ABSTRACT
Objective: Vanishing white matter (VWM) is a genetic brain white matter disorder caused by mutations in eIF2B. eIF2B is central in the integrated stress response (ISR), during which its activity is inhibited by various cellular stresses. VWM is a chronic progressive disease with episodes of rapid neurological deterioration provoked by stresses. VWM patients and VWM mouse models show ISR deregulation in brain, correlating with chronic disease development. ISR inhibition ameliorates the chronic disease in VWM mice. The subacute deteriorations have not been modeled yet. We hypothesized that ISR activation could worsen disease progression in mice and model the episodic neurological deterioration.
Method: We chose to activate the ISR by subjecting wild-type (wt) and VWM mice to an isocaloric low protein diet. This model would allow us to investigate the contribution of ISR activation in subacute decline in VWM.
Results: We found that the low protein diet did not significantly affect amino acid levels nor ISR levels in wt and VWM mouse brain. Our study serendipitously led to the discovery of increased levels of glycine, asparagine and Fgf21 mRNA in VWM mouse brain irrespective of the dietary protein content. Strikingly, the ISR was not activated by the low protein diet in the liver of VWM in contrast to wt mice, due to a modest ISR deregulation in this organ.
Discussion: A model for subacute neurological deterioration in VWM was not established. Possibly, ISR deregulation in VWM results in reduced ISR responsiveness.
Introduction
Vanishing white matter (VWM) is a chronic progressive neurological disease with rapid worsening of the disease provoked by stressors, especially febrile infections.Citation1,Citation2 Progression of the chronic disease is inversely correlated with the age of onset.Citation3 VWM is caused by mutations in any of the five subunits of eIF2B with a reported genotype-phenotype correlation.Citation3,Citation4 eIF2B is essential for the protein synthesis and is a key factor of the integrated stress response (ISR).Citation5 This ISR is activated by various types of proteotoxic stimuli, each activating a kinase that phosphorylates the α subunit of eIF2, e.g. protein kinase R (PKR) activated by viral infections, or general control non-derepressible 2 (GCN2) by shortage of amino acids.Citation6 Phosphorylated eIF2 reduces eIF2B activity,Citation5 which decreases general protein synthesis rates, yet increases the synthesis of specific proteins such as the transcription factor ATF4.Citation7,Citation8 These specific proteins induce a change in the transcription profile as a part of the ISR.Citation6 Expression of this 'ISR transcriptome' is initially aimed to protect cells and restore proteostasis, but leads to cell death when the stress is long lasting or severe.
In 82% of VWM patients disease course has been reported to exacerbate, with episodic rapid neurological deterioration that may end in loss of ambulation, coma and death.Citation3 Coma and death were observed most frequently in patients with early onset.Citation3 Febrile infections are reported as provoking trigger for episodic deterioration in 80% of patients with exacerbated disease course.Citation3 Such episodes are complex. Typically, the dietary intake of the patient is greatly decreased due to reduced food intake and vomiting.Citation1 When the patient is admitted, the fluid and caloric intake is typically at least in part guaranteed by a glucose infusion. Possibly eIF2B activity is declined during such episodes by cumulative effects of infection, fever and reduced intake of proteins. Which factors contribute to the rapid neurological deterioration and how much they contribute are unclear. Usually, patients do not fully recover after the episode and may even die.
Recently, we have developed representative mouse models for VWM with a clinical phenotype and brain pathology replicating the chronic part of the human disease.Citation9 We reported ISR deregulation in astrocytes in VWM mouse and VWM patient brain, where an increased expression of the ATF4-regulated transcriptome and significantly reduced eIF2α phosphorylation were found.Citation10 This transcriptome is predominantly expressed in VWM astrocytes starting at a pre-symptomatic stage and its expression level correlates positively with disease stage and severity.Citation10 eIF2α phosphorylation is reduced, probably as a consequence of an increased GADD34-mediated negative feedback loop.Citation10–13 This reduction may be a compensatory measure for intrinsically reduced activity of mutated eIF2B. Our findings point to a constitutively and increasingly deregulated ISR in VWM that contribute to the chronic neurological deterioration. Treatment of VWM mice with an ISR inhibitor ISRIB or 2BAct prevented development of clinical signs in VWM mice, including ataxia and reduced body weight.Citation10,Citation14 It is unclear if ISR deregulation contributes to patients’ episodic deterioration upon febrile infections and other stresses and if ISR inhibition ameliorates also this feature of the disease.
