ABSTRACT
Objective
Determine vitamin B12 threshold levels below which additional testing of methylmalonic acid (MMA) and/or homocysteine (Hcy) is useful to diagnose metabolic vitamin B12 deficiency in patients with polyneuropathy, and how vitamin B12, MMA and Hcy levels relate to the effect of supplementation therapy.
Methods
In a retrospective cohort study of 331 patients with polyneuropathy, vitamin B12, MMA and Hcy were measured. Linear regression models with vitamin B12 as dependent and Hcy or MMA as covariate were compared, to assess which was best related to vitamin B12. Threshold vitamin B12 levels for metabolic deficiency (defined as elevatede metabolites) were determined using logistic regression with elevated metabolites as dependent and vitamin B12 as covariate. A structured interview was conducted in 42 patients to evaluate response to vitamin B12 supplementation.
Results
MMA was best related to vitamin B12. Using elevated MMA for metabolic deficiency, we found 90% sensitivity at a vitamin B12 threshold level <264 pmol/L (358 pg/mL) and 95% sensitivity at <304 pmol/L (412 pg/mL). Improvement after supplementation was reported by 19% patients and stabilization by 24%. 88% of patients with improvement and 90% with stabilization either had absolute deficiency (Vitamin B12 < 148 pmol/L) or metabolic deficiency (elevated MMA and vitamin B12 ≥ 148 pmol/L). There were no additional patients with improvement or stabilization with isolated elevated Hcy.
Conclusion
Testing of MMA has additional value in identifying patients with clinically relevant metabolic deficiency when vitamin B12 is below 304 pmol/L (412 pg/mL). Supplementation can be effective in patients with absolute and metabolic deficiency.
Introduction
In the workup of polyneuropathy, neurologists rely on the interpretation of laboratory results to identify (treatable) causes. Absolute vitamin B12 deficiency, commonly defined as a total serum vitamin B12 level of <148 pmol/L (200 pg/mL), along with clinical evidence of disease, is a common and treatable cause of polyneuropathy [Citation1–3]. The clinical relevance of metabolic vitamin B12 deficiency, defined as low-normal total serum vitamin B12 with elevated metabolites methylmalonic acid (MMA) or homocysteine (Hcy), is unclear. Previous studies in patients with polyneuropathy do not provide sufficient data on how to define metabolic vitamin B12 deficiency, which threshold levels for vitamin B12 should be used, nor which metabolites should be determined: MMA and/or Hcy [Citation4–8]. As a result, evidence-based guidelines either provide no or only limited guidance on testing for metabolic vitamin B12 deficiency in patients with polyneuropathy [Citation9–12]. Currently used diagnostic criteria for metabolic vitamin B12 deficiency are mostly based on studies with hematological or asymptomatic participants [Citation13]. The relevance and accuracy of these criteria in patients with polyneuropathy is uncertain, as polyneuropathy often develops independently of hematological abnormalities, and the relationship between vitamin B12 levels and the effect of supplementation seems less clear in patients with polyneuropathy than in hematological patients [Citation8,Citation14–16].
The aim of this study was to establish whether threshold levels for vitamin B12 can be defined below which it is useful to test for MMA and/or Hcy to diagnose metabolic vitamin B12 deficiency, and how vitamin B12, MMA and homocysteine levels relate to the effect of supplementation in patients with polyneuropathy.
Materials and methods
Study population
From patients records we identified 542 adult (18 years or older) first-referral outpatients diagnosed with polyneuropathy between 1 January 2013 and 1 January 2018, for whom combined laboratory test results for vitamin B12, MMA, Hcy and folate within a 60 days’ timespan were available. Exclusion criteria were: folate deficiency (<6.8 μmol/L, n = 51), vitamin B6 (pyridoxal 5’-phosphate) deficiency (<110 nmol/L, n = 2), self-reported vitamin B supplement use up to 1 year prior to laboratory investigations (n = 113), elevated (>3SD above the mean) blood levels of vitamin B12, folate or vitamin B6 suggestive of vitamin supplement use (n = 27), or decreased renal function as indicated by age-adjusted estimated glomerular filtration rate (eGFR; n = 18) [Citation17]. Thus we included 331 patients in the final analysis.
