Abstract
Objectives: Green tea infusion contains a complex mixture of polyphenolic compounds that were shown to provide health benefits. It was previously demonstrated that (-)-epigallocatechin-3-gallate, one of the major polyphenols present in green tea, has a suppressing effect on various aspects of pathogenesis in models of Huntington’s disease (HD), an inherited neurodegenerative disorder. In this study, we aimed to investigate, whether green tea infusion prepared as for human consumption has similar positive effects.
Methods: We used a transgenic Drosophila model of HD to study the effects of green tea on mutant Huntingtin induced phenotypes. We tested the effects of green tea infusion on mutant Huntingtin induced neurodegeneration, impaired motor performance, reduced viability and lifespan by pseudopupil assay, climbing assay, eclosion and survival tests, respectively. We used immunoblots to measure Huntingtin protein levels and tested generic health benefits of green tea by longevity analysis.
Results: We found that green tea supplementation reduced mutant Huntingtin induced neurodegeneration in Drosophila and positively impacted the longevity of mutant Huntingtin expressing flies. However, green tea did not rescue reduced viability of Drosophila expressing mutant Huntingtin or increased longevity of wild-type fruit flies.
Discussion: Our results indicate that green tea consumption might have a modest positive effect on symptoms of HD.
Introduction
Huntington’s disease (HD) is a late-onset, dominantly inherited neurodegenerative disorder caused by elongation of a polymorphic CAG triplet repeat in the first exon of the huntingtin (HTT) gene that is translated to an elongated polyglutamine (polyQ) repeat in the mutant Huntingtin (Htt) protein.Citation1 The mutant protein is susceptible to aggregate formation and triggers a multifaceted pathogenesis that leads to the degeneration of medium spiny neurons in the striatum, motor, cognitive and psychiatric symptoms, and finally death.Citation1,Citation2 The pathogenic mechanisms of HD include impairment in Brain-Derived Neurotrophic Factor signaling,Citation3 complex dysfunction of the proteostasis network,Citation4 transcriptional dysregulation,Citation5 and mitochondrial impairment.Citation6 Previous studies indicated that epigallocatechin-gallate (EGCG), a polyphenol compound found in green tea, has a rescue effect on mitochondrial impairment induced oxidative stressCitation7 and Htt aggregationCitation8 in some HD models. Pretreatment with EGCG reduced oxidative damage, striatal damage, and behavioral symptoms in rats treated with 3-nitropropionic acid, a mitochondrial complex II inhibitor, causing degeneration of striatal cells and symptoms similar to HD.Citation7 Another study showed that EGCG inhibited aggregation of mutant Huntingtin in a dose dependent manner in vitro by interfering with conformational rearrangements of the protein; reduced aggregate load and toxicity in a yeast HD model; and reduced disease phenotypes in a Drosophila model of HD.Citation8 Further studies indicated that beside Huntingtin EGCG also provided protection against other aggregation prone proteins, alpha-synuclein and amyloid-beta, implicated in Parkinson’s and Alzheimer’s disease, respectively.Citation9,Citation10
Although EGCG is the most abundant polyphenolic compound in green tea, infusion also contains caffeine and several other polyphenols such as epicatechin gallate, epigallocatechin, and epicatechinCitation11 that might also participate in modulating pathogenetic processes. Therefore, in this study, we aimed to investigate whether green tea infusion prepared as for human consumption also has suppressing effect on HD pathogenesis. For this purpose, we applied a transgenic Drosophila model of the disease, which is based on neuronal expression of the first exon of human Htt with an elongated polyglutamine domain.Citation12 Our results suggest that green tea consumption might have a positive modulatory effect in HD.
Methods
Drosophila strains and media
Neuron-specific GAL4 driver strain w P{GawB}elavC155 was from the Bloomington Drosophila Stock Center. w1118; UAS-Httex1p-Q93 and w1118; UAS-Httex1p-Q20 transgenic lines expressing the first exon of the human huntingtin gene with pathological or normal polyglutamine repeat length, respectively, were published previously.Citation12 Longevity study was performed with inbred w1118 flies isogenized by pair-mating for 20 generations.
