlations of mycotoxins levels in urine or serum within the autistic group, GLIO in urine associated with Sa/So in serum (ρ = 0.5147, P = 0.0288) and Sa/So in urine associated with OTA in serum (ρ = –0.3233, P = 0.0477).
Assessing correlations between mycotoxins and other variables, GLIO levels in serum correlated with IgGs against orange, with ECP and CER values (see for ρ and P values). OTA levels in serum were correlated with IgG values against casein, orange, Asp. Nidulans, and with MAL values (see for ρ and P values). GLIO levels in urine were correlated with codfish IgG values (ρ = –0.3647, P = 0.0092). Sa/So ratios in urine were correlated with gluten, wheat and orange IgG values and CER values (see for ρ and P values). These correlations indicate a positive or negative (ρ values positive or negative) relationship with the associated variable; ρ values are all above 0.3 and ECP with GLIO and MAL with OTA, which have shown the highest ρ values, strengthen the hypothesis that these oxidative and inflammation markers have a kind of correlation with the toxins.
Table 8 Summary table of the correlations found statistically significant between mycotoxins (in serum and urine) and variables; ρ and P values are reported
Discussion
Summary of main findings
We developed an analytical method with low LoQ and good analytical precision and accuracy to detect mycotoxins in serum and urine from a small group of autistic children (displaying no syndromic genetic variants by CGH analysis) and healthy controls (siblings and unrelated subjects). We first found a significant association between OTA in biologic fluids and GLIO in serum when autistic group was compared with healthy children.
In spite of the small size of the groups, comparing the results obtained in ASD children vs. unrelated controls, a significant difference of OTA in urine and serum (P = 0.0002 and P = 0.0017, respectively) was found, as well as significant difference of GLIO in urine (P = 0.0401). Comparing the results obtained in ASD children vs. siblings and unrelated healthy controls together, a significant difference of OTA in urine (P = 0.0081) was found. Regardless of comparable diet regimes, and consequently comparable levels of exposure, average values in ADS group are always lower suggesting altered biotransformation and metabolism of the toxins that might not follow the traditional route of exposure.
Recently, Duringer et al.Citation22 also tested this hypothesis by screening for urinary mycotoxins in autistic patients and healthy controls but failed to find any association. Indeed, they tested a very small group of ASD patients (n = 25) and controls (n = 29) for 87 urinary mycotoxins (via liquid chromatography–tandem mass spectrometry), thus they presented a good screening of the toxins, but LoQs were high for a test in urine or serum (10 times higher: 5 ng/ml against 50 ng/ml). Moreover, since the recruited patients had not been tested for genetic risk factors, the study could have included subjects with genetic syndromes, thus invalidating the statistical analysis.
To elucidate if OTA is a mere temporal artefact or has a role in the aggravation of autistic symptoms, we have reviewed the literature about the toxicological effects of OTA. Interestingly, it is found that OTA inhibits some autism-related genes and, moreover, exerts a male-specific toxicity,Citation23–Citation25 recalling the sex bias of autism. We also try to explain how, in which conditions, and at which age OTA could lead to neurodevelopment disorders.
OTA is a small organic molecule, consisting of an isocoumarin moiety linked to a phenylalanine (phe) molecule. The phe of OTA competes with phe in binding to the phe-metabolizing enzymes phenylalanine hydroxylase (PAH), thus causing its inhibition.Citation26 In support of this finding, a simultaneous administration of OTA and an antagonist, such as aspartame,Citation26 reduces the inhibition of PAH restoring the physiological activity of the enzyme and reversing the mycotoxin toxicity. In physiological condition, PAH converts phe into the amino acid tyrosine necessary for the biosynthesis of catecholamines, i.e. the neurotransmitters dopamine, noradrenaline, and adrenaline. Dopamine is, moreover, involved in social behaviour,Citation27 since it interacts with oxytocin (OT), a hormone with a key role in social communication; thus, PAH inhibition leads to a decrease in catecholamines and consequent interfering in OT activity. Interestingly, autistic children have significant lower level of OT in plasma and are characterized by deficit in social communication. Then again, genetic mutations of PHA, which provoke the deficiency of the enzyme, lead to PKU, a metabolic disorder characterized by mental retardation often associated with psychiatric disorders, seizures, behavioural problems and, in ∼25% of patients, autistic traits.Citation28 In this scenario, it is speculated that some autistic features can be derived from PHA inhibition that, in turn, could depend on genetic mutations or OTA binding.
