ABSTRACT
Objectives: Brain-derived neurotrophic factor (BDNF) plays an essential role in brain and metabolic health. The fact that higher concentrations are associated with improved cognitive performance has resulted in numerous intervention trials that aim at elevating BDNF levels. This systematic review provides an overview of the relation between various nutritional factors and BDNF concentrations in controlled human intervention studies. Methods: A systematic search in May 2020 identified 48 articles that examined the effects of dietary patterns or foods (n = 3), diets based on energy intake (n = 7), vitamins and minerals (n = 7), polyphenols (n = 11), long-chain omega-3 polyunsaturated fatty acids (n = 5), probiotics (n = 8), and miscellaneous food supplements (n = 7). Results: In particular, studies with dietary patterns or foods showed increased peripheral BDNF concentrations. There are also strong indications that polyphenols tend to have a positive effect on BDNF concentrations. Four of the 11 included studies with a polyphenol intervention showed a significant increase in BDNF concentrations, one study showed an increase but this was not statistically analyzed, and two studies showed a trend to an increase. Discussion: The two polyphenol classes, phenolic acids, and other phenolic compounds were responsible for the significant effects. No clear effect was found for the other dietary factors, which might also be related to whether serum or plasma was used for BDNF analysis. More work is needed to understand the relation between peripheral and central BDNF concentrations.
Introduction
Brain-derived neurotrophic factor (BDNF) is a protein that is mainly produced in the central nervous system [Citation1]. Its function depends on the stage of brain development. In early life, BDNF plays an important role in neural development and functioning, whereas in adult life it is involved in processes such as synaptic transmission and synaptic plasticity, which contribute to cognitive function [Citation2]. Indeed, previous studies have shown that lower BDNF concentrations are associated with cognitive impairment [Citation3] and higher BDNF concentrations with improved cognitive performance [Citation4]. It is well-known that lifestyle factors such as diet are contributing to mental and cognitive health [Citation5]. Therefore, specific dietary changes could be an effective way to exert an effect on BDNF and thereby preserving and improving cognitive and metabolic health. In more detail, it seems that dietary factors can influence cognition via pathways related to energy metabolism and synaptic plasticity [Citation6], in which BDNF could play a role.
BDNF can also be found in the periphery since it can cross the blood–brain barrier, albeit this has only been demonstrated in animal studies [Citation7]. However, this circulating peripheral BDNF is not only brain-derived since it can also be produced by peripheral tissues including muscle, thymus, heart, liver, vascular smooth muscle cells, lung, and spleen [Citation8]. This peripheral BDNF production seems to be similar between animals and humans [Citation9]. In the periphery, the majority of BDNF is stored in platelets while the rest circulates in plasma [Citation8]. There are indications that peripheral and central BDNF concentrations are positively associated [Citation10], which suggests that concentrations of BDNF in the blood can be regarded as indicative of concentrations of BDNF in the central nervous system, though this conclusion remains open for discussion.
Although it has received the most attention, BDNF is not only relevant from the cognitive health perspective as evidence is also growing that BDNF also plays a role in other more peripheral oriented processes. Lower peripheral BDNF concentrations are not only associated with an impaired cognitive performance [Citation11], but, for example, also with a higher body weight [Citation12]. In this context, a combined central and peripheral role for BDNF was found in energy homeostasis, a finding which was first demonstrated in rats [Citation13]. In that study, intraventricularly administered BDNF resulted in decreased energy intake and consequently body weight loss. More recently, it has been suggested that BDNF also acts as a metabolic modulator in humans by controlling and affecting patterns of food intake, physical activity, and glucose metabolism [Citation14]. Moreover, BDNF mediates energy metabolism not only via the brain but also via peripheral neurons and target organs that are involved in maintaining energy balance. These various sites of action and functions imply that BDNF could influence appetite, insulin sensitivity, and parasympathetic cardiovascular tone. If that is indeed the case, then there is a possibility that elevated BDNF concentrations could counteract the development of obesity and the metabolic syndrome [Citation15].
Interventions aimed at increasing BDNF concentrations seem an attractive target to maintain or even improve cognitive performance as well as metabolic health. Despite numerous observations that specific dietary ingredients affect BDNF concentrations, the question remains whether these dietary factors indeed modulate BDNF concentrations. In this systematic review, we provide an overview of the potential relation between the consumption of different dietary factors and BDNF concentrations in controlled human intervention studies.
Methods
Search strategy
The systematic review was based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist [Citation16]. This systematic review was not pre-registered with PROSPERO. A systematic literature search was performed on May 27th 2020 in three databases: Cochrane Central Register of Clinical Trials, Ovid MEDLINE, and Embase. Search terms consisted of (brain-derived neurotrophic factor or BDNF) combined with (diet or dietary or food or nutrient or nutrition or nutritional or supplement or supplements or supplementation or intake). Duplicates were removed and those studies that only included human were filtered out. However, this filter was impossible in Cochrane where all records had to be retained.
Selection criteria
The first phase of the selection procedure consisted of screening the titles and abstracts. This selection was performed independently by two researchers. When inconclusive, articles’ eligibility was discussed by both researchers until agreement was reached. Articles were included if they met the following criteria: (1) intervention studies performed in human adult subjects (aged ≥ 18 years); (2) intervention with a nutritional component; (3) plasma or serum concentrations of BDNF are provided; (4) original research (i.e. no letters, conference proceedings or reviews); (5) written in English; (6) no duplicates. The second phase of the selection procedure consisted of reading full texts to assess their eligibility. Articles were excluded when no control group had been used.