The diagnosis of polyneuropathy was confirmed by a neuromuscular specialist and was based on a history of distal symptoms (numbness, paresthesia, pain or weakness) with distal sensory or sensorimotor signs and/or decreased/absent reflexes upon neurological examination, and confirmation by standardized nerve conduction studies [Citation18,Citation19]. Patients whose findings on nerve conduction studies were normal, were only included if clinical signs were unequivocally consistent with polyneuropathy and if other potential causes of the neurological deficits (e.g., myelopathy or lumbar spinal stenosis) were excluded. All patients underwent an extensive diagnostic workup to identify underlying etiologies, including medical history taking, kinship history and blood investigations including at least: blood count, erythrocyte sedimentation rate, glucose, HbA1c, renal function, liver enzymes, vitamin B1, vitamin B6, folic acid, vitamin B12, homocysteine, methylmalonic acid, thyroid-stimulating hormone, serum M-protein. Additional investigations, such as lumbar punction or genetic testing, were done when clinically indicated. Patients with small fibre neuropathy were excluded.
Approval to conduct this study was received from the hospital's ethical standards committee; the medical ethical committee assessed that this study does not fall under WMO legislation.
Assessments
From the electronic patient files, we extracted clinical data, which included age, sex, duration of symptoms, medical history, alcohol intake, smoking status, medication, vitamin supplement use, type of symptoms, findings from neurological examination, results of laboratory tests and additional investigations, final etiological diagnosis, duration and method of vitamin B12 supplementation.
Laboratory tests
Total vitamin B12 was measured in plasma with Beckman Coulter DXI immunoenzymatic assay. A threshold of <148 pmol/L (<200 pg/mL) was used for absolute vitamin B12 deficiency, in accordance with most literature and the national Dutch general practitioners’ guidelines [Citation13,Citation20].
MMA was measured in plasma by liquid chromatography-tandem mass spectroscopy. A threshold of >0.29 μmol/L was used for elevated MMA based on the literature and a validation study in our hospital, carried out in 100 randomly selected adult patients with normal metabolites [Citation13].
Hcy was measured with an Atellica immunoassay. Immediately after obtaining the sample, blood was centrifuged, plasma removed and frozen. A threshold of >14.0 μmol/L was used for elevated Hcy, based on test manufacturer validation in 119 healthy donors with no history of heart disease.
Effect of vitamin B12 supplementation
According to the electronic patient files, 64 patients had been prescribed vitamin B12 supplementation. We excluded 19 patients: 1 patient had died; other reasons for exclusion were refusing to participate (n = 6), being unreachable by telephone (n = 9), not having received supplementation (n = 1), language barrier (n = 2). Additionally, three patients were excluded because we were unable to establish the effect of treatment: two reported improvement of sensory symptoms but deterioration of motor symptoms and one improved but had received intravenous immunoglobulins simultaneously with supplementation. Thus we could include 42 patients for the evaluation of the effect of supplementation. Limited data on the effect of supplementation were available in the electronic patient files, because our hospital is a referral center and follow-up is usually carried out by the general practitioner or referring neurologist. Therefore, patients who had been prescribed vitamin B12 supplementation were contacted by telephone for a structured interview on the effect of supplementation. Patients were asked about the duration and method of supplementation, whether vitamin B12 levels had been checked after supplementation and reported as normal by the treating physician, whether and which symptoms had improved, stabilized or deteriorated. We assessed effect of supplementation as improvement, stabilization, or deterioration of symptoms.
Statistical analysis
All statistical analyses were performed in R-studio (version 1.1.456). Results were considered statistically significant when the P-value was less than 0.05. Participant characteristics between patients with (metabolic) vitamin B12 deficiency and those without were compared with either the χ2 or Fisher's exact test for categorical variables and student's t-test or one-way ANOVA test, with natural logarithm transformation of right-skewed data when applicable for continuous variables.
To assess which metabolite (Hcy or MMA) was best related to vitamin B12, we compared the AIC and adjusted R2 of a linear regression model with log-transformed vitamin B12 level as dependent variable and either dichotomized Hcy or MMA (elevated or normal) as covariate. We chose to dichotomize MMA and Hcy as ‘elevated’ or ‘normal’, as this is how the results of the levels of these metabolites are used in clinical practice for diagnosing metabolic vitamin B12 deficiency. A difference between AIC's of two points or more was considered relevant. Additionally, we calculated Pearson correlations between vitamin B12 and Hcy and between vitamin B12 and MMA (all after log transformation).