The current study aimed to investigate if amino acid restriction modulates expression of the ATF4-regulated transcriptome in brain, resulting in episodic deterioration of the clinical phenotype in VWM mice. These mice are homozygous for a pathogenic mutation in eIF2B associated with an early onset disease course in humans.Citation9 If the diet would cause rapid and evident decline in the mouse motor behavior or induce a coma, this phenotype could model the episodic neurological deterioration in VWM patients. We subjected wild type (wt) and early-symptomatic mutant eIF2B (2b5ho) mice to an isocaloric diet with protein levels reduced to 5% (normal protein levels are 15–20%). The diet is isocaloric to prevent a general starvation response instead of a shortage of amino acids and has been shown to induce an ISR in mice.Citation15 We assessed the dietary effects on body weight and walking behavior and euthanized the mice after three weeks to assess ISR markers in several organs, including the central nervous system (CNS). We did not find diet-induced reductions in amino acid concentrations and ISR modulation in wt nor 2b5ho brain. Strikingly, we found evidence for differential ISR activation by the low protein diet in liver in wt versus 2b5ho mice, with 2b5ho mice being unresponsive. The latter may be a result from a subtle ISR deregulation in 2b5ho mouse liver.
Materials and methods
Animals
Animal experiments were carried out under the Dutch and European law and with approval of the local animal care and use committee of VU Amsterdam (FGA 11-05). Four-month-old male wt and 2b5ho mice were used (n=3 per genotype, per condition). The 2b5ho mouse is homozygous for the Arg191His mutation in eIF2Bϵ corresponding to the Arg195His mutation in VWM patients, which is associated with a severe variant in disease.Citation16 All animals were weaned at P21 and had ad libitum access to food and water. The mice were housed with a 12 h light/dark cycle. One week before the experiment each mouse was housed individually in a standard open cage with an a gnawing stick and ample nesting material (). Body weight and food intake of each mouse were measured daily from 5 days before the start of the diet (). At day 0 the diet was changed to a control diet (20% protein) or an isocaloric low protein diet (5% protein). To maintain isocaloric composition the carbohydrate content was increased from 70% to 85% in the low protein diet ( and ). From day 0 the mice were observed daily to investigate phenotypic changes due to the diet such as walking and overall wellbeing.
Figure 1. Overview of experimental setup. One week before the experiment, four-month-old wt and 2b5ho mice were housed individually. At this age 2b5ho mice are early-symptomatic and show ISR deregulation in brain. At the start of the diet, 3 mice of either genotype either received a low (5% protein) or a normal protein diet (20% protein). Body weight and food intake were measured daily from day 0 onwards. The mice were monitored for changes in behavior.
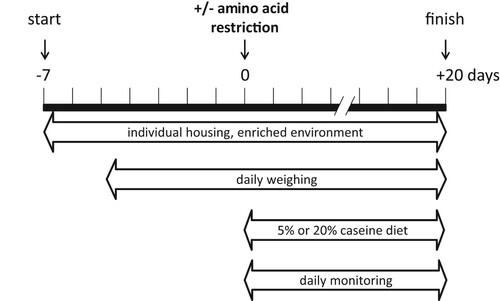
Table 1. Composition of the normal and low protein diet.
Western blot
Mice were sacrificed by cervical dislocation at the indicated ages. Organs (brain, spinal cord, liver, kidney, muscle) were isolated, snap-frozen in liquid nitrogen and stored at −80°C until further use. Preparation of lysates and Western blots and analyses of the results were as described.Citation10,Citation17
RNA isolation, cDNA synthesis and qPCR
Trizol samples were used for RNA isolation, cDNA synthesis and qPCR as described.Citation10,Citation17 Gapdh mRNA expression was used for normalization in brain samples as describedCitation10,Citation17 and Akt mRNA was used for normalization in liver samples. The expression of reference mRNAs was tested not to co-vary with genotype or diet. Primers are listed in .
Table 2. qPCR Primers.
Measurements of amino acid concentrations
Samples were prepared from sagittal brain halves of each mouse and amino acids concentrations were determined according to.Citation18 Free amino acids were measured with a dedicated amino acid analyzer (Biochrom 30). The Biochrom 30 dedicated Amino Acid analyzer measures free amino acids after their individual separation on a column filled with a cation-exchange resin. Detection of the amino acids is accomplished by an online-derivatisation of the analytes with ninhydrin, followed UV-absorbance detection at two wavelengths, i.e. 570 and 440 nm. The tested amino acids are: alanine, arginine, asparagine, aspartic acid, glutamine, glutamic acid, glycine, histidine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine and tyrosine.
Statistical analyses
Statistical analyses of body weight, food intake and food intake corrected for body weight were assessed with a three-way ANOVA (genotype, diet, time) followed by Tukey. Statistical analyses for amino acid concentration measurements were performed with a two-way ANOVA followed by Sidaks. Statistical analyses of qPCR and Western blot results were performed as described.Citation17 The program Factor was used to correct for differences between experiments but not between other conditions (genotype, treatments), if RNA or protein samples were tested in more than 1 qPCR plate or 1 SDS-PAGE Western blot.Citation19 Graphpad Prism was used for all statistical tests. Raw data are shown per figure in Suppl. Data Files 1–5. Differences were significant when p < 0.05.