Data collection
The data from the selected articles were extracted to create an overview that included: publication information (year of publication, first author); study and subject characteristics (design, sample size, specification of subgroups, mean age, gender); characteristics of the BDNF measurement (assay, unit, plasma or serum); and specifications of the intervention. If BDNF concentrations were expressed in ng/ml, units were converted to pg/ml. A software caliper package was used when data were only displayed in graphs (Onde Rulers; Ondesoft, Beijing, China). The intervention effect of studies with parallel designs was defined as the difference between the changes from baseline and intervention in the experimental and placebo groups. The intervention effect of crossover studies was defined as the difference between values obtained at the end of the experimental and control periods. In order to assess the methodological quality of the selected intervention studies, the Jadad score was calculated [Citation17]. This five-point scale reviews the randomization process, blinding and the description of withdrawals (Supplemental Table 1).
Results
Search results
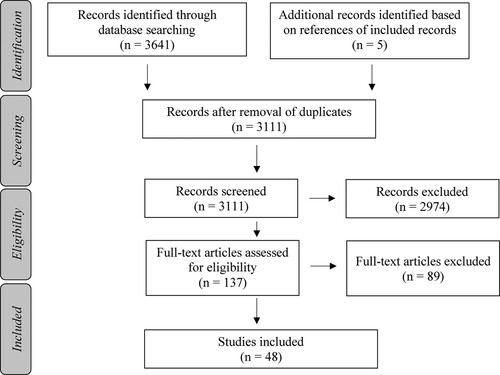
The literature search retrieved 3641 articles from the selected databases. Screening of titles and abstracts resulted in the exclusion of 2974 articles based on the predefined selection criteria. After reviewing the full texts of the remaining 132 articles, another 89 articles were excluded as they did not meet the selection criteria. Based on a search through references of the included articles five additional articles were included. Ultimately 48 articles met the inclusion criteria (), which were then clustered based on the type of nutritional intervention. This resulted in the following clusters: dietary patterns or foods (n = 3); diets based on energy intake (n = 7); and supplements (n = 38). The latter category was further divided into the following sub-clusters: vitamins and minerals (n = 7); polyphenols (n = 11); long-chain omega-3 polyunsaturated fatty acids (n = 5); probiotics (n = 8); and miscellaneous: protein (extracts) or lipids (n = 7).
Dietary patterns & foods
One study evaluated changes in BDNF concentrations based on dietary patterns, while two studies evaluated changes based on whole foods (). In a study by Sánchez-Villegas et al. [Citation18], subjects with a high cardiovascular risk consumed a Mediterranean diet supplemented with virgin olive oil or mixed nuts or a low-fat control diet. Energy intake was not controlled for and subjects were divided into two groups according to the observed weight changes. No significant differences in plasma BDNF concentrations were observed between the three diets, and these effects did not depend on changes in body weight. In a second study, Sandberg et al. [Citation19] showed that consuming a whole grain rye kernel-based bread increased plasma BDNF concentrations in apparently healthy subjects when compared with white wheat flour-based control bread. Finally, a study in mild cognitively impaired women revealed that consuming mold-fermented cheese as compared with non-mold-fermented cheese led to a significant increase in serum BDNF concentrations [Citation20].
Table 1. Characteristics of the studies included per cluster in the systematic review.
Energy intake
In seven studies, energy intake was changed by various types of fasting and caloric restrictions (). The interventions were conducted in apparently healthy subjects [Citation21–23], subjects with overweight [Citation24] or obesity [Citation25,Citation26], and subjects with a mental disorder [Citation27]. Only in this last study did a hypocaloric diet significantly increase serum BDNF concentrations, with the other six studies observing no effects.
Supplements
Vitamins and minerals
Seven studies were identified that had evaluated the effects of supplementation with either vitamins or minerals (). Four studies examined the effects of zinc but all in different populations: in women with premenstrual syndrome [Citation28], diabetic subjects [Citation29], subjects diagnosed with depression [Citation30], and obese subjects [Citation31]. Zinc supplementation showed a significant increase in serum BDNF concentrations in the premenstrual syndrome and obese population, while the other two populations did not appear to respond significantly to zinc. Two studies examined the effect of vitamin D3 in apparently healthy subjects. No significant differences between the intervention and control groups were found by Pirotta et al. [Citation32], but a significant decrease in serum BDNF concentrations was observed in the supplemented group in the study by Walentukiewicz et al. [Citation33]. In the latter study, however, the difference in changes between the intervention and control arms was not statistically analyzed. Finally, one study examined the separate and combined effects of both zinc and vitamin D3 in subjects with depressive symptoms [Citation34]. Here, both the zinc arm and the combined zinc and vitamin D3 arm showed a trend to decreased serum BDNF concentrations, but again the study, unfortunately, did not statistically analyze the difference in changes between the groups.