After excluding patients with absolute deficiency (vitamin B12 < 148 pmol/L), we used logistic regression with the presence of elevated metabolites (MMA, Hcy, or Hcy and/or MMA) as the dependent variable, i.e. as the gold standard for metabolic deficiency, and vitamin B12 levels as covariate. We calculated threshold vitamin B12 levels to obtain 90% and 95% sensitivity, and determined the optimum trade-off between sensitivity and specificity for the different reference metabolites. Results were plotted as Receiver Operating Characteristic (ROC) curves and we estimated the respective area under the curve (AUC). We preferred a high level of sensitivity compared to high specificity to minimize the probability of missing patients with elevated reference metabolite(s) and, therefore, potentially missing metabolic vitamin B12 deficiency. For several consecutive ranges of vitamin B12 threshold levels, we calculated the prevalence of elevated reference metabolite(s), the number of missed cases of patients with elevated metabolite(s), and the number needed to test to diagnose (NND) one patient with elevated metabolite(s).
Finally, we assessed what percentage of patients, who improved and stabilized after vitamin B12 supplementation, had a metabolic deficiency, defined as vitamin B12 levels ≥ 148 pmol/L (200 pg/mL) and either elevated MMA or Hcy. A similar analysis was conducted for absolute vitamin B12 deficiency, defined as <148 pmol/L (<200 pg/mL) independent of metabolite level. We compared the distribution of vitamin B12, MMA and Hcy levels, method of supplementation and the prevalence of elevated metabolites and absolute deficiency between patients with improvement, stabilization or deterioration.
Method of supplementation, the prevalence of absolute deficiency and elevated metabolites were compared between patients with improvement, deterioration and stabilization using Fisher exact test, and distribution of vitamin B12, MMA and Hcy was compared using student's t-test after natural logarithm transformation.
Prevalence of elevated metabolites, metabolic vitamin B12 deficiency (using the different definition as described at ROC analysis), effect of vitamin B12 supplementation were compared between patients with and without a known etiology for polyneuropathy (patients with cryptogenic axonal polyneuropathy or CAP) using χ2 test, and distribution of vitamin B12 and metabolites using student's t-test after natural logarithm transformation. The comparison between patients with and without a known etiology was carried out, because the role of vitamin B12 deficiency in patients with another known etiology is less clear. Patients with vitamin B12 < 148 pmol/L were excluded from these analyses as absolute deficiency is an exclusion criterion for the diagnosis CAP.
Results
The clinical and laboratory characteristics of all 331 patients are presented in .
Table 1. Clinical and laboratory characteristics of 331 patients with a polyneuropathy.
Laboratory results
Vitamin B12 levels ranged from 77 to 657 pmol/L. In patients with elevated MMA, vitamin B12 levels were below 319 pmol/L; in patients with elevated Hcy, below 616 pmol/L. shows that both metabolites increased with decreasing levels of vitamin B12. shows the prevalence of elevated metabolites in ascending categories of vitamin B12 levels; these decreased for both metabolites as levels of vitamin B12 increased.
Figure 1. Metabolite levels and vitamin B12 levels. (a) Dot plot of vitamin B12 and methylmalonic acid levels with trend line, Pearson correlation after logarithm transformation: −0.39 (95% CI −0.48- −0.29). (b) Dot plot of vitamin B12 and homocysteine levels with trend line, Pearson correlation after logarithm transformation: −0.32 (95% CI −0.41- −0.22).
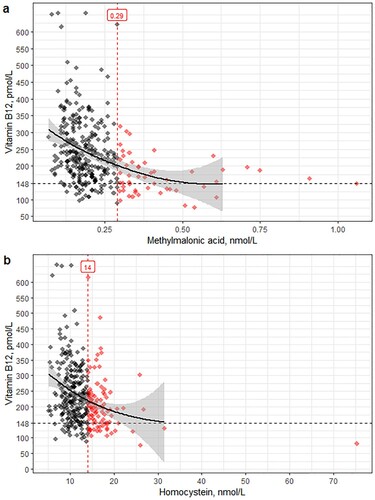
Table 2. Prevalence of elevated metabolites per vitamin B12 category.