Results
Body weight, food intake and walking behavior are similar between diets
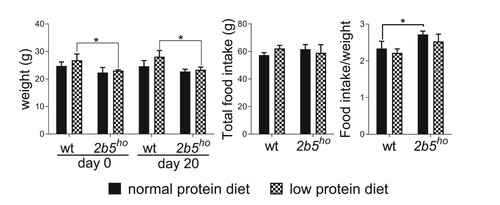
Body weight of wt and 2b5ho mice did not change significantly by the low protein diet (). Also, food intake was similar for both wt and 2b5ho mice and was not affected by the low protein diet (). The body weight of 2b5ho mice was lower than wt mice, as reported previously.Citation9,Citation10 Despite their reduced body weight, 2b5ho mice ate similar amounts of food compared to wt mice, which means that the food intake of 2b5ho mice per gram body weight was 15% higher than of wt mice (). Statistical analyses supported these observations and provided most statistically significant changes in factors genotype (p = 0.0133, Suppl. Data File 1) and day of experiment (p = 0.0008, Suppl. Data File 1). When assessing food intake throughout the experiment, we noticed that food intake decreased during the course of the experiment, suggestive of acclimatization to the novel diet. An interaction between diet and genotype was not found (p = 0.4807, Suppl. Data File 1). During the experiment a diet-related change in neurological behavior was not observed. Loss of ambulation, coma or death did not occur. The 2b5ho mice displayed a broad-based hind gait, which did not evidently change during the course of the experiment and was therefore not assessed in motor skill tests.
Figure 2. The low protein diet did not affect body weight and food intake of wt and 2b5ho mice. Body weight was lower in 2b5ho mice than wt mice at the start of the experiment (day 0). The body weight did not signficantly change during the experiment (day 20). Both genotypes ate comparable amounts of food during the experiment (total food intake). The body weight and food intake were similar between the two diets. Statistical analyses of diet-related changes are shown in Suppl. Data File 1. Graphs show average ± sd (n=3 per group). *, p < 0.05.
ISR markers in the CNS are not affected by the low protein diet
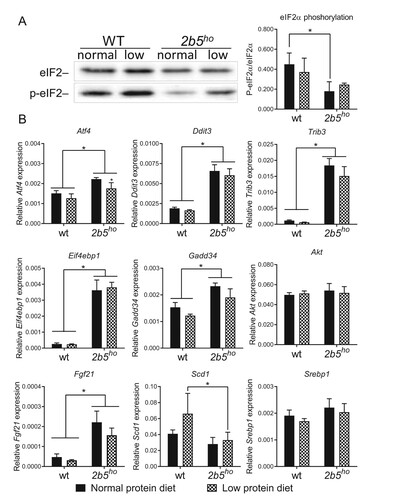
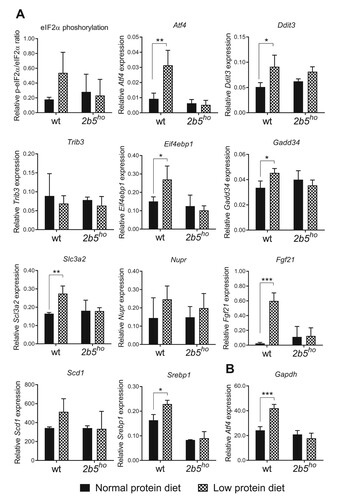
We investigated the effect of the low protein diet on ISR markers in 2b5ho mouse brain. We investigated eIF2α phosphorylation and mRNA levels of Atf4, Ddit3, Trib3, Gadd34 and Eif4ebp1. eIF2α phosphorylation was reduced, while the ATF4-regulated mRNA markers were increased in brain of 2b5ho compared to wt mice when fed the normal protein diet, as shown before.Citation10 The low protein diet did not consistently alter the phosphorylation of eIF2α or the expression of ATF4-regulated mRNAs in wt or 2b5ho mouse brain (). Some ATF4-dependent mRNA markers were slightly decreased in brains of wt and 2b5ho mice fed the low protein diet (e.g. Ddit3, Trib3, Gadd34). This decrease was not consistent amongst all tested markers and did not correspond with absence of changes in the eIF2α phosphorylation status.