Polyphenols
The 11 studies included in the polyphenol cluster investigated the effect of supplementation of foods or supplements rich in phenols or flavonols (). Therefore, the type of polyphenols studied was markedly different. One study examined polyphenols from Eriobotrya japonica Lindley [Citation35], two studies seaweed rich in polyphenols [Citation36,Citation37], two studies resveratrol [Citation38,Citation39], one study ellagic acid [Citation40], two studies (nano-)curcumin [Citation41,Citation42], one study Ginkgo Biloba [Citation43], one study green tea [Citation44], and one study dark chocolate [Citation45]. A combination of resveratrol and quercetin showed no effect on serum BDNF concentrations in overweight subjects [Citation39]. The remaining interventions were mainly performed in apparently healthy subjects in whom again resveratrol [Citation38], but also a Ginkgo biloba extract [Citation43] showed no significant change in serum BDNF concentrations, neither did dark chocolate [Citation45]. Although the study evaluating the effect of green tea did not statistically analyze the difference in changes between the groups, evaluating the data as such indicated that the intervention had no effect on serum BDNF concentrations [Citation44]. In contrast, supplementation with a plant extract of leaves of Eriobotrya japonica Lindley in apparently healthy subjects [Citation35] and supplementation with nano-curcumin in subjects with metabolic syndrome [Citation41] showed a trend to increased serum and plasma BDNF concentrations, respectively. Furthermore, supplementation with seaweed in apparently healthy subjects resulted in a significant increase in serum BDNF concentrations [Citation36,Citation37]. Supplementation with ellagic acid significantly increased plasma BDNF concentrations in overweight subjects but not in healthy subjects [Citation40]. Finally, curcumin supplementation was found to increase plasma BDNF concentrations in subjects with major depressive disorder [Citation42].
Long-chain omega-3 polyunsaturated fatty acids
Five studies have been identified that examined the effects of long-chain omega-3 polyunsaturated fatty acid supplements (). No significant differences in serum BDNF concentrations were found after the interventions with the different long-chain omega-3 polyunsaturated fatty acids in subjects with diabetes [Citation46], subjects with depression [Citation47], trauma patients [Citation48], and overweight subjects [Citation49]. The study in apparently healthy subjects [Citation50] did not statistically analyze the difference in changes between the intervention and control arms, but as the change in serum BDNF concentrations in both groups was similar and small in relation to the reported group averages and standard deviations, this would likely not have reached significance.
Probiotics
The eight studies in the probiotics cluster evaluated the effects of various bacterial supplements (). Pinto-Sanchez et al. [Citation51] and Riezzo et al. [Citation52] found no significant effects of a single strain Bifidobacterium longum NCC3001 or Lactobacillus reuteri DSM-17938 probiotic approach on serum BDNF concentrations in subjects with irritable bowel syndrome or constipation, respectively, though Riezzo et al. [Citation52] did not statistically analyze the changes versus control. West et al. [Citation53] investigated both the single strain (Bifidobacterium animalis subsp. lactis BI-04) and double strain (Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis BI-07) probiotic approach in apparently healthy subjects, and similarly found no significant changes in plasma BDNF concentrations. Haghighat et al. [Citation54] examined both synbiotics (prebiotics and probiotics) and probiotics in hemodialysis patients with depressive symptoms. However, only in the synbiotics supplemented group they did find a significant increase in serum BDNF concentrations. Probiotic supplementation with Lactobacillus plantarum C29 did not affect serum BDNF concentrations in mild cognitive impaired subjects, but again no statistical test was applied to compare the difference in change [Citation55]. Probiotic supplementation with different doses of Lactobacillus helveticus IDCC3801-fermented milk showed no overall effect on plasma BDNF concentrations in apparently healthy subjects [Citation56]. Finally, probiotic supplementation with Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI in apparently healthy subjects did result in a significant increase in serum BDNF concentrations [Citation57], but supplementation with Lactobacillus helveticus and Bifidobacterium longum in subjects with depressive symptoms did not [Citation58].
Miscellaneous
The remaining cluster included seven studies with plant and protein extracts or supplements rich in lipids (). A single dose of ayahuasca increased serum BDNF concentrations in a combined population of apparently healthy subjects and depressed subjects [Citation59]. A blend of high-purity caviar-derived DNA, collagen elastin and protein extracts from sturgeon did change serum BDNF concentrations in apparently healthy subjects [Citation60]. Frail older persons who consumed milk fat globule membrane supplements showed no significant changes in serum BDNF concentrations [Citation61]. Furthermore, supplementation with royal jelly did not significantly increase serum BDNF concentrations in overweight subjects [Citation62], nor did the nutraceutical combination used in depressed subjects [Citation63]. However, a study with two different doses of macular xanthophyll in apparently healthy subjects revealed a significant increase in serum BDNF concentrations when the two groups were combined [Citation64]. Finally, another study in apparently healthy subjects found β-alanine supplementation to have no significant effect on plasma BDNF concentrations [Citation65].
Risk of bias assessment
The assessment regarding the methodological quality of the studies resulted in sufficient Jadad scores for the dietary patterns and foods cluster and poor Jadad scores for the energy intake cluster (Supplemental Table 1). Regarding the poor scores for the studies in these clusters, it should be taking into account that no points could be awarded for blinding regarding the intervention with dietary patterns or whole foods, or restriction in energy intake. Therefore, it can be questioned if this outcome is a real representation of quality for these particular clusters. The overall quality of the supplement studies in the vitamins and minerals sub-cluster is good, in the polyphenol sub-cluster sufficient to good with two studies scoring below average, in the long-chain omega-3 polyunsaturated fatty acids sub-cluster good except for one study, in the probiotics sub-cluster good, and in the miscellaneous sub-cluster one sufficient and the remaining studies good.