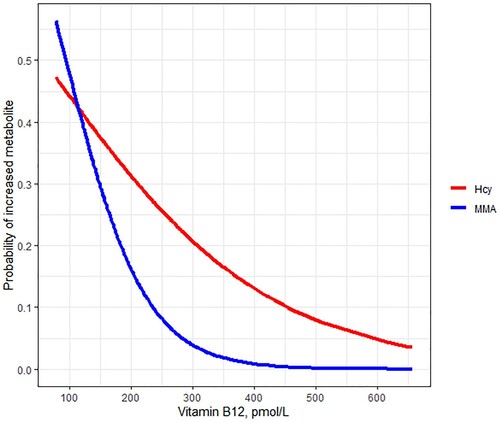
Comparing AIC's for the linear model of vitamin B12 and elevated MMA to the model of vitamin B12 and elevated Hcy shows that MMA is better related to vitamin B12 levels (AIC 130.73 and 138.97, R2 0.055 and 0.026, respectively, for MMA and Hcy). Pearson correlation between vitamin B12 and MMA was −0.39 (95% confidence interval −0.48 - −0.29), and −0.32 (95% confidence interval −0.41 - −0.22) between vitamin B12 and Hcy, all after natural logarithm transformation. shows that the probability of increased metabolites decreases with increasing vitamin B12 levels and illustrates that vitamin B12 has better discriminative ability for the risk of increased MMA than for increased Hcy. In patients with vitamin B12 levels >300 pmol/L, the probability of elevated MMA is very low, but there is still a probability of over 0.2 of elevated Hcy.
Vitamin B12 threshold levels
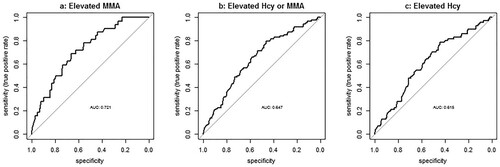
shows results for the 285 included patients from logistic regression for vitamin B12 as covariate using three different gold standards for metabolic deficiency as reference: elevated MMA, elevated Hcy, and elevated MMA and/or Hcy combined.AUC curves are shown in .
Table 3. Logistic regression for Vitamin B12 as covariate, after excluding vitamin B12 < 148 pmol/L (200 pg/mL), n = 285.
The best trade-off between sensitivity and specificity results in relatively low sensitivity rates (69–80%). Elevated Hcy or elevated MMA and/or Hcy combined show higher threshold levels of vitamin B12 to achieve the same sensitivities compared to elevated MMA alone, and result in a higher prevalence of ‘metabolic deficiency’. Using MMA as a reference, 95% sensitivity was reached at 304 pmol/L vitamin B12 threshold. We, therefore, defined metabolic deficiency as vitamin B12 levels between 148 and 304 pmol/L with elevated MMA. shows that clinical phenotype does not differ between patients with absolute, metabolic or no vitamin B12 deficiency, and that patients with absolute deficiency have a higher MCV, other red blood indices did not differ between groups.
Table 4. Comparison of clinical phenotype and laboratory results between patients with absolute, metabolic and no vitamin B12 deficiency.
Effect of vitamin B12 supplementation
Of the 42 included patients most received intramuscular injections of 1000 µg once or twice weekly as the method of supplementation (79%), otherwise they received 1000 µg of oral daily supplementation. The mean duration of supplementation was 114 weeks (SD 98). Supplementation had been discontinued in 9/42 patients, most of whom reported that vitamin B12 had normalized (78%).