Drosophila strains were maintained on standard yeast-cornmeal-sucrose medium at 25°C unless otherwise noted. Drosophila medium used for the experiments contained 9.3 g/l agar, 61.2 g/l cornmeal, 129.4 g/l glucose, 32.4 g/l dry yeast, and 0.1% Nipagin. Green tea containing medium (GTM) and control medium (CM) was prepared by cooking the required amount of media mix in green tea infusion or in deionized water, respectively. Green tea infusion was prepared from commercially available unflavored green tea leaves (Lipton green tea, Unilever) as recommended for human consumption by the manufacturer: 1.5 g tea leaves were extracted in 200 ml of 90°C deionized water for 2 minutes.
Viability and longevity analysis
To analyze effects on mutant Huntingtin induced reduced viability w P{GawB}elavC155 males were mated with w; UAS-Httex1p-Q93 or control w; UAS-Httex1p-Q20 females in vials containing control Drosophila medium or medium prepared with green tea infusion. Crosses were done at 22 or 24°C, the total number of flies used for the analyses was 778 and 1420, respectively. The number of progeny in each F1 genotype category was counted and viability was expressed as the ratio of eclosed mutant Huntingtin expressing w P{GawB}elavC155/w; UAS-Httex1p/+ females to w; UAS-Httex1p/+ non-expressing male siblings. For statistical analysis of viability data two-way analysis of variance (ANOVA) (under R 3.4.3) was used.
For longevity analysis of transgenic flies, w P{GawB}elavC155/w; UAS-Httex1p-Q93/+ and w P{GawB}elavC155/w; UAS-Httex1p-Q20/+ females were raised on CM and transferred to GTM or CM medium as adults. Flies were kept at 24°C, passed to fresh vials (10–34 flies per each vial) three times per week and the number of survivors recorded daily. The number of flies inspected was: n=356 (HttQ93 on CM), n=345 (HttQ93 on GTM), n=122 (HttQ20 on CM), and n=118 (HttQ20 on GTM). For longevity analysis of non-transgenic flies at least 175 freshly eclosed isogenized w1118 male flies were sorted to vials containing GTM or CM, six vials each, 27–30 flies per each vial. Flies were kept at 25°C, passed to fresh vials and the number of survivors scored three times per week (every second or third day). Flies from outlier vials (with restricted mean lifespan below Q1–1.5 IQR or above Q3+1.5 IQR, where Q1 stands for the first quartile, Q3 for the third quartile of data and IQR for the Q1–Q3 interquartile range) were excluded from further analysis. For the analysis of survival data, the OASIS 2 applicationCitation13 and TSHRC package under R 3.4.3 were used. Two Stage Hazard Rate ComparisonCitation14 was used to compare hazard rate functions through lifespan while modified Mann–Whitney U test was used to compare survival functions at time points corresponding to specific mortality levels.
Measurement of motor activity
Motor activity was measured by climbing assay,Citation15 which is based on the characteristic negative geotaxis of adult Drosophila. Age synchronized three-day-old adults were transferred to graduated glass cylinders in cohorts of 9–10 flies and their upward climbing after being knocked down was recorded on video. Motor activity was characterized by the speed of climbing calculated based on the average of vertical distance climbed by each cohort in the first 7 seconds. Three cohorts (29–30 flies) of Httex1pQ20 and five cohorts (50 flies) of Httex1pQ93 expressing flies were tested on both CM and GTM medium, each cohort was measured five times.
Measurement of neurodegeneration
To measure neurodegeneration, pseudopupil assayCitation16 was used. Freshly removed heads of six-day-old flies were immobilized on microscopic slides with nail polish and the number of intact rhabdomeres per ommatidium in the compound eye were counted under a Nikon Eclipse 80i microscope using a 50× oil immersion lens. At least 25 ommatidia per eye and at least eight eyes per treatment were scored.