Discussion
Higher BDNF concentrations have been associated with both improved brain health [Citation6] and metabolic health [Citation14]. Therefore, interventions that could potentially elevate BDNF concentrations have attracted considerable attention. We have evaluated here the possible relation between consuming different nutrients and BDNF concentrations in controlled human intervention studies. To do so, we have categorized the available literature into seven (sub-)clusters. In general, two of three studies in the whole foods cluster and four of eleven studies in the polyphenol sub-cluster showed significant positive effects on BDNF concentrations. The studies in the cluster based on energy intake, and the supplement sub-clusters for vitamins and minerals, long-chain omega-3 polyunsaturated fatty acids, probiotics, and miscellaneous showed no consistent effects.
Four of the 11 studies in the polyphenol cluster showed a significant increase in BDNF concentrations, one study showed an increase in BDNF concentrations but did not statistically analyze this, and two studies showed a trend to an increase. The overall quality of the studies in this cluster was sufficient to good based on the Jadad scores (Supplemental Table 1). It thus seems that polyphenols are molecules of interest which could have a potentially positive effect on BDNF. Epidemiological studies and randomized controlled trials have indeed shown that an elevated intake of polyphenols can be linked to neuroprotective effects associated with improved cognitive function [Citation66]. Based on polyphenols’ chemical structure, they can be classified into flavonoids, phenolic acids, stilbenes, lignans, and other phenolic compounds [Citation67]. Of the 11 polyphenol studies included here, four evaluated the effects of flavonoids (Eriobotrya japonica Lindley, Ginkgo biloba, green tea, dark chocolate), one evaluated the effects of phenolic acids (ellagic acid), two evaluated the effects of stilbenes (resveratrol), and five evaluated the effects of other phenolic compounds (fermented Laminaria japonica A., (nano-)curcumin).
Phenolic acids and other phenolic compounds were responsible for the significant changes in BDNF concentrations. A potential mechanism underlying the beneficial effects of polyphenols might be a direct modulation of the cAMP-response element-binding protein (CREB) signaling pathways which are linked to BDNF expression [Citation68]. However, activation of this CREB-mediated mechanism of action has generally been ascribed to flavonoids [Citation69]. Although Eriobotrya japonica Lindley showed no significant effect on BDNF, it did show a trend to increased BDNF concentrations [Citation35]. However, the other studies that applied the other flavonoids, Ginkgo biloba, green tea, and dark chocolate, showed no effect on BDNF concentrations [Citation43,Citation45]. The reason for this lack of an effect could be due to the low bioavailability of flavonoids in humans [Citation70].
Another neurotrophic signaling pathway that can be modulated by polyphenols, in particular phenolic acids, is the Akt pathway, which ultimately leads to an increase in BDNF concentrations [Citation71]. This pathway could be the mechanism of action of ellagic acid which showed an effect on BDNF concentrations, at least in overweight subjects [Citation40]. Both the Akt and CREB pathway are stimulated by curcumin [Citation72], resulting in an expected increase in BDNF concentration as observed in depressed subjects in the study by Yu et al. [Citation42] and a trend to an increase in metabolic syndrome subjects in the study by Osali et al. [Citation41]. Despite several studies highlighting the positive effect of resveratrol supplementation on cognitive improvement [Citation73], it does not seem to be mediated via elevated BDNF concentrations [Citation38,Citation39].
Of the active polyphenol compounds responsible for the significant effect on BDNF concentration upon supplementation with fermented Laminaria japonica A. [Citation36,Citation37], the seaweed specific phlorotannins could play a role [Citation67] as the same pathway is stimulated as is seen after flavonoid exposure [Citation74]. However, since a specialized fermentation process had been applied to the seaweed, the supplements not only contained the phlorotannin polyphenols but were also enriched with gamma-aminobutyric acid (GABA; [Citation75]). GABA has been recognized as stimulating BDNF expression via the same CREB mechanism, at least during early development [Citation76]. It is possible therefore that besides the phlorotannin polyphenols, the GABA content in seaweed could also have played a role in increasing BDNF concentrations, yet it has not been shown that GABA has the same effect on BDNF expression in adult life. Furthermore, the high fiber content in seaweed [Citation77] may also have contributed to the positive effects on BDNF concentration, since Sandberg et al. [Citation19], as presented in the dietary patterns and foods cluster, showed that a fiber-rich diet also elevated BDNF concentrations. Another mechanism that could be of importance is adult hippocampal neurogenesis since both BDNF and various dietary interventions have been shown to affect the level of neurogenesis in the adult hippocampus [Citation78].
Regarding the dose of the polyphenol supplements used in the interventions, it is notable that those studies that did not show an effect on BDNF concentrations used a lower dose (38-575 mg) than the studies that did show an effect (>1000 mg; Supplemental Table 2). The doses in those studies that did show an effect were comparable to the average dietary polyphenol intake in Europe [Citation79], suggesting that the negative findings in the other studies may have been related to the low doses used. In summary, based on the included studies in this cluster it can be postulated that polyphenols, more specifically the phenolic acids and other phenolic compounds, have a beneficial effect on neurotrophic factors such as BDNF. The underlying mechanisms, as far as evaluated in humans, appear to be similar to the CREB and Akt pathways as described in cell and animal studies [Citation71].