shows the effect of supplementation with the distribution of vitamin B12 deficiency and elevated metabolites. Improvement after supplementation was reported by 19% of the 42 patients, by 22% of patients with absolute deficiency (vitamin B12 < 148 pmol/L), and by 20% of patients with metabolic deficiency (vitamin B12 ≥ 148 pmol/L and elevated MMA). Stabilization was reported by 24% of patients, by 26% with absolute deficiency and by 30% with metabolic deficiency. Sensory symptoms most commonly improved or stabilized upon supplementation treatment (in 14 out of 18 patients). Motor symptoms improved in two patients, and two patients reported the stabilization of both sensory and motor symptoms. Vitamin B12 ranged from 82 to 197 pmol/L in patients with improvement or stabilization, and 77–222 pmol/L in patients with deterioration. Most patients with improvement (7/8, 88%) or stabilization (9/10, 90%) either had an absolute deficiency (respectively 71%; 60%) or a metabolic deficiency (respectively 25%; 30%). There were no additional patients with improvement or stabilization with isolated elevated Hcy. If only elevated Hcy was used, the number of patients with improvement identified would be one fewer than with MMA. Most patients with improvement who had normal metabolites had an absolute vitamin B12 deficiency (<148 pmol/L; 3/4). The patient with improvement with both normal metabolites and vitamin B12 ≥ 148 pmol/L had vitamin B12 levels of 152 pmol/L.
Table 5. Effect of vitamin B12 supplementation and prevalence of elevated metabolites, n (%).
As shown in , the prevalence of elevated metabolites did not differ between patients with improvement, deterioration and stabilization. There was no difference between patients with improvement, deterioration and stabilization in: prevalence of absolute deficiency (present in 62,5%, 50%; and 60% respectively; p = 0.76, Fisher's exact test), method of supplementation (oral supplementation in respectively 37.5%, 12.5%, and 30.0%; p = 0.23, Fisher's exact test), vitamin B12 levels (median levels 141.5, 142.9 and 145.8 pmol/L respectively; p = 1.0, ANOVA after log transformation), MMA (median 0.27, 0.33 and 0.35 μmol/L respectively; p = 0.45, ANOVA after log transformation), and Hcy levels (median 13.9, 15.2 and 20.4 μmol/L respectively, p = 0.64, ANOVA after log transformation).
Cryptogenic axonal polyneuropathy (CAP)
The prevalence of elevated metabolites, metabolic vitamin B12 deficiency, effect of vitamin B12 supplementation and distribution of vitamin B12 and metabolites did not differ between patients with CAP (n = 128) and patients with a known etiology (n = 152), as shown in . The vitamin B12 threshold levels were similar, but slightly lower in patients with CAP compared to the results from the total group in . For example, at 90% sensitivity using increased MMA as reference Vitamin B12 threshold level was 245 pmol/L in patients with CAP.
Table 6. Results of patients with CAP compared to patients with polyneuropathy with a known underlying etiology (non-CAP).
Discussion
In this retrospective study, we determined serum vitamin B12, methylmalonic acid and homocysteine levels in 331 patients with polyneuropathy. We found that serum vitamin B12 levels >148 pmol/L (>200 pg/mL) in the presence of increased methylmalonic acid levels justify supplementation, whilst the measurement of homocysteine levels had no additional value. In patients with an absolute deficiency (vitamin B12 < 148 pmol/L or below a local lower limit of normal) supplementation can be effective regardless of metabolite levels.
MMA is most appropriate for diagnosing clinically relevant metabolic vitamin B12 deficiency, as it has less confounders that can cause false-positive results than Hcy. Reported confounders of Hcy include: decreased renal function, folate and pyridoxine deficiency, alcohol overuse, smoking, hypothyroidism, age and hypovolemia [Citation2,Citation21,Citation22]. In contrast, confounders of MMA are limited to decreased renal function, age, hypovolemia and HIBCH polymorphisms [Citation2,Citation23]. However, eGFR, age and vitamin B12 levels have been shown to explain only part of the variation in MMA levels, which could mean that other unknown factors can be of influence [Citation23].