Determination of polyphenol content
Total polyphenol concentration of green tea infusion was determined by Folin–Ciocalteau method,Citation17 with modifications. Tea infusion samples were centrifuged then the clear supernatant was diluted 10× with H2O. 20 µl diluted samples were added to 780 µl H2O and mixed with 50 µl Folin–Ciocalteau’s phenol reagent (Merck). After 5 minutes 150 µl 20% sodium carbonate solution was added and after 2 hours incubation at room temperature sample absorbances were determined at 765 nm with a Cecil CE 1021 spectrophotometer. Sample concentrations were calculated by setting sample absorbance values to a gallic acid (Sigma) calibration curve (50–500 μg/ml, R2=0.999) and expressed as gallic acid equivalents.
Immunoblots
Drosophila heads were separated and homogenized in sonication buffer (50 mM Tris-HCl pH 7.9, 2 mM EDTA, 50 mM NaCl, 0.5 mM DTT, and 1× Protease inhibitor cocktail set I (Calbiochem)) using plastic pestle, then samples were denatured by boiling in Laemmli sample buffer containing 5% β-mercaptoethanol. After centrifugation for 10 minutes at 13000 RPM sample supernatants were loaded in 10% polyacrylamide gel and proteins were separated by electrophoresis in discontinuous Tris-glycine-SDS buffer system. After electrotransfer to Amersham Protran Premium 0.45 μm nitrocellulose membrane (GE Healthcare Life Sciences), membranes were blocked in 5% nonfat milk and incubated with the following primary and secondary antibody combinations in the indicated dilutions: rabbit polyclonal anti-Huntingtin (1:1000, Viva Bioscience, VB3130) with goat-anti-rabbit IgG-HRP (1:4000, Dako, P0448), monoclonal anti-α-tubulin (1:2500, Sigma, T9026) with rabbit-anti-mouse IgG-HRP (1:5000, Dako, P0260). Immunoblots were developed with Immobilon Western Chemiluminescent HRP substrate (Millipore) and imaged with a C-DiGit chemiluminescent blot scanner (Li-Cor Biosciences). At least five biological replicates per condition were analyzed.
Results
Viability of mutant Huntingtin expressing Drosophila is not influenced by green tea
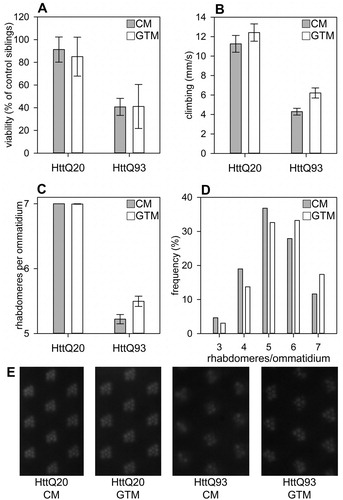
The main aim of our study was to investigate whether green tea infusion prepared as for human consumption affects mutant Huntingtin induced pathology in a Drosophila model of HD. Tea infusion was prepared from a world-wide distributed brand, Lipton Green Tea (Unilever), by extracting 1.5 mg tea leaves in 200 ml water as recommended by the manufacturer. The total polyphenol content of the infusion was determined by Folin–Ciocalteau methodCitation17 to be 1.712±0.077 mg/ml (average±sd, n=5) gallic acid equivalent. We administered green tea to Drosophila by using the infusion to prepare medium that fruit flies consume as larvae during development and also as adults. To investigate whether green tea infusion has an impact on toxicity induced by mutant Huntingtin, we compared the viability of flies expressing transgenes containing the first exon of human Htt with an elongated (Httex1p-Q93) or normal length (Httex1p-Q20) polyglutamine domain under the control of the elav-GAL4 pan-neuronal driver on either GTM or CM. The GAL4/UAS transgene expression system is temperature sensitive,Citation18 and Huntingtin protein levels in head samples are positively correlated with culturing temperature if transgenes are driven by elav-GAL4 (Pearson correlation coefficient r=0.917, P=0.00049, Supplementary Fig. 1). Therefore, we performed crosses both at 22 and 24°C and assessed Htt induced toxicity by determining the eclosion rate of Htt expressing flies compared to non-expressing control siblings. At 22°C the viability of Httex1p-Q93 and Httex1p-Q20 expressing flies was similar on both GTM and CM indicating that at 22°C the mutant Htt transgene did not have significant toxic effects (data not shown). At 24°C however, the viability rate of Httex1p-Q93 expressing flies (40.7±7.5%, average±SEM, n=6) was significantly lower (P=0.00379, two-way ANOVA) than that of Httex1p-Q20 expressing flies (91.2±11%, n=6) indicating that at this temperature mutant Htt exerted its toxic effects (A). However, we found no significant difference in the eclosion rates of Huntingtin expressing flies raised on GTM vs. CM (eclosion rates of 85±17.1% (n=6 vials (384 flies)) on GTM vs. 91.2±11% (n=6 (325 flies)) on CM in case of Httex1p-Q20; and 41.1±17.8% (n=7 (300 flies)) on GTM vs. 40.7±7.5% (n=6 (411 flies)) on CM in case of Httex1p-Q93), suggesting that green tea supplementation does not rescue Htt induced overt toxicity (A).