As well as the polyphenol sub-cluster, interesting observations have also been found in the dietary patterns and foods cluster. The quality of the studies in this cluster was sufficient based on the Jadad scores (Supplemental Table 1), when taking into account no points could be awarded for blinding regarding the intervention with dietary patterns or whole foods. The intake of whole-grain rye kernel-based bread resulted in increased plasma BDNF concentrations despite the short duration of the intervention [Citation19]. It could be that the gut-brain axis is involved in increasing BDNF, amongst others because the microbiota composition changed after rye kernel bread had been consumed, which was positively correlated with the increase in circulating BDNF concentrations [Citation80]. This is supported by the increase in short-chain fatty acids in response to rye-based products [Citation81], as well as by improved cognitive performance that is correlated to the amount of fiber in the diet [Citation82]. Similarly, supplementation with a mixed-grain diet in adolescents has also been seen to result in increased plasma BDNF concentrations [Citation83], though unfortunately changes in microbiota were not analyzed. The suggestion that changes in the diet could affect BDNF via modulating microbiota composition is appealing and is supported by several studies that have shown a link between microbiota composition, BDNF concentrations [Citation84], and cognition [Citation85]. This link can also be seen in the respective studies from Haghighat et al. [Citation54] and Kim et al. [Citation57] where the microbiota populations changed upon supplementation. In this context, it is significant that the studies in the probiotics sub-cluster showed no significant changes in BDNF concentrations [Citation51,Citation53]. However, it should be acknowledged that in those interventions the microbiota profiles also remained stable [Citation51], except for one genus [Citation55].
When evaluating the observed effects on BDNF concentrations in these intervention studies, it should be noted that a number of issues make interpreting the data complex. First, there is enormous variation in peripheral BDNF concentrations between studies, something which could depend on whether it was measured either in serum or in plasma (Supplemental Table 2). Since platelets release BDNF during the clotting process, serum contains higher concentrations of BDNF than plasma [Citation8]. These differences in BDNF concentrations between serum and plasma can be up to 100- to 200-fold [Citation86]. Moreover, clotting time also affects serum BDNF concentrations, whereas the centrifugation protocol also affects plasma BDNF concentrations [Citation87]. Due to the low association between serum and plasma BDNF concentrations, it is difficult to generalize the findings of peripheral measurements [Citation10]. Differences between plasma and serum BDNF analysis might also explain the rather unexpected finding that the studies in the long-chain omega-3 polyunsaturated fatty acid sub-cluster showed no effect on BDNF concentrations, even though omega-3 fatty acids have been found to have neuroprotective effects [Citation88]. In addition, the expected link between dietary omega-3 fatty acid intake and BDNF concentrations has been shown in rat studies [Citation89], and more recently in humans [Citation90]. Interestingly, in the latter study, BDNF concentrations were measured in plasma unlike those interventions described in the sub-cluster where BDNF was always analyzed in serum. As fish oil lowers platelet aggregation [Citation91], it could be that the BDNF stored in platelets was not secreted as easily during the clotting procedure after fish oil supplementation and, therefore, any potential increase in BDNF concentrations would not have become visible when analyzed in serum. Such a reduced BDNF release from platelets has also been shown upon an oral dose of the anticoagulant drug clopidogrel [Citation92]. In addition to this potential serum effect, the absence of an effect in the reviewed fish oil intervention studies could also be related to the different populations studied. Fish oil supplements improved cognitive function in subjects with cognitive decline but not in healthy subjects [Citation93], which could explain why Witte et al. [Citation50] found no changes in BDNF concentrations.
Second, the majority of the immunoassays used to analyze BDNF concentrations cannot distinguish between precursor BDNF (proBDNF) and mature BDNF (mBDNF; [Citation94]), despite the fact that the two variants are functionally different [Citation2]. Both are biologically active, yet proBDNF has a negative influence and mBDNF a positive influence on synaptic plasticity and, ultimately, memory and cognition. It could be that either one mediates the dietary effect on cognition [Citation95]. For example, extracellular zinc activated metalloproteinase is involved in cleaving proBDNF into mBDNF [Citation96].
Third, it is unclear whether peripheral BDNF concentrations as measured in the intervention studies included in this systematic review reflect central BDNF concentrations. There are certainly indications for such a relation since animal studies have shown positive correlations between peripheral and central BDNF concentrations, which was apparently evolutionary conserved across species [Citation97]. However, from a structural and functional perspective, the blood–brain barrier in humans does not resemble those in animals [Citation98]. Although evidence from human samples is limited, a positive correlation has been found between BDNF concentrations in cerebrospinal fluid and plasma in psychotic subjects [Citation99]. Future research should focus on analyzing both peripheral (serum and plasma) and central BDNF to determine whether it is possible to estimate central BDNF from peripheral BDNF concentrations in other populations. There is also a pressing need to understand whether interventions induce similar changes in both compartments and whether there is a difference between peripheral plasma and serum concentrations in these correlations.
In conclusion, we have demonstrated that dietary interventions can elevate circulating BDNF concentrations. In particular, certain polyphenols, such as phenolic acids and phenolic compounds, seem to have this effect, which could be beneficial in improving metabolic and cognitive health. The effect of dietary ingredients from other (sub-)clusters was, however, inconsistent. Notwithstanding, it should be noted that reviewing studies that describe such effects on BDNF concentrations is highly complex, with findings varying depending on the type of material sampled for BDNF analysis and the BDNF variant measured. Further evaluations of nutritional interventions targeting an increase in BDNF concentrations, particularly focusing on the link between peripheral and central effects, are warranted.
Supplemental Material
Download MS Word (78.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Notes on contributors
Elske Gravesteijn
Elske Gravesteijn is a PhD student at the department of Nutrition and Movement Sciences, NUTRIM School of Nutrition and Translational Research in Metabolism of Maastricht University. Her research interest is to investigate the effects of almond consumption on glucose metabolism, vascular function and cognition.