Figure 3. ATF4-regulated and lipogenesis markers in brain of 2b5ho mice were not significantly induced by the low protein diet. A: Western blot analysis of eIF2α and phosphorylated eIF2α Ser51 (p-eIF2α) confirm reduced eIF2α phosphorylation in 2b5ho brain, which is not significantly affected by the low protein diet. B: qPCR analyses shows that expression of ISR-regulated Atf4, Ddit3, Trib3, Eif4ebp1 and Gadd34 and Fgf21 mRNAs is increased in 2b5ho compared to wt brain tissue (p < 0.01-0.001, not marked) and is not increased by the low protein diet. Atf4 mRNA in 2b5ho brain is subtly decreased in mice fed the low protein diet. Reference mRNA Akt expression was similar in all tested conditions. None of the tested mRNAs increased in response to the low protein diet. Statistical analyses of diet-related changes are shown in Suppl. Data File 2. Graphs show average ± sd (n=3 per group). *, p < 0.05.
We investigated the ATF4-regulated mRNA markers in spinal cord, as this tissue is also affected in VWM and may allow detection of subtle changes.Citation14,Citation20 The tested ATF4-regulated markers were increased in 2b5ho compared to wt mice, as reported previously.Citation14 Their expression remained unaffected by the reduced protein diet (). Similar to the brain samples, some ATF4-dependent markers were slightly decreased (e.g. Ddit3 and Trib3) and again this decrease was not consistent amongst all markers ().
Figure 4. Expression of ATF4-regulated and lipogenesis mRNA markers in spinal cord of 2b5ho mice was not affected by the low protein diet. Increased levels of ISR-regulated Atf4, Ddit3, Trib3, Fgf21 and decreased levels of Scd1 mRNAs were detected in the spinal cord of 2b5ho mice with qPCR. None of the tested mRNAs increased in response of the low protein diet. Statistical analyses of diet-related changes are shown in Suppl. Data File 3. Graphs show average ± sd (n=3 per group). *, p < 0.05.
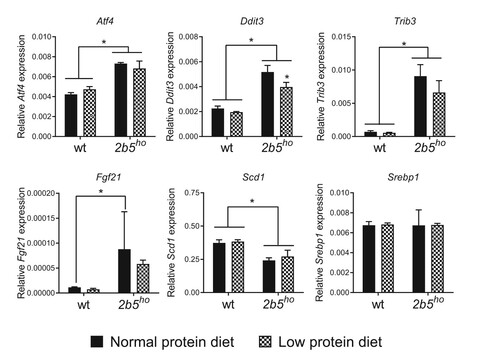
The low protein diet previously resulted in ISR activation in liver in mice.Citation15,Citation21 Interestingly, in these experiments the mRNA encoding fibroblast growth factor 21 (Fgf21) was significantly increased in expression in liver upon the low protein diet, while the mRNA levels of acyl-CoA desaturase 1 (Scd1) and sterol regulatory element – binding protein1 (Srebp1) were decreased.Citation15,Citation21 Fgf21, Scd1 and Srepb1 are lipogenesis markers and their expression is regulated by ATF4.Citation22,Citation23 We measured Fgf21, Scd1 and Srebp1 mRNA expression in the brain and spinal cord as these markers may be more efficiently measured than the mRNA markers used thus far. We found that Fgf21 expression was increased in the CNS of 2b5ho mice ( and ). Scd1 expression in spinal cord was lower in 2b5ho mice than in wt mice and was not affected by the low protein diet in either genotype ( and ). Expression of Srebp1 was not significantly different in wt and 2b5ho mice fed a normal or low protein diet. In summary, the low protein diet did not affect the expression of the investigated ATF4-regulated markers, including the lipogenesis markers Fgf21, Scd1 and Srebp1 in wt nor 2b5ho CNS, suggesting the low protein diet did not induce a continuous ISR in the CNS.
Amino acids concentrations in brain tissue are not affected by the low protein diet
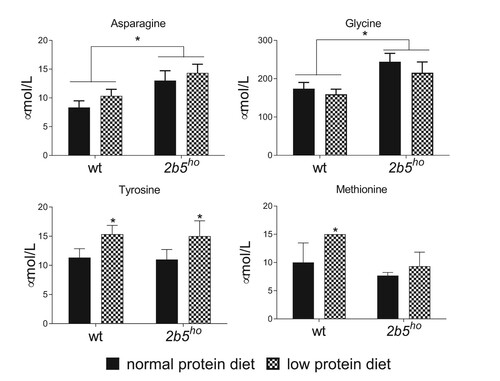
To investigate if the low protein diet affected the amino acid concentration in wt and 2b5ho mouse brain, we measured the abundance of twenty amino acids. None of the measured amino acids in brain was decreased by the low protein diet, irrespective of the mouse genotype. Tyrosine significantly increased in in response to the low protein diet and methionine was increased in wt brain in response to the low protein diet (). The amino acids asparagine and glycine were significantly increased in 2b5ho mouse brain compared to wt mouse brain irrespective of the diet (). The absence of a global decrease in all amino acid levels explains the lack of ISR modulation in mouse brain by the low protein diet.