Figure 1 Green Tea Infusion does not affect overall Toxicity but ameliorates neurodegenerative Phenotypes of Flies expressing mutant Htt. A. Relative viability of flies expressing Htt with an elongated polyQ repeat (Httex1p-Q93) is significantly reduced (P=0.00379, two-way ANOVA) compared to that of control flies expressing Htt with a short polyQ repeat (Httex1p-Q20). Reduced viability of Httex1p-Q93 flies is not rescued by green tea supplementation. CM: control medium, GTM: green tea containing medium. Viability of Htt expressing flies is shown as the ratio of eclosed transgene expressing flies in percent of non-expressing control siblings, error bars represent SEM, n (vials/flies): Q20 CM=6/325, Q20 GTM=6/384, Q93 CM=6/411, Q93 GTM=7/300. B. Httex1p-Q93 flies have significantly reduced (P=6.39×10−8, two-way ANOVA) climbing ability compared to Httex1p-Q20 control siblings, and motor performance is significantly (P=0.015, two-way ANOVA) enhanced by green tea supplementation, however, this effect is not specific for Httex1p-Q93 expression. Bars show the average distance climbed vertically per seconds, error bars represent SEM. C. In the eyes of six-day-old Httex1p-Q93 expressing adults significantly fewer (P=2×10−16, two-way ANOVA) visible rhabdomeres are present per ommatidium than in similarly treated Httex1p-Q20 control. Green tea supplementation significantly ameliorates degeneration of photoreceptor neurons (P=0.01, two-way ANOVA), and this effect is specific for mutant Huntingtin (interaction P=0.0039, two-way ANOVA). Bars show the average of the average number of rhabdomeres per ommatidium in inspected eyes, error bars represent SEM. D. In the retina of Httex1p-Q93 expressing Drosophila kept on GTM medium the percentage of ommatidia with 6 or 7 intact rhabdomeres is increased compared to ommatidia of flies kept on CM medium (P=0.0076, Chi-squared test). Bars represent the frequency of ommatidia containing the indicated number of rhabdomeres. E. Representative images of retina of flies expressing mutant (HttQ93) or control (HttQ20) Huntingtin kept on CM or GTM medium. In healthy retina, seven visible rhabdomeres are present in each ommatidium.
Green tea infusion ameliorates neurodegeneration of mutant Huntingtin expressing Drosophila
Expression and accumulation of mutant Htt in neurons leads to neuronal dysfunction and degeneration.Citation12,Citation15 Therefore, we aimed to investigate whether green tea infusion improves the functional integrity of the nervous system by determining changes in motor activity. Impaired motor control, involving impaired voluntary movements, and uncontrolled involuntary writhing movements, are characteristic features of HD that are in part recapitulated in the Drosophila model. Fruit flies exhibit negative geotaxis and climb upwards on vertical surfaces that can be used to analyze their motor performance by climbing assay.Citation14 To characterize motor impairment caused by mutant Htt we determined the speed of vertical climbing in a glass cylinder and found that Httex1p-Q93 expressing flies raised on CM climbed slower (4.29±0.36 mm/s, average±SEM, n=5 vials (50 flies)) than control Httex1p-Q20 expressing flies (11.26±0.86 mm/s, n=3 vials (29 flies)) suggesting that the functional integrity of the neuro-muscular system is compromised in flies expressing mutant Huntingtin (B). In parallel we also compared the effect of green tea supplementation on climbing ability of Httex1p-Q93 flies and found that flies raised and kept on GTM climbed faster (6.21±0.52 mm/s, n=5 vials (50 flies)) than Httex1p-Q93 flies raised and kept on CM (4.29±0.36 mm/s, n=5 vials (50 flies)). We used two-way ANOVA for the statistical analysis of the data and found that the effect of both the type of Htt transgene (P=6.39 ×10−8) and the type of fly medium (P=0.015) on climbing ability was statistically significant. However, the interaction term of the two factors was not statistically significant, thus based on our data the positive effect that green tea infusion has on climbing ability is independent of the type of the expressed Huntingtin transgene.