As MMA is a more costly assay, it seems prudent to restrict testing to those patients who are most likely to have an elevated MMA. In our hospital, this was the case when vitamin B12 levels were <264 pmol/L (threshold level with 90% sensitivity for identifying elevated MMA). The generalizability of the vitamin B12 threshold values from our analysis has limitations, because normal ranges for metabolites and vitamin B12 differ between hospitals and depend on methods of measurement [Citation13]. When we compared vitamin B12 levels in patients who underwent testing in both our hospital and in the referring hospital, we found that levels were lower in our hospital (n = 112, median vitamin B12 = 226 vs. 272 pmol/L, p < 0.01). A previous study in patients with polyneuropathy found a slightly higher vitamin B12 threshold level of <290 pmol/L to obtain 90% sensitivity for elevated MMA (>0.39 μmol/L), also underlining that threshold values may differ between laboratories. There were additional factors in this study that could contribute to the difference, namely: the higher threshold level of abnormal MMA, and the inclusion of patients using vitamin supplements and with folate deficiency [Citation6]. Taking these limitations into account, we propose using a vitamin B12 threshold of 304 pmol/L below which testing for MMA is useful, which resulted in 95% sensitivity in our population. When MMA is elevated, supplementation should be considered. An exception should be made for patients with polyneuropathy and history of nitrous oxide abuse, in whom MMA should always be tested, as vitamin B12 levels can remain high despite serious metabolic deficiency [Citation24].
A limitation of this study is the low number of patients eligible for evaluating the effect of vitamin B12 supplementation, as patients with vitamin B12 levels >200 pmol/L and elevated MMA were often not treated with supplementation. This limited our ability to draw conclusions about the effectiveness of supplementation in these patients, who may benefit from treatment as previously reported [Citation8,Citation25]. Due to these low numbers, we were unable to carry out subgroup analysis on possible differences between the effectiveness of supplementation between patients with different additional underlying etiologies of polyneuropathy. To our knowledge, this has not been studied before and would be an interesting direction for future research. Additionally, the effect of supplementation was a self-reported outcome that might be less reliable due to possible placebo effect and recall bias. However, patient-reported outcomes are considered to be increasingly important in research [Citation26].
Due to the retrospective nature of this study, there is a risk of recall bias and selection bias; for example, in the selection of patients who underwent all laboratory investigations (vitamin B12, MMA, Hcy and folate). However, on comparing vitamin B12 levels between patients who underwent all laboratory investigations and those who did not, we found no difference. We referred patients back to their general practitioner for the evaluation of the underlying cause of the vitamin B12 deficiency. Therefore, we could not analyse possible differences in the effectiveness of supplementation between methods of supplementation for different underlying etiologies of vitamin B12 deficiency. Though, based on previous studies we do not expect any differences, as oral and intramuscular supplementation have been shown to be equally effective regardless of the underlying etiology of vitamin B12 deficiency [Citation27]. The prevalence of vitamin B12 deficiency might differ between ethnical groups, due to different dietary intake or metabolism. Due to missing information on the ethnicity of the included patients, we were unable to compare our results between ethnical groups. Therefore, our results might not be generalizable to populations with a different ethnical or racial composition.
A strength of this study is the large number of patients with polyneuropathy who underwent all laboratory tests to assess metabolic B12 deficiency. In most previous studies, metabolites were only assessed in patients with low or low-normal vitamin B12 levels, or those with a known risk factor for vitamin B12 deficiency [Citation4–8]. Additionally, the risk of confounding of the laboratory results was reduced by excluding patients with decreased renal function, folate deficiency and vitamin B supplementation use.
This and a previous study show that some patients with both normal metabolites and normal vitamin B12 levels can respond to supplementation [Citation14,Citation25]. This appears to contradict the notion that clinically relevant deficiency is practically ruled out in patients with normal metabolites [Citation4]. The paradox could have several explanations, such as a placebo effect, intraindividual variation in MMA, Hcy and vitamin B12 levels, altered vitamin B12 metabolism, altered availability of vitamin B12 due to disorders of transcobalamins, or alternative pharmacological properties of vitamin B12 [Citation25,Citation28]. This underlines the fact that a perfect gold standard for (metabolic) vitamin B12 deficiency is lacking and that further trials and studies are needed to better understand this paradox.