Figure 5. Amino acid concentrations in brain were not decreased by a low protein diet for three weeks. Amino acid concentrations were determined in brain tissue. Asparagine and glycine were significantly increased in 2b5ho brain. None of the measured amino acids decreased due to the low protein diet. Statistical analyses of diet-related changes are shown in Suppl. Data File 4. Graphs show average ± sd (n=3 per group). *, p < 0.05.
Dietary ISR activation in liver was is absent in 2b5ho mice
To investigate the possibility that the low protein diet was not effective in eliciting an ISR at all, we assessed eIF2α phosphorylation as well as the expression of several ATF4-dependent mRNA markers in liver, as this organ previously showed activation of the ISR in mice fed a low protein diet or a diet without leucine.Citation15,Citation24 eIF2α phosphorylation appeared modestly increased by the low protein diet in wt liver only, although not significantly (p = 0.14, Suppl. Data File 5; A). The Gapdh reference mRNA co-varied with the low protein diet in wt mice and we therefore selected Akt mRNA as reference mRNA for this organ (B). The expression levels Atf4, Ddit3, Eif4ebp1, Gadd34, Fgf21, Srebp1 and Slc3a2 mRNA were significantly increased in wt mice fed the low protein diet (A). Remarkably, expression of these ATF4-regulated markers remained unchanged in 2b5ho liver in response to the low protein diet. Other ATF4-regulated mRNA markers (Trib3, Scd1 and Nupr) were not significantly changed by the diet in either genotype (A, Suppl. Data File 5). We did not find altered expression for these markers in kidney and skeletal muscle in wt or 2b5ho mice (data not shown). Other organs were not analyzed. Interestingly, some ISR markers showed small differences in expression between wt and 2b5ho liver, when fed the normal protein diet (e.g. Ddit3, Gadd34, Fgf21 mRNA upregulated and Srebp1 downregulated in 2b5ho compared to wt liver, A). To confirm ISR changes in liver tissue we measured expression levels of several ATF4-regulated mRNAs in liver of additional wt and 2b5ho mice that were not part of the low protein diet experiment. Although this experiment did not confirm a consistently deregulated expression of all tested ISR mRNA markers in 2b5ho compared to wt liver, increased expression of Ddit3, Gadd34 and Fgf21 in 2b5ho liver was confirmed (Suppl. Table 1). We reanalyzed the expression of ISR markers in liver of wt and 2b5ho mice fed normal levels of proteins, after combining the raw data from these two groups shown in and Suppl. Table 1 to increase statistical power. This approach showed statistically significantly altered expression of ISR markers Ddit3, Gadd34, Fgf21, Scd1 and Srebp1 and normal levels of eIF2α phosphorylation in 2b5ho liver (Suppl. Fig. 1). These findings indicate a subtle ISR deregulation in this organ of 2b5ho mice.
Figure 6. The low protein diet activates an ISR in wt liver but not in 2b5ho liver. In wt liver the low protein diet increased eIF2α phosphorylation (p-eIF2α/eIF2α) and expression of Atf4, Ddit3, Eif4ebp1, Gadd34, Fgf21, Srebp1 and Slc3a2 mRNA indicative of ISR activation. Low protein diet-induced changes in ATF4-regulated and lipogenesis markers were not observed in 2b5ho livers. Under normal protein intake Ddit3, Gadd34, and Fgf21 mRNA levels appear to be higher and Srebp1 mRNA levels lower in 2b5ho compared to wt livers. Statistical analyses of diet-related changes are shown in Suppl. Data File 5. Graphs show average ± sd (n=3 per group). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Summary of main findings
We predicted that the low protein diet would accelerate neurological deterioration in 2b5ho mice. This acceleration did not occur, likely because the system buffered against large reductions in circulating amino acids, thereby protecting the brain from amino acid restriction and ISR activation.Citation25 This combination of dietary and genetic manipulations does not provide the envisioned mouse model of subacute neurological deterioration in VWM. Assessment of ISR markers in liver yielded remarkable findings. In this organ, ISR activation in response to the low protein diet was evident in wt mice, but not in 2b5ho mice. We observed increased expression of Ddit3, Gadd34 and Fgf21 and normal levels of eIF2α phosphorylation in 2b5ho liver when dietary protein intake was normal, which reflects subtle ISR deregulation in this organ. If this deregulated state affects the basal function of this organ remains to be further investigated. Liver pathology has been reported in VWM patients with a severe phenotype.Citation26–28
The deregulated ISR in 2b5ho liver may have hampered sensing of ISR-triggering stimuli (in this study reduced protein intake) or activation of the ISR transcriptome in liver. The expression of the ATF4-regulated transcriptome is most prominent in the CNS and may affect astrocytic functions most significantly. Alternatively, ATF4 expression may have cell-type dependent effects leading to pathology specifically in astrocytes. As in liver, the deregulated ISR in astrocytes may make them unresponsive to ISR triggering stimuli, possibly contributing to both chronic and subacute neurological deterioration.