Our next goal was to determine whether green tea supplementation influences degeneration of neurons in the Drosophila HD model. For this purpose, we applied the pseudopupil assay.Citation16 In the compound eyes of fruit flies each optical unit, called ommatidium, contains seven visible rhabdomeres, light gathering structures of photoreceptor neurons. Degeneration of photoreceptor neurons in Drosophila expressing mutant Htt in the nervous system is apparent by decreased number of rhabdomeres per ommatidia. Six-day-old elav-GAL4/w; UAS-Httex1p-Q20/+ fruit flies that were raised on CM and were transferred to CM or GTM medium after eclosion showed no sign of neurodegeneration (C and E). However, when we compared similarly treated six-day-old elav-GAL4/w; UAS-Httex1p-Q93/+ fruit flies we could observe degeneration of photoreceptor neurons and found that neurodegeneration was less severe in the eyes of flies kept on GTM (5.5±0.08 rhabdomeres per ommatidium, average±SEM, n=10) than in the eyes of flies kept on CM (5.20±0.07, n=8) (C and E). Accordingly, frequency distribution of ommatidia with specific number of rhabdomeres shows that in the eyes of Httex1p-Q93 expressing flies kept on GTM ommatidia with less than six rhabdomeres are less frequent, while ommatidia with six or seven rhabdomeres are more frequent than in the eyes of siblings kept on CM (P=0.0076, Chi-squared test, D). By analyzing our data with two-way ANOVA we found that the effect of both the type of Htt transgene (P=2×10−16) and the type of fly medium (P=0.01), and also their interaction term (P=0.0039) was statistically significant. Thus, our data show that green tea infusion suppresses neurodegeneration induced by mutant Htt.
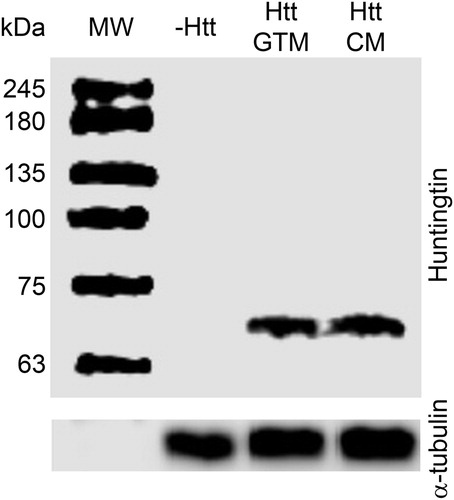
Having found that green tea infusion partially suppressed neurodegeneration of Httex1p-Q93 expressing fruit flies we were interested to know whether these effects could be attributed to changes in the level of mutant Huntingtin. We performed immunoblots on head extracts of elav-GAL4/w; UAS-Httex1p-Q93/+ females reared on CM or GTM medium with an antibody raised against the N-terminal part of human Htt and found that green tea supplementation did not alter the level of mutant Htt (). The absence of bands in the negative control (second lane on ), head extract of w/w; UAS-Httex1p-Q93/+ adults, shows that the anti-Htt antibody used does not recognize the endogenous Drosophila Huntingtin protein, and that Httex1p-Q93 is not expressed in the absence of a GAL4 driver.