As recently demonstrated, OTA modulates microRNA (abbreviated miRNA) expression. MiRNAs are small non-coding RNAs that epigenetically silence gene expression by binding mRNA targets. In vitro, OTA increases the expression of mir-200cCitation29 and mir-132.Citation29,Citation30 Interestingly, these miRNAs participate in neurodevelopment and synaptic plasticity,Citation31–33 and their dysregulations are reported in several neurological or psychiatric conditions, including autism.Citation32,Citation34,Citation35 Specifically, mir-132 is involved in the reciprocal regulation of autism-related genes MeCP2 and PTEN. Over-expression of mir-132 decreases MecP2 expression that leads to an increase on mir-137 that, in turn, targets and inhibits PTEN. Mutations in MeCP2 cause Rett syndrome and severe autism as well as abnormalities in PTEN lead to neurological and psychiatric conditions including ASD.Citation36 We can speculate that knockdown of MeCP2 (and all the resulting damages) could be due to genetic mutations of MeCP2 or to OTA-induced over-expression of mir-132. On the other hand, mir-200c is predicted by bioinformatics tools and validated by Next Generation Sequencing to inhibit the expression of Neuroligin4X (http://mirtarbase.mbc.nctu.edu.tw/),Citation37 a gene involved in synaptic plasticity and mutated in ∼1% of autistic population.Citation38 Recalling the male prevalence of ASD, it is noteworthy that both MECP2 and neuroligin4X are mapped on X chromosome, thus making males more prone to the effects of their downregulation.
The sex bias of ASD could also be explained by the sex differences in human xenobiotic metabolism. Indeed, sex differences in OTA degradation and pharmacokinetics have been described in humans and rats: contrary to females, males do not express the OTA detoxifying enzymes CYP2C9, CYP2C12, and CYP3A4, leading to an almost male-specific OTA toxicity.Citation23,Citation24
As for conditions making children particularly susceptible to OTA neurotoxic effects, it is emphasized that the first years of life represent a very critical period for the immune protection system. When weaning begins, at the age of about 6 months, the maternal immune protection, first vested during pregnancy and then by breast-feeding, decreases while the child own immune system is not yet mature. On the other hand, the gut microbiota, which has a great impact on health, is neither mature nor stable and the xenobiotic metabolizer cytochrome p450 is not completely effective yet. This makes children susceptible to infection diseases often treated with oral broad-spectrum antibiotics. These antimicrobial drugs can further unbalance and impair intestinal microbiota leading to dysbiosis, leaky gut, GI disorders, and inflammation. Intestinal permeability leads antigenic and toxic macromolecules to pass the intestinal wall barrier and enter the blood stream further involving immune system.Citation39 At neurodevelopmental level, specific time windows of acquisition of particular cognitive and sensitive processes characterize this critical period. Time windows occur in a hierarchical sequence: from primary senses acquisition up to intellectual ability. Once a specific window of acquisition is missed because of interferences in sense or social experiences, it cannot be recuperated nor senses or skills be acquired.Citation39
Leaky gut and GI inflammation can have a strong interplay with food sensitivities; the gut microbiota contributes to many GI functions (integrity of epithelial barrier, drug, and nutrient metabolism) and may be disturbed by infections of GI diseases. Not only drugs but also toxins may perturb homeostatic equilibrium and exert a toxic effect with worst consequences in predisposed patients.Citation40 In this scenario, OTA is not proposed to trigger autism, indeed it is suggested to play a relevant role on autistic symptoms only in children with predisposing conditions such as to be males in the first period of life and suffering from dysbiosis and leaky gut. Moreover, possible genetic susceptibility to environmental agents might contribute to the disorder onset.
In this study, the autistic group showed positive correlations among Sa/So ratios in urine and IgG values against wheat and gluten, and OTA values in serum and IgG values against casein and orange. The gluten-free and casein-free diet is a recurring finding in the literature with regard to autistic children with mixed results. It is noteworthy that children are used to consume much milk-based and wheat-based foods often coming to excesses and it is found that diets excluding those highly consumed foods improve GI symptoms.Citation41–43
No values of GLIO in urine have ever been reported before, since this toxin has been studied in serum and bronchoalveolar lavage fluids exclusively in invasive aspergillosis-infected adult patients.Citation44 This is the first study reporting a range of values of GLIO in children. GLIO has been appointed as a potent immunosuppressive mycotoxin with suppressive effects also to mast cells playing a crucial role in the production of histamine and other inflammatory mediators. It can be hypothesized that the synergistic action of food-specific IgG antibody and GLIO can deteriorate the inflammatory modulation, deteriorating the intestinal inflammation. The disulphide bridge of GLIO is recognized to be responsible for inactivation of proteins with thiol moieties and of generation of reactive oxygen species. A GLIO–glutathione conjugation might be consistent with the hypothesis of GSH deficit as potential source of neuropathological abnormalities.