Our study also showed increased glycine and asparagine levels in 2b5ho brain and increased Fgf21 expression in brain and liver of 2b5ho mice, irrespective of dietary protein intake. Increased glycine levels in cerebrospinal fluid have been reported for VMW patients.Citation29 Glycine may be increased as a result of the ATF4-induced expression of serine biosynthesis genes and Shmt2 in 2b5ho brain as we postulated previously.Citation10 Shmt2 encodes an enzyme that converts serine into glycine.Citation30 The increased asparagine levels may be similarly explained: asparagine is synthesized by asparagine synthase (ASNS) and its mRNA is induced by ATF4.Citation31,Citation32
The increased levels of Fgf21 mRNA in 2b5ho brain and liver are likely caused by ATF4.Citation22,Citation32 FGF21 likely plays a role in energy homeostasis, as it increases energy expenditure in obese rats.Citation25,Citation33,Citation34 Interestingly, this increased energy expenditure overlaps with the phenotype of the 2b5ho mice: they are lighter than wt mice, despite a similar food intake (,Citation9). Further investigation into FGF21 protein levels needs to be conducted before firmer conclusions can be drawn. Removal or inhibition of FGF21 from mice may be informative on the role of FGF21 in the phenotype of 2b5ho mice.
The strengths and limitations of the study
Overall the amino acid levels in brain did not change, indicating that the tested low protein diet is not effective in long-term modulation of the eIF2B activity in brain in vivo. One may argue that the number of animals per group has hampered detection of reduced amino acid changes. We argue that this is not likely the case, since our findings are in agreement with two previous studies, indicating that the experimental set-up has been sound.Citation35,Citation36 Tyrosine and methionine have been reported to increase in response to the low protein diet.Citation35 This study reported unchanged amino acid levels and unchanged expression of the ATF4-regulated serine biosynthesis pathway in brain in response to a low protein diet.Citation35 A recent study described the unexpected increase of amino acid levels in brain, including tyrosine and methionine, in response to a low protein diet.Citation36 An explanation for these specific changes was not reported.
Some studies detected ISR changes at an early time point after the diet change, which we may have missed in the current study. Variable responses to low protein diets have been reported for liver and brain, as well as other organs, also upon acute dietary alterations (<2 days).Citation37,Citation38 Investigations at an early time point after the diet change are precarious, as our data indicate that the food intake increased during the first 6 days after the change in diet (day 0–6, , Suppl. Data File 1). Nevertheless, these potential early ISR-related effects that may or may not have occurred in the mice in our study did not induce an event in 2b5ho mice reminiscent of subacute deterioration in VWM patients.
The implications for future research and clinical practice
We distill from the current study that the ISR status in brains of wt and 2b5ho mice is not evidently influenced by a low protein diet after a period of three weeks. Subtle weight gain was observed in 2b5ho mice placed on a low protein diet, which has been observed during disease amelioration and is not expected to occur during neurological deterioration.Citation10,Citation14 These findings confirm that reduced protein intake is unlikely to exacerbate murine VWM disease progression. The CNS is probably protected from significant amino acid reduction.Citation35 Possibly, the dietary protein intake needs further reduction before having an ISR-activating and deteriorating neurological effect.Citation37,Citation38 In liver from 2b5ho mice, we found a mild expression of a few ISR markers when given the normal diet and poor ISR activation in response to the low protein diet. These findings suggest that the ISR is subtly deregulated in 2b5ho liver as a result of the eIF2B mutation, which makes this organ unresponsive to ISR-triggering stimuli. It remains uncertain if these conclusions can be directly extrapolated onto VWM patients.
The question if and how the ISR deregulation in VWM mice and VWM patients contributes to episodic neurological deterioration still remains unanswered, due to the lack of a representative model. A model to study the events of episodic deterioration is urgently needed to develop therapy targeting the drastic and irreversible effects of stressors like febrile infections. Mimicking episodic deterioration in VWM mice might be more successful by provoking a febrile infection. If such a model would be successful, it can be determined which pharmacological interventions are effective.
Supplemental Material
Download Zip (713.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Lisanne E. Wisse
Lisanne E. Wisse studied Biopharmaceutical Sciences at the University of Leiden (NL). She obtained her PhD in 2018 at the Amsterdam UMC (VUmc) on molecular mechanisms underlying vanishing white matter. She currently works at the Department of Clinical Genetics (Amsterdam UMC (VUmc)).