Figure 2 Green Tea does not influence Htt Protein Levels. A representative immunoblot with antibody raised against the N-terminal part of human Htt (top panel) shows unaltered levels of mutant Htt in head extracts of Httex1p-Q93 expressing flies reared on GTM or CM (lanes 3 and 4, respectively; at least five biological replicates per treatment were inspected). In the absence of the elav-GAL4 neuronal driver (lane 2), the Httex1p-Q93 transgene is not expressed. Immunoblot with anti-α-tubulin antibody (bottom panel) serves as loading control.
Green tea moderately increases lifespan of mutant Huntingtin expressing Drosophila but does not increase longevity of wild-type flies
One of the characteristic consequences of neuronal expression of mutant Huntingtin in Drosophila is reduced lifespan.Citation12 To test whether green tea supplementation rescues mutant Huntingtin induced early death we raised flies that expressed Httex1p-Q93 or Httex1p-Q20 under the control of elav-GAL4 on CM and kept them as adults on CM or GTM medium. Lifespan data of Httex1p-Q93 flies were collected from two replicated experiments performed at different times. We confirmed that the elongated polyglutamine domain in mutant Huntingtin reduces longevity: on CM Httex1p-Q20 expressing flies had a median lifespan of 54 days, while Httex1p-Q93 flies had a 15 days median lifespan (P=1.8×10−54, Mann–Whitney U test). Furthermore, while all Httex1p-Q93 expressing flies deceased during the first 29 days, more than 83% of Httex1p-Q20 expressing flies survived this time period on both medium (A and B). Httex1p-Q93 expressing Drosophila show a characteristic survival curve with an early and a late period of increased mortality. By analyzing the survival data of Httex1p-Q93 expressing flies (B) we found a significant difference in the mortality rate functions of flies kept on CM or GTM medium (P=0.025, Two Stage Hazard Rate Comparison). Flies kept on GTM reached mortality rates of 75 and 90% later (23 days on GTM vs. 22 days on CM, P=0.0218 and 26 days on GTM vs. 24 days on CM, P=0.0003, respectively; Mann–Whitney U test), while at mortality rates of 25 and 50% there was no statistically significant difference between the two treatment groups and their restricted mean lifespan was not significantly different either. The observation that GTM had a positive effect on survival only in older adults might be explained by the fact that all inspected flies developed on CM and were separated to treatment groups only as adults. Based on these results we concluded that green tea infusion had a small but significant positive effect on the adult survival of Drosophila expressing mutant Huntingtin in the nervous system.
Figure 3 Green Tea Infusion increases the Lifespan of mutant Huntingtin expressing Drosophila. The two graphs on top show percent survival as function of days of adult Drosophila females expressing A. Httex1p-Q20 (control) or B. Httex1p-Q93 transgenes (combined results of experiments performed at two different times are shown) in the nervous system kept on either CM or GTM. Median lifespan of Httex1p-Q93 expressing flies is significantly reduced compared to that of Httex1p-Q20 controls (P=1.8×10−54, Mann–Whitney U test). On GTM medium Httex1p-Q93 expressing flies reach 75 and 90% mortality rates significantly later than on CM (P=0.0218 and P=0.0003, respectively; Mann–Whitney U test). C. Wild-type fruit flies have a significantly (P=1.1×10−7, Two Stage Hazard Rate Comparison) shorter lifespan on GTM than on CM. The graph shows percent survival as function of days of isogenic w1118 fruit flies kept at 25°C.