Ronald P. Mensink
Ronald P. Mensink is Professor of Molecular Nutrition at Maastricht University with emphasis on lipid metabolism. His research interest is the relationships between nutritive and nonnutritive diet components with chronic metabolic stress, in particular cardiovascular disease.
Jogchum Plat
Jogchum Plat is Professor of Physiology of Nutrition at Maastricht University. Within the department of Nutrition and Movement Sciences, he works on the theme of metabolic health which is at the core of health and disease and amongst others an important factor in the development of cardiovascular disease. Prof Plat is known from his work on plant sterols and stanols in the management of dyslipidemia, which contributed to the current position of these compounds in most international guidelines. Additionally, Prof Plat works on understanding the potential benefits of other foods and dietary ingredients such as beta-glucans, nuts, protein hydrolysates and other non-nutrients. Prof Plat was elected chair (2013–2016) of the Dutch Academy of Nutrition Sciences. He is current head of the department of Nutrition and Movement Sciences and is leader of Division 1 “Obesity, Diabetes and Cardiovascular Heath” of the NUTRIM School of Nutrition and Translational Research in Metabolism of Maastricht University.
References
- Sakharnova TA, Vedunova MV, Mukhina IV. Brain-derived neurotrophic factor (BDNF) and its role in the functioning of the central nervous system. Neurochem J. 2012;6(4):251–9.
- Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–93.
- Yu H, Zhang Z, Shi Y, Bai F, Xie C, Qian Y, et al. Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry. 2008;69:1104–11.
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363.
- Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579–92.
- Gomez-Pinilla F, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16(6):726–33.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–61.
- Fujimara H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–34.
- Maisonpierre PC, Le Beau MM, Espinosa R III, Ip NY, Belluscio L, De la Monte SM, et al. Human and rat brain-derived neurotrophic factor and neurotrophin-3, gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558–68.
- Serra-Millas M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J Psychiatry. 2016;6(1):84–101.
- Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113.
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–23.
- Lapchak PA, Hefti F. BDNF and NGF treatment in lesioned rats, effects on cholinergic function and weight gain. NeuroReport. 1992;3:405–8.
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98.
- van Praag H, Fleshner M, Schwartz MW, Exercise MM, Intake E. Glucose homeostasis, and the brain. J Neurosci. 2014;34(46):15139–49.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Br Med J. 2009;339:b2535.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of report of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
- Sanchez-Villegas A, Galbete C, Martinez-Gonzalez MA, Martinez JA, Razquin C, Salas-Salvado J, et al. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: The PREDIMED-NAVARRA randomized trial. Nutr Neurosci. 2011;14(5):195–201.
- Sandberg JC, Bjorck IME, Nilsson AC. Increased plasma brain-derived neurotrophic factor 10.5 h after intake of whole grain rye-based products in healthy subjects. Nutrients. 2018;10:8.
- Suzuki T, Kojima N, Osuka Y, Tokui Y, Takasugi S, Kawashima A, et al. The effects of mold-fermented cheese on brain-derived neurotrophic factor in community-dwelling older Japanese women with mild cognitive impairment: a randomized, controlled, crossover trial. J Am Med Dir Assoc. 2019;20(12):1509–14e2.
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–34.
- Cherif A, Meeusen R, Farooq A, Briki W, Fenneni MA, Chamari K, et al. Repeated sprints in fasted state impair reaction time performance. J Am Coll Nutr. 2017;36(3):210–7.
- Khoshandam Ghashang S, Hamdan I, Lichtinghagen R, Gutenbrunner C, Nugraha B. Alterations of brain-derived neurotrophic factor and creatinine during ramadan fasting: a prospective, controlled clinical trial. Iranian Red Crescent Med J. 2019;21(5):e88324.
- Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108:933–45.
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring. 2016;24(9):1874–83.
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714–27.
- Guimaraes LR, Jacka FN, Gama CS, Berk M, Leitao-Azevedo CL, Belmonte de Abreu MG, et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1595–8.
- Jafari F, Amani R, Tarrahi MJ. Effect of zinc supplementation on physical and psychological symptoms, biomarkers of inflammation, oxidative stress, and brain-derived neurotrophic factor in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2020;194(1):89–95.
- Kheirouri S, Naghizadeh S, Alizadeh M. Zinc supplementation does not influence serum levels of VEGF, BDNF, and NGF in diabetic retinopathy patients: a randomized controlled clinical trial. Nutr Neurosci. 2019;22(10):718–24.
- Ranjbar E, Shams J, Sabetkasaei M MMS, Rashidkhani B, Mostafavi A, et al. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr Neurosci. 2014;17(2):65–71.
- Solati Z, Jazayeri S, Tehrani-Doost M, Mahmoodianfard S, Gohari MR. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: a double-blind, randomized, placebo-controlled trial. Nutr Neurosci. 2015;18(4):162–8.
- Pirotta S, Kidgell DJ, Daly RM. Effects of vitamin D supplementation on neuroplasticity in older adults: a double-blinded, placebo-controlled randomised trial. Osteoporos Int. 2015;26(1):131–40.
- Walentukiewicz A, Lysak-Radomska A, Jaworska J, Prusik K, Prusik K, Kortas JA, et al. Vitamin D supplementation and nordic walking training decreases serum homocysteine and ferritin in elderly women. Int J Environ Res Public Health. 2018;15:10.