Denise Visser
Denise Visser obtained a MSc in Neurosciences (research) and is currently a PhD candidate at the Department of Radiology & Nuclear Medicine at the Amsterdam UMC (location VUmc) studying in vivo accumulation of amyloid and tau proteins in neurodegenerative disorders.
Timo J. ter Braak
Timo ter Braak obtained his BSc in Biomedical Science at the Hogeschool Utrecht (NL) in 2014 and is currently working as a research technician in the Department of Pathology of the Amsterdam UMC (Cancer Center Amsterdam). His research focuses on improving cancer diagnostics.
Abdellatif Bakkali
Abdellatif Bakkali, BSc, works as analytical chemist on metabolic deceases.
Eduard A. Struys
Eduard A. Struys, PhD, works as analytical chemist on metabolic deceases.
Christopher D. Morrison
Christopher Morrison has a general research interest in whole animal neuroendocrinology and physiology, especially as applied to the neuronal regulation of feeding behavior, body weight homeostasis, reproduction, growth, and metabolism. His work has recently focused on dietary protein content and its effects on food intake and body weight. Dietary protein restriction significantly alters body composition, metabolism and food intake, but the mechanisms through which protein intake is detected and regulated are largely unknown. Recent work in the Morrison lab has discovered novel pathways contributing to the detection of protein restriction, and in particular has identified the circulating hormone FGF21 as the first known endocrine signal of protein restriction. Ongoing work is focusing on both the mechanism through which dietary protein regulates FGF21 and the mechanisms through which FGF21 coordinates adaptive changes in food intake and metabolism in response to protein restriction. In addition, separate experiments seek to identify novel pathways connecting dietary protein intake to metabolism, feeding behavior and longevity.
Marjo S. van der Knaap
Marjo van der Knaap, MD PhD, is full professor in Pediatric Neurology at the Vrije Universiteit Medical Center and the Vrije Universiteit in Amsterdam and has a long track record in leukodystrophies, especially MRI pattern recognition, definition of novel disorders and identification of novel genetic defects for those disorders. She participated in definition of VWM as new disease and her group identified the 5 genes mutated in VWM. Her further studies have focused on the pathophysiology of VWM and developing treatment. In 2000, she founded the Center for Childhood White Matter Disorders in Amsterdam, now called the Amsterdam Leukodystrophy Center. She received numerous international awards for her work; in 2008 she received the prestigious Dutch Spinoza award.
Truus E. M. Abbink
Truus E.M. Abbink obtained her PhD in 2001 at the University of Leiden (NL) on plant disease resistance mechanisms and viral replication. During her postdocs at the Amsterdam Medical Center (NL) and Addenbrooke's Hospital University of Cambridge (UK), she specialized into HIV RNA biology. Within her current position as principal investigator at the Amsterdam Leukodystrophy Center, her expertise has changed focus towards investigations on leukodystrophy disease mechanisms. Her main research goal is to gain insight into VWM disease mechanisms, mostly to generate openings for treatment and identify biomarkers.
References
- van der Knaap MS, Barth PG, Gabreels FJ, Franzoni E, Begeer JH, Stroink H, et al. A new leukoencephalopathy with vanishing white matter. Neurology. 1997;48:845–55.
- van der Knaap MS, Pronk JC. Scheper GC: vanishing white matter disease. Lancet Neurol. 2006;5:413–23.
- Hamilton EMC, van der Lei HDW, Vermeulen G, Gerver JAM, Lourenco CM, Naidu S, et al. The natural history of vanishing white matter. Ann Neurol. 2018;84:274–88.
- van der Knaap MS, Leegwater PA, Konst AA, Visser A, Naidu S, Oudejans CB, et al. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264–70.
- Proud CG. Regulation of eukaryotic initiation factor eIF2B. Prog Mol Subcell Biol. 2001;26:95–114.
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17:1374–95.
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33.
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–74.
- Dooves S, Bugiani M, Postma NL, Polder E, Land N, Horan ST, et al. Astrocytes are central in the pathomechanisms of vanishing white matter. J Clin Invest. 2016;126:1512–24.
- Abbink TEM, Wisse LE, Jaku E, Thiecke MJ, Voltolini-Gonzalez D, Fritsen H, et al. Vanishing white matter: deregulated integrated stress response as therapy target. Ann Clin Transl Neurol. 2019;6:1407–22.
- Novoa I, Zeng H, Harding HP, Ron D.Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–22.
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–303.