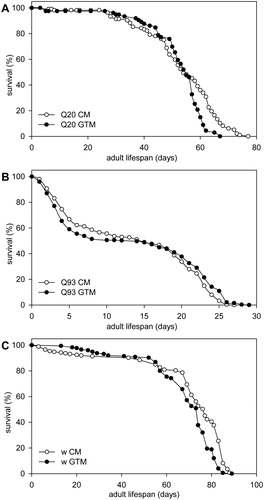
Green tea contains a variety of polyphenolic molecules that where shown previously to have anti-oxidative and anti-aging properties and therefore might provide generic health benefits to adult organisms. Accordingly, previous studies reported that green tea polyphenol extractsCitation19,Citation20 or purified EGCGCitation21 extended the longevity of fruit flies. Therefore, we aimed to test whether green tea infusion provides a generic health benefit to Drosophila that would have obscured the results gained from experiments in the HD model. For this, we determined the lifespan of isogenic w1118 (white eyed, otherwise wild-type) flies on control and green tea supplemented Drosophila medium at 25°C. By comparing the lifespans of at least 175 male flies per treatment we found that the green tea infusion used in the test did not have a positive effect on the longevity of w1118 flies (C), in fact flies kept on GTM had slightly shorter lifespan than flies kept on CM with 68.14 vs. 70.23 days restricted mean lifespan and 74 vs. 77 days median lifespan, respectively. Flies kept on GTM had significantly shorter lifespan than controls at tested mortality rates of 25, 50, 75, and 90% (P=4.2×10−5, P=2.3×10−6, P=6×10−9, and P=6×10−4; Mann–Whitney U test), and the mortality rate functions of flies on the two medium were also significantly different (P=1.1×10−7, Two Stage Hazard Rate Comparison). Similarly, flies expressing nonpathogenic Httex1p-Q20 reached mortality rates of 75% (P=0.0001, Mann–Whitney U test) and 90% (P=0.0196, Mann–Whitney U test) earlier on GTM than on CM (A). Thus, we concluded that green tea supplementation does not have a generic positive effect on viability that would have masked its potential effects on Htt toxicity.
Discussion
HD is a devastating neurodegenerative disorder for which there is no effective cure yet. Although treatment strategies aimed at eliminating the cause of disease by suppressing the expression of the mutant gene copy are under developmentCitation22, current therapies are limited to treat disease symptoms. In the present study, we investigated whether green tea infusion, a widely available and consumed beverage containing a complex mixture of bioactive compounds could provide beneficial effects in a transgenic animal disease model of HD. For the experiments, we utilized a well-characterized Drosophila model of HD that was used successfully in the past to characterize both genetic and pharmacological interventions.Citation8,Citation12,Citation15 Our data show that green tea infusion prepared as for human consumption does ameliorate neurodegeneration and reduced lifespan caused by mutant Huntingtin, suggesting that green tea consumption might have a modest positive effect on symptoms of HD. Green tea infusion also improved motor performance in our model but based on statistical analysis this effect was not specific for the expression of the mutant form of the Htt protein. These results are in line with previous observations showing that EGCG, the most abundant polyphenolic compound in green tea ameliorated HD phenotypes in yeast and animal models.Citation7,Citation8 Overt toxicity induced by mutant Huntingtin as measured by the viability of Htt expressing Drosophila, however, did not change in our experiments suggesting that the cellular effects of bioactive compounds in green tea are not crucial in HD pathogenesis and the beneficial effects green tea consumption can provide are limited.
To investigate whether the neuroprotective effects of green tea in the HD model are specific for mutant Huntingtin induced pathogenesis or could be attributed to a generic health benefit gained by consumption of the infusion rich in antioxidants we tested the effect of green tea on adult lifespan. We found that green tea prepared as for human consumption did not provide life-prolonging effects in Drosophila, leading us to conclude that green tea infusion does not have a generic positive effect on viability that would mask its potential effects on Htt toxicity. This result was surprising, however, as previous studies reported increased lifespan in Drosophila treated with green tea polyphenol extractsCitation19,Citation20 or EGCG.Citation21 The discordance between our results and the ones from the studies cited above could arise, on one hand, from different composition and concentration of active components in the various extracts. On the other hand, however, it is important to note that the genetic background of Drosophila used in the study and fly husbandry might also have a crucial effect on longevity. In our hands, the mean lifespan of both fruit flies kept on GTM or CM media was at least 9 days longer than the extended mean lifespan of treated flies in the aforementioned articles. Thus, it is feasible that green tea polyphenols are capable of increasing (restoring) lifespan only in case it is reduced by genetic background or environmental conditions.
Disclaimer statements
Contributors None.
Funding This work was supported by the Hungarian National Research, Development and Innovation Office (NKFIH) under Grant K-112294 and Grant GINOP-2.3.2-15-2016-00032.
Conflicts of interest None.
Ethics approval None.
Supplementary material
Supplemental data for this article can be accessed 10.1080/1028415X.2018.1484021.