- Yosaee S, Soltani S, Esteghamati A, Motevalian SA, Tehrani-Doost M, Clark CCT, et al. Effects of zinc, vitamin D, and their co-supplementation on mood, serum cortisol, and brain-derived neurotrophic factor in patients with obesity and mild to moderate depressive symptoms: a phase ii, 12-wk, 2 ( 2 factorial design, double-blind, randomized, placebo-controlled trial. Nutrition. 2020;71:110601.
- Choi E-K, Ko M-H, Park S-H, Ha K-C, Baek H-I, Kim Y-J, et al. Eriobotrya japonica improves cognitive function in healthy adolescents: a 12-week, randomized double-blind. placebo-controlled clinical trial. Int J Pharmacol. 2016;12(4):370–8.
- Choi W-C, Reid SNS, Ryu J-K, Kim Y, Jo Y-H, Jeon BH. Effects of γ-aminobutyric acid-enriched fermented Sea Tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in Middle aged women. Algae. 2016;31(2):175–87.
- Reid SNS, Ryu JK, Kim Y, Jeon BH. The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evid-Based Complement Altern Med. 2018;2018:8109621.
- Huhn S, Beyer F, Zhang R, Lampe L, Grothe J, Kratzsch J, et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults – a randomized controlled trial. Neuroimage. 2018;174:177–90.
- Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23):7862–70.
- Liu Y, Yu S, Wang F, Yu H, Li X, Dong W, et al. Chronic administration of ellagic acid improved the cognition in middle-aged overweight men. Appl Physiol Nutr Metab. 2018;43(3):266–73.
- Osali A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol Metab Syndr. 2020;12:26.
- Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clini Psychopharmacol. 2015;35(4):406–10.
- Sadowska-Krepa E, Klapcinska B, Pokora I, Domaszewski P, Kempa K, Podgorski T. Effects of six-week Ginkgo biloba supplementation on aerobic performance, blood pro/antioxidant balance, and serum brain-derived neurotrophic factor in physically active men. Nutrients. 2017;9:8.
- Sadowska-Krepa E, Domaszewski P, Pokora I, Zebrowska A, Gdanska A, Podgorski T. Effects of medium-term green tea extract supplementation combined with crossfit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: a pilot study. J Int Soc Sports Nutr. 2019;16(1):13.
- Sumiyoshi E, Matsuzaki K, Sugimoto N, Tanabe Y, Hara T, Katakura M, et al. Sub-chronic consumption of dark chocolate enhances cognitive function and releases nerve growth factors: a parallel-group randomized trial. Nutrients. 2019;11:11.
- Bot M, Pouwer F, Assies J, Jansen EH, Beekman AT, de Jonge P. Supplementation with eicosapentaenoic omega-3 fatty acid does not influence serum brain-derived neurotrophic factor in diabetes mellitus patients with major depression: a randomized controlled pilot study. Neuropsychobiology. 2011;63(4):219–23.
- van der Burg KP, Cribb L, Firth J, Karmacoska D, Mischoulon D, Byrne GJ. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur J Nutr. 2020;59(6):2439–2447.
- Matsuoka Y, Nishi D, Tanima Y, Itakura M, Kojima M, Hamazaki K, et al. Serum Pro-BDNF/BDNF as a treatment biomarker for response to docosahexaenoic acid in traumatized people vulnerable to developing psychological distress: a randomized controlled trial. Transl Psychiatry. 2015;5:e596.
- Sedláček P, Plavinova I, Langmajerova J, Dvorakova J, Novak J, Trefil L, et al. Effect of N-3 fatty acids supplementation during life style modification in women with overweight. Centr Eur J Public Health. 2018;26(4):265–71.
- Witte AV, Kerti L, Hermannstadter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014;24(11):3059–68.
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–59.e8.
- Riezzo G, Chimienti G, Orlando A, D'Attoma B, Clemente C, Russo F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef Microbes. 2019;10(2):137–47.
- West NP, Horn PL, Barrett S, Warren HS, Lehtinen MJ, Koerbin G, et al. Supplementation with a single and double strain probiotic on the innate immune system for respiratory illness. Eur e-J Soc Clin Nutr Metab. 2014;9(5):e178–e84.
- Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. 2019;4:1–10.
- Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019;11:2.
- Chung Y-C, Jin H-M, Cui Y, Kim DS, Jung JM, Park J-I, et al. Fermented milk Of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods. 2014;10:465–74.
- Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling elderly: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol: Ser A Biol Sci Med Sci. 2020;76(1):32–40.
- Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51(8):810–21.
- de Almeida RN, Galvao ACM, da Silva FS, Silva E, Palhano-Fontes F, Maia-de-Oliveira JP, et al. Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol. 2019;10:1234.
- Chui DH, Marcellino M, Marotta F, Sweed H, Solimene U, Vignali AI, et al. A double-blind, RCT testing beneficial modulation of BDNF in middle-aged, life style-stressed subjects: a clue to brain protection? J Clin Diagn Res. 2014;8(11):MC01-6.
- Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, et al. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled. Follow-Up Trial. Public Libr Sci One. 2015;10(2):e0116256.
- Petelin A, Kenig S, Kopinc R, Dezelak M, Cernelic Bizjak M, Jenko Praznikar Z. Effects of royal jelly Administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evid-Based Complement Altern Med. 2019;2019:4969720.