- Terumitsu-Tsujita M, Kitaura H, Miura I, Kiyama Y, Goto F, Muraki Y, et al. Glial pathology in a novel spontaneous mutant mouse of the Eif2b5 gene: a vanishing white matter disease model. J Neurochem. 2019. doi:https://doi.org/10.1111/jnc.14887
- Wong YL, LeBon L, Basso AM, Kohlhaas KL, Nikkel AL, Robb HM, et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife. 2019;8. doi:https://doi.org/10.7554/eLife.42940
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–22.
- Black DN, Watters GV, Andermann E, Dumont C, Kabay ME, Kaplan P, et al. Encephalitis among Cree children in northern Quebec. Ann Neurol. 1988;24:483–9.
- Wisse LE, Penning R, Zaal EA, van Berkel CGM, Ter Braak TJ, Polder E, et al. Proteomic and metabolomic analyses of vanishing white matter mouse astrocytes reveal deregulation of ER functions. Front Cell Neurosci. 2017;11:411.
- Schutz PW, Struys EA, Sinclair G, Stockler S. Protective effects of d-3-hydroxybutyrate and propionate during hypoglycemic coma: clinical and biochemical insights from infant rats. Mol Genet Metab. 2011;103:179–84.
- Ruijter JM, Thygesen HH, Schoneveld OJ, Das AT, Berkhout B, Lamers WH. Factor correction as a tool to eliminate between-session variation in replicate experiments: application to molecular biology and retrovirology. Retrovirology. 2006;3:2.
- Leferink PS, Breeuwsma N, Bugiani M, van der Knaap MS, Heine VM. Affected astrocytes in the spinal cord of the leukodystrophy vanishing white matter. Glia. 2018;66:862–73.
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16:707–16.
- Miyake M, Nomura A, Ogura A, Takehana K, Kitahara Y, Takahara K, et al. Skeletal muscle-specific eukaryotic translation initiation factor 2 alpha phosphorylation controls amino acid metabolism and fibroblast growth factor 21-mediated non-cell-autonomous energy metabolism. FASEB J. 2016;30:798–812.
- Chen H, Yuan R, Zhang Y, Zhang X, Chen L, Zhou X, et al. ATF4 regulates SREBP1c expression to control fatty acids synthesis in 3T3-L1 adipocytes differentiation. Biochim Biophys Acta. 2016;1859:1459–69.
- Guo FF., Cavener DR. The GCN2 eIF2 alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–14.
- Antflick JE, Baker GB, Hampson DR. The effects of a low protein diet on amino acids and enzymes in the serine synthesis pathway in mice. Amino Acids. 2010;39:145–53.
- van der Knaap MS, van Berkel CG, Herms J, van Coster R, Baethmann M, Naidu S, et al. eIF2B-related disorders: antenatal onset and involvement of multiple organs. Am J Hum Genet. 2003;73:1199–207.
- Unal O, Ozgen B, Orhan D, Tokatli A, Hismi BO, Dursun A, et al. Vanishing white matter with hepatomegaly and hypertriglyceridemia attacks. J Child Neurol. 2013;28:1509–12.
- Lee JS, Lee S, Choi M, Lim BC, Choi J, Kim KJ, et al. eIF2B-related multisystem disorder in two sisters with atypical presentations. Eur J Paediatr Neurol. 2017;21:404–09.
- van der Knaap MS, Wevers RA, Kure S, Gabreels FJ, Verhoeven NM, van Raaij-Selten B, et al. Increased cerebrospinal fluid glycine: a biochemical marker for a leukoencephalopathy with vanishing white matter. J Child Neurol. 1999;14:728–31.
- Narkewicz MR, Thureen PJ, Sauls SD, Tjoa S, Nikolayevsky N, Fennessey PV. Serine and glycine metabolism in hepatocytes from mid gestation fetal lambs. Pediatr Res. 1996;39:1085–90.
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33.
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90.
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26:22–9.
- Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24:555–65.
- Munzberg H, Qualls-Creekmore E, Berthoud HR, Morrison CD, Yu S. Neural control of energy expenditure. Handb Exp Pharmacol. 2016;233:173–94.
- Vogel KR, Arning E, Bottiglieri T, Gibson KM. Multicompartment analysis of protein-restricted phenylketonuric mice reveals amino acid imbalances in brain. J Inherit Metab Dis. 2017;40:227–35.
- Chaveroux C, Carraro V, Canaple L, Averous J, Maurin AC, Jousse C, et al. In vivo imaging of the spatiotemporal activity of the eIF2alpha-ATF4 signaling pathway: insights into stress and related disorders. Sci Signal. 2015;8:rs5.
- Yamamoto J, Kamata S, Miura A, Nagata T, Kainuma R., Ishii I. Differential adaptive responses to 1-or 2-day fasting in various mouse tissues revealed by quantitative PCR analysis. Febs Open Bio. 2015;5:357–68.