As regards the clinical parameters, bearing in mind that no paediatric values are available for mycotoxins in biologic fluids, we consider the group of data gathered in this study as dataset of reference to elucidate the role of environmental risk factors in ASD and clarify biological causes.
We also scrutinized Sa/So ratio, since this has been associated with exposure to FBs; however, results are difficult to interpret and the lack of references and consistent data renders this ratio controversial.Citation21 We found a very low level of Sa in comparison to So; indeed, as reported, males have a lower Sa than females.Citation45 Interestingly, administration of green tea polyphenols, the prebiotic inulin, fructo-oligosaccharides, galacto-oligosaccharides and resistant starchesCitation46 as well as probiotics such as Lactobacillus and Bifidobacteria has been suggested to counteract FB1 exposure. These microbial strains act at various levels on the intestinal mucosa and produce GABA that can restore GABAergic signalling altered in autism.Citation47
From the metagenomic analyses of OTA–gut microbiota interaction, it has emerged that Lactobacillus is the genus used to detoxify OTA in vivo. Starting from this finding, we suggest that Lactobacillus probiotic administration could be a possible adjuvant for OTA detoxification and improvement of ASD symptoms in those autistic children with high level of OTA in the body fluids.Citation48,Citation49
Strengths and limitations
A method for the detection of low quantity of mycotoxin in the body fluids was first-tuned and then applied to the analysis of mycotoxins in ASD patients and controls, allowing a precise detection and quantification of GLIO, OTA, ZEA, α and β-ZEL, and Sa/So.
The possible mechanism of action of OTA in the pathobiology of ASD has been proposed, argued, and substantiated through an extensive and careful study of the literature.Citation11,Citation23,Citation26,Citation29,Citation30,Citation34,Citation39
Moreover, the study has collected a good number of parameters that may be of general interest in understanding the pathobiology of the disorder and proposes some dietary and therapeutic interventions to counteract the deleterious effects of OTA.
Then again, a small size of samples has been analysed and a more extensive recruitment and sampling of both patients and controls is required to confirm these first results.
Implication for future research
Evidence of a possible role of OTA in triggering symptoms of autism came out in this pilot study that requires to be explored in a more structured study on a larger series of patients and controls. The administration of possible adjuvant to detoxify and/or counteract OTA deleterious effects could be also considered in future research. A study including also other important mycotoxins and their metabolites (namely, aflatoxin B1, aflatoxin M1, deoxynivalenol, and a direct measurement of FB1) should be carried out to explore the involvement of this group of xenobiotics.
Disclaimer statements
Contributors None.
Funding Ministero della Salute (RC-2008 5-03), responsible Dr ME Raggi, IRCCS E. Medea; Banca di Credito Cooperativo dell'Alta Brianza (BCC dell'Alta Brianza) Alta-Brianza, responsible Dr ME Raggi, IRCCS E. Medea.
Conflicts of interest None.
Ethics approval None.
ORCID
Barbara De Santis http://orcid.org/0000-0002-2700-7566
Giorgio Moretti http://orcid.org/0000-0002-2700-7566
Maria Clara Bonaglia http://orcid.org/0000-0002-7121-7712
References
- Schaaf C, Zoghbi H. Solving the autism puzzle a few pieces at a time. Neuron 2011;70(5):806–8. doi: 10.1016/j.neuron.2011.05.025
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord 2005;35:103–16. doi: 10.1007/s10803-004-1038-2
- Fulceri F, Morelli M, Santocchi E, Cena H, Del Bianco T, Narzisi A, et al. Gastrointestinal symptoms and behavioral problems in preschoolers with Autism Spectrum Disorder. Dig Liver Dis 2016;48(3):248–54. doi: 10.1016/j.dld.2015.11.026
- Christensen D, Baio J, Braun K, Bilder D, Charles J, Constantino J, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. NeuroToxicology 2008;29(1):190–201. doi: 10.1016/j.neuro.2007.09.010
- Werling D, Geschwind D. Understanding sex bias in autism spectrum disorder. Proc Natl Acad Sci 2013;110(13):4868–9. doi: 10.1073/pnas.1301602110
- Kinney DK, Barcha DH, Chayka B, Napoleon S, Munir KM. Environmental risk factors for autism: do they help cause de novo genetic mutations that contribute to the disorder? Med Hypotheses 2010;74(1):102–6. doi:10.1016/j.mehy.2009.07.052 doi: 10.1016/j.mehy.2009.07.052
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 2010;31(1):71–82. doi:10.1093/carcin/bgp264 doi: 10.1093/carcin/bgp264
- FAO. (1988). Jelinek CF. Distribution of mycotoxin – an analysis of worldwide commodities data, including data from FAO/WHO/UNEP food contamination monitoring programme. Joint FAO/WHO/UNEP Second International Conference on Mycotoxins. Bangkok, Thailand, September 28 to October 3, 1987.