Supplemental Material
Download TIFF Image (136.8 KB)Acknowledgements
The authors thank the Bloomington Drosophila Stock Center and J. Lawrence Marsh (University of California, Irvine) for providing Drosophila stocks.
ORCID
László Bodai http://orcid.org/0000-0001-8411-626X
References
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, et al. Huntington disease. Nat Rev Dis Primer. 2015;26:15005. doi: 10.1038/nrdp.2015.5
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. 2010;90(3):905–81. doi: 10.1152/physrev.00041.2009
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311–22. doi: 10.1038/nrneurol.2009.54
- Finkbeiner S, Mitra S. The ubiquitin-proteasome pathway in Huntington’s disease. The Scientific World Journal. 2008;8:421–33. doi: 10.1100/tsw.2008.60
- Sipione S, Rigamonti D, Valenza M, Zuccato C, Conti L, Pritchard J, et al. Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum Mol Genet. 2002;11(17):1953–65. doi: 10.1093/hmg/11.17.1953
- Milakovic T, Johnson GVW. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280(35):30773–82. doi: 10.1074/jbc.M504749200
- Kumar P, Kumar A. Protective effects of epigallocatechin gallate following 3-nitropropionic acid-induced brain damage: possible nitric oxide mechanisms. Psychopharmacology. 2009;207(2):257–70. doi: 10.1007/s00213-009-1652-y
- Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet. 2006;15(18):2743–51. doi: 10.1093/hmg/ddl210
- Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, et al. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A. 2010;107(17):7710–5. doi: 10.1073/pnas.0910723107
- Williams RB, Gutekunst WR, Joyner PM, Duan W, Li Q, Ross CA, et al. Bioactivity profiling with parallel mass spectrometry reveals an assemblage of green tea metabolites affording protection against human huntingtin and alpha-synuclein toxicity. J Agric Food Chem. 2007;55(23):9450–6. doi: 10.1021/jf072241x
- Li D-W, Zhu M, Shao Y-D, Shen Z, Weng C-C, Yan W-D. Determination and quality evaluation of green tea extracts through qualitative and quantitative analysis of multi-components by single marker (QAMS). Food Chem. 2016;197:1112–20. doi: 10.1016/j.foodchem.2015.11.101
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413(6857):739–43. doi: 10.1038/35099568
- Han SK, Lee D, Lee H, Kim D, Son HG, Yang J-S, et al. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget. 2016;7(35):56147–52.
- Qiu P, Sheng J. A two-stage procedure for comparing hazard rate functions. J R Stat Soc Ser B Stat Methodol. 2008;70(1):191–208.
- Agrawal N, Pallos J, Slepko N, Apostol BL, Bodai L, Chang L-W, et al. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc Natl Acad Sci U S A. 2005;102(10):3777–81. doi: 10.1073/pnas.0500055102
- Song W, Smith MR, Syed A, Lukacsovich T, Barbaro BA, Purcell J, et al. Morphometric analysis of huntington’s disease neurodegeneration in Drosophila. Methods Mol Biol. 2013;1017:41–57. doi: 10.1007/978-1-62703-438-8_3
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–78. doi: 10.1016/S0076-6879(99)99017-1
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genes N Y N 2000. 2002;34(1–2):1–15.
- Li YM, Chan HYE, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutr Food Res. 2007;51(5):546–54. doi: 10.1002/mnfr.200600238
- Lopez T, Schriner SE, Okoro M, Lu D, Chiang BT, Huey J, et al. Green tea polyphenols extend the lifespan of male Drosophila melanogaster while impairing reproductive fitness. J Med Food. 2014;17(12):1314–21. doi: 10.1089/jmf.2013.0190
- Wagner AE, Piegholdt S, Rabe D, Baenas N, Schloesser A, Eggersdorfer M, et al. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget. 2015;6(31):30568–78. doi: 10.18632/oncotarget.5215
- Lu X-H, Yang XW. “Huntingtin holiday”: progress toward an antisense therapy for Huntington’s disease. Neuron. 2012;74(6):964–6. doi: 10.1016/j.neuron.2012.06.001