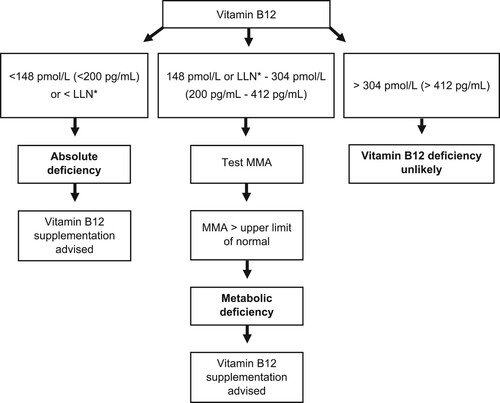
Our study supports the usefulness of testing for, and treating, metabolic vitamin B12 deficiency in patients with polyneuropathy. We found that 9% of patients with polyneuropathy in our cohort classify as having metabolic vitamin B12 deficiency, of which 43% could potentially benefit from supplementation (with 11% reporting improvement and 16% stabilization). Considering the high prevalence of polyneuropathy this is an important finding with implications at the population level. As shown in , we advise a stepwise approach in diagnosing vitamin B12 deficiency. The initial step consists of determining vitamin B12 levels and starting supplementation in those patients with absolute deficiency. Next, performing additional testing of MMA when vitamin B12 levels are between the local lower limit of normal and 304 pmol/L (412 pg/mL), and considering starting supplementation in patients with metabolic deficiency, defined as elevated MMA when vitamin B12 levels are above the lower limit of normal.
Figure 3. ROC curves for elevated metabolites as dependent and vitamin B12 levels as covariate. Methylmalonic acid >0.29 μmol/L as dependent. Methylmalonic acid >0.29 μmol/L or homocysteine >14 μmol/L as dependent. Homocysteine >14 μmol/L as dependent. Abbreviations: MMA, methylmalonic acid; Hcy, homocysteine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Pseudonimized data are available upon reasonable request.
Additional information
Funding
Notes on contributors
Janna K. Warendorf
Janna Warendorf studied medicine at the Academic Medical Centre Amsterdam. She started her PhD on the improvement of diagnosis and treatment of chronic idiopathic axonal polyneuropathy in 2016 at Utrecht University. She presented her research at several international conferences. During her PhD, she started her residency in Neurology in 2017 at the University Medical Centre Utrecht and has a special interest in neuromuscular disorders.
Perry T.C. van Doormaal
Perry van Doormaal is a neurologist and is specialized in neuromuscular diseases, especially neuropathies. During his PhD research, he investigated the role of environmental and genetic factors in amyotrophic lateral sclerosis, for which he was awarded the biannual CU Ariens Kappers Prize in 2016 from the Dutch Society for Neurology, for the best neurological research in the Netherlands during the prior two years. During his postdoctoral fellowship at the Johns Hopkins School of Medicine, he focused on risk factors for axonal neuropathy and neuropathic pain, what he continues to investigate at the University Medical Center Utrecht. He is also a clinical neurologist at Tergooi Hospitals in Hilversum, the Netherlands
Alexander F.J.E. Vrancken
Alexander Vrancken obtained his PhD degree at Utrecht University (2007) on diagnostic and treatment strategies in chronic axonal polyneuropathy. He has obtained several research grants from Prinses Beatrix Foundation and ZonMW to continue research on epidemiology, risk factors and etiology of chronic idiopathic axonal polyneuropathy (CIAP) and immune-mediated polyneuropathies. He collaborates in the worldwide task force on vasculitic neuropathy and MGUS neuropathy. His clinical work as a neurologist is specialized in the field of neuromuscular diseases and clinical neurophysiology of the peripheral nervous system at the University Medical Centre Utrecht in the Netherlands.
Nanda M. Verhoeven-Duif
Nanda Verhoeven-Duif studied chemistry at Utrecht University and obtained her PhD in the field of genetic metabolic diseases at the VU University Amsterdam. Hereafter, she was trained and registered both as a clinical chemist and as laboratory specialist in biochemical genetics. Since 2015, she is a professor in diagnostics of metabolic diseases and head of the diagnostic metabolic laboratory of the University Medical Centre Utrecht. Her research group has a focus on the discovery of new diseases and their characterization, making use of model systems and metabolomics approaches. She has been chairman of the board of the Dutch society for metabolic diseases and board member of the Dutch consortium United for Metabolic Diseases that aims to improve lives of patients and families with metabolic disease. In 2019, she became faculty member of the European training advisory committee that provides training programs for medical and laboratory specialists from over the world in the field in genetic metabolic diseases.
Ruben P.A. van Eijk
Ruben van Eijk studied medicine at the University of Utrecht. During his medical studies, he worked as a student researcher at various research groups and developed a growing interest in the underlying medical