- Sarris J, Byrne GJ, Stough C, Bousman C, Mischoulon D, Murphy J, et al. Nutraceuticals for major depressive disorder – more is not merrier: an 8-week double-blind, randomised, controlled trial. J Affect Disord. 2019;245:1007–15.
- Stringham NT, Holmes PV, Stringham JM. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol Behav. 2019;211:112650.
- Varanoske AN, Wells AJ, Boffey D, Harat I, Frosti CL, Kozlowski GJ. Effects of high-dose, short-duration beta-alanine supplementation on cognitive function, mood, and circulating brain-derived neurotropic factor (BDNF) in recreationally-active males before simulated military operational stress. J Diet Suppl. 2020;6:1–22.
- Cox KHM, Scholey A. Polyphenols for brain and cognitive health. Recent Adv Polyphenol Res. 2016;5:259.
- Gomez-Guzman M, Rodriguez-Nogales A, Algieri F, Galvez J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar Drugs. 2018;16:8.
- Rendeiro C, Vauzour D, Rattray M, Waffo-Teguo P, Merillon JM, Butler LT, et al. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. Public Libr Sci One. 2013;8(5):e63535.
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45(3):295–305.
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. Am Soc Nutr Sci. 2000;130(8S Suppl):2073S–85S.
- Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Dev Ther. 2016;10:23–42.
- Sarraf P, Parohan M, Javanbakht MH, Ranji-Burachaloo S, Djalali M. Short-term curcumin supplementation enhances serum brain-derived neurotrophic factor in adult men and women: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Res. 2019;69:1–8.
- Cicero AFG, Ruscica M, Banach M. Resveratrol and cognitive decline: a clinician perspective. Arch Med Sci. 2019;15(4):936–43.
- Um MY, Lim DW, Son HJ, Cho S, Lee C. Phlorotannin-rich fraction from Ishige Foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J Funct Foods. 2018;40:110–6.
- Lee B-J, Kim J-S, Kang YM, Lim J-H, Kim Y-M, Lee M-S, et al. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus Brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010;122(1):271–6.
- Obrietan K, Gao X, Van den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism – a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88(2):1005–15.
- Kim JY, Kwon YM, Kim IS, Kim JA, Yu DY, Adhikari B, et al. Effects of the Brown seaweed Laminaria japonica supplementation on serum concentrations of IgG, triglycerides, and cholesterol, and intestinal microbiota composition in rats. Front Nutr. 2018;5:23.
- Poulose SM, Miller MG, Scott T, Shukitt-Hale B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr. 2017;8(6):804–11.
- Zamora-Ros R, Knaze V, Rothwell JA, Hemon B, Moskal A, Overvad K, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016;55(4):1359–75.
- Prykhodko O, Sandberg J, Burleigh S, Bjorck I, Nilsson A, Fak Hallenius F. Impact of rye kernel-based Evening meal on microbiota composition of young healthy lean volunteers with an emphasis on their hormonal and appetite regulations, and blood levels of brain-derived neurotrophic factor. Front Nutr. 2018;5:45.
- Sandberg JC, Bjorck IM, Nilsson AC. Rye-based evening meals favorably affected glucose regulation and appetite variables at the following breakfast; a randomized controlled study in healthy subjects. Public Libr Sci One. 2016;11(3):e0151985.
- Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr. 2015;145(1):143–9.
- Chung YC, Park CH, Kwon HK, Park YM, Kim YS, Doo JK, et al. Improved cognitive performance following supplementation with a mixed-grain diet in high school students: a randomized controlled trial. Nutrition. 2012;28(2):165–72.
- Breugelmans T, De Winter BY, Smet A. The microbiota-gut-brain axis in gastrointestinal inflammation and neurological comorbidities. Microbiota Health Dis. 2019;1:e201.
- Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Diet, gut microbiota and cognition. Metab Brain Dis. 2017;32(1):1–17.
- Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–30.
- Gejl AK, Enevold C, Bugge A, Andersen MS, Nielsen CH, Andersen LB. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci Rep. 2019;9(1):9655.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21(10):1457–67.
- Pawelczyk T, Grancow-Grabka M, Trafalska E, Szemraj J, Zurner N, Pawelczyk A. An increase in plasma brain-derived neurotrophic factor levels is related to N-3 polyunsaturated fatty acid efficacy in first episode Schizophrenia: secondary outcome analysis of the OFFER randomized clinical trial. Psychopharmacology (Berl. 2019;236(9):2811–22.
- Kristensen SD, Schmidt EB, Dyerberg J. Dietary supplementation with N-3 polyunsaturated fatty acids and human platelet function: a review with particular emphasis on implications for cardiovascular disease. J Intern Med. 1989;225:141–50.
- Stoll P, Plessow A, Bratke K, Virchow JC, Lommatzsch M. Differential effect of clopidogrel and aspirin on the release of BDNF from platelets. J Neuroimmunol. 2011;238(1-2):104–6.
- Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012;33:1482.e17–.e29.
- Lim Y, Zhong JH, Zhou XF. Development of mature BDNF-specific Sandwich ELISA. J Neurochem. 2015;134(1):75–85.
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155(3):751–9.
- Hwang JJ, Park MH, Choi SY, Koh JY. Activation of the Trk signaling pathway by extracellular zinc. role of metalloproteinases. J Biol Chem. 2005;280(12):11995–2001.
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–53.
- Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, et al. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. Public Libr Sci One. 2016;11(9):e0163037.
- Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13(4):535–9.