- Ohta K, Maekawa M, Katagiri R, Ueta E, Naruse I. Genetic susceptibility in the neural tube defects induced by ochratoxin A in the genetic arhinencephaly mouse, Pdn/Pdn. Congenit Anom (Kyoto) 2006;46(3):144–8. doi: 10.1111/j.1741-4520.2006.00117.x
- Paradells S, Rocamonde B, Llinares C, Herranz-Pérez V, Jimenez M, Garcia-Verdugo JM, et al. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J Appl Toxicol 2015;35(7):737–51. doi:10.1002/jat.3061.PubMedPMID:25256750 doi: 10.1002/jat.3061
- Kunio D, Koji U. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 2011;12:5213–37. doi:10.3390/ijms12085213 doi: 10.3390/ijms12085213
- Li Q, Zhou JM. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016;324:131–9. doi:10.1016/j.neuroscience.2016.03.013 doi: 10.1016/j.neuroscience.2016.03.013
- Marasas WFO, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr 2004;134:711–6. doi: 10.1093/jn/134.4.711
- Paroni R, Fermo I, Molteni L, Folini L, Pastore MR, Mosca A, et al. Lactulose and mannitol intestinal permeability detected by capillary electrophoresis. J Chromatogr B 2006;834:183–7. doi: 10.1016/j.jchromb.2006.02.050
- Commission Regulation (EC). 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Official Journal of the European Union L. 70/12;9.3.2006.
- Beom SS, Seok HH, Sang WH, Hyoung JK, Jong BL, Hae-Seong Y, et al. Determination of zearalenone by liquid chromatography/tandem mass spectrometry and application to a pharmacokinetic study LC/MS/MS analysis of zearalenone. Biomed Chromatogr 2009;23:1014–21. doi:10.1002/bmc.1217 doi: 10.1002/bmc.1217
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 2006;39(2):82–91. doi: 10.1016/j.ymeth.2006.05.004
- Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S, De Saeger S. Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environ Int 2015;84:82–9. doi:10.1016/j.envint.2015.06.011 doi: 10.1016/j.envint.2015.06.011
- Shephard GS, Van Der Westhuizen L, Sewram V. Biomarkers of exposure to fumonisin mycotoxins: a review. Food Addit Contam 2007;24(10):1196–201. doi: 10.1080/02652030701513818
- Duringer J, Fombonne E, Craig M. No association between mycotoxin exposure and autism: a pilot case-control study in school-aged children. Toxins 2016;8(7):224. doi: 10.3390/toxins8070224
- Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 2009;76(2):215–28. doi:10.1124/mol.109.056705 doi: 10.1124/mol.109.056705
- Mor F, Kilic MA, Ozmen O, Yilmaz M, Eker I, Uran K. The effects of orchidectomy on toxicological responses to dietary ochratoxin A in Wistar rats. Exp Toxicol Pathol 2014;66(5–6):267–75. doi:10.1016/j.etp.2014.04.002 doi: 10.1016/j.etp.2014.04.002
- Ueta E, Kodama M, Sumino Y, Kurome M, Ohta K, Katagiri R, et al. Gender-dependent differences in the incidence of ochratoxin A-induced neural tube defects in the Pdn/Pdn mouse. Congenit Anom (Kyoto) 2010;50(1):29–39. doi:10.1111/j.1741-4520.2009.00255.x doi: 10.1111/j.1741-4520.2009.00255.x
- Zanic-Grubisić T, Zrinski R, Cepelak I, Petrik J, Radić B, Pepeljnjak S. Studies of ochratoxin A-induced inhibition of phenylalanine hydroxylase and its reversal by phenylalanine. Toxicol Appl Pharmacol 2000;167(2):132–9. doi: 10.1006/taap.2000.8987
- Gunaydin LA, Deisseroth K. Dopaminergic dynamics contributing to social behavior. Cold Spring Harb Symp Quant Biol 2014;79:221–7. doi:10.1101/sqb.2014.79.024711 doi: 10.1101/sqb.2014.79.024711
- Saad K, Hammad E, Abdel-Rahman A, Sobhy K. Autistic symptoms in late diagnosed phenylketonuric children in upper Egypt. J Neurol Res North Am 2013;3–4:122–9.
- Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Boesch-Saadatmandi C, Rimbach G, et al. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res 2013;57(3):504–15. doi:10.1002/mnfr.201200456 doi: 10.1002/mnfr.201200456
- Dai Q, Zhao J, Qi X, Xu W, He X, Guo M, et al. MicroRNA profiling of rats with ochratoxin A nephrotoxicity. BMC Genomics 2014;15(1):333. doi:10.1186/1471-2164-15-333 doi: 10.1186/1471-2164-15-333
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010;65:373–84. doi: 10.1016/j.neuron.2010.01.005
- Jin J, Cheng Y, Zhang Y, Wood W, Peng Q, Hutchison E, et al. Interrogation of brain miRNA and mRNA expression profiles reveals a molecular regulatory network that is perturbed by mutant huntingtin. J Neurochem 2012;123(4):477–90. doi:10.1111/j.1471-4159.2012.07925.x doi: 10.1111/j.1471-4159.2012.07925.x
- Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K, Dawson TM. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE 2010;5(11):e15497. doi:10.1371/journal.pone.0015497 doi: 10.1371/journal.pone.0015497
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, et al. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics 2008;9(3):153–61. doi:10.1007/s10048-008-0133-5 doi: 10.1007/s10048-008-0133-5
- Sarachana T, Zhou R, Chen G, Manji HK, Hu VW. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med 2010;2(4):23. doi:10.1186/gm144 doi: 10.1186/gm144
- Lyu JW, Yuan B, Cheng TL, Qiu ZL, Zhou WH. Reciprocal regulation of autism-related genes MeCP2 and PTEN via microRNAs. Sci Rep 2016;4(6):20392. doi:10.1038/srep20392 doi: 10.1038/srep20392
- Chou C, Chang N, Shrestha S, Hsu S, Lin Y, Lee W, et al. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res 2016;44(D1):D239–47. doi: 10.1093/nar/gkv1258
- Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep 2009;9(3):188–97. doi: 10.1007/s11910-009-0029-2
- Mezzelani A, Landini M, Facchiano F, Raggi ME, Villa L, Molteni M, et al. Environment, dysbiosis, immunity and sex-specific susceptibility: a translational hypothesis for regressive autism pathogenesis. Nutr Neurosci 2015;18(4):145–61. doi:10.1179/1476830513Y.0000000108 doi: 10.1179/1476830513Y.0000000108
- Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioessays 2014;36:933–9. doi:10.1002/bies.201400075 doi: 10.1002/bies.201400075
- Isolauri E, Rautava S, Kalliomäki M. Food allergy in irritable bowel syndrome: new facts and old fallacies. Gut 2004;53(10):1391–3. doi: 10.1136/gut.2004.044990
- Cai C, Shen J, Zhao D, Qiao Y, Xu A, Jin S, et al. Serological investigation of food specific immunoglobulin G antibodies in patients with inflammatory bowel diseases. PLoS ONE 2014;9(11):e112154. doi:10.1371/journal.pone.0112154 doi: 10.1371/journal.pone.0112154
- Ligaarden SC, Lydersen S, Farup PG. Igg and IgG4 antibodies in subjects with irritable bowel syndrome: a case control study in the general population. BMC Gastroenterol 2012;12:667. doi:10.1186/1471-230X-12-166.
- Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, et al. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun 2005;73:635–7. doi: 10.1128/IAI.73.1.635-637.2005
- Solfrizzo M, Avantaggiato G, Visconti A. Rapid method to determine sphinganine/sphingosine in human and animal urine as a biomarker for fumonisin exposure. J Chromatogr B Biomed Sci Appl 1997;692(1):87–93. doi: 10.1016/S0378-4347(96)00502-6
- Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, et al. FAO technical meeting on prebiotics. J Clin Gastroenterol 2008;42(Suppl 3 Pt 2):S156–9. doi: 10.1097/MCG.0b013e31817f184e
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 2012;113(2):411–7. doi:10.1111/j.1365-2672.2012.05344.x. Epub 2012 Jun 15. Erratum in: J Appl Microbiol 2014;116(5):1384–6. doi: 10.1111/j.1365-2672.2012.05344.x
- Guo M, Huang K, Chen S, Qi X, He X, Cheng WH, et al. Combination of metagenomics and culture-based methods to study the interaction between ochratoxin a and gut microbiota. Toxicol Sci 2014;141(1):314–23. doi:10.1093/toxsci/kfu128 doi: 10.1093/toxsci/kfu128
- Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract 2011;2011:1–8. doi:10.1155/2011/161358 doi: 10.1155/2011/161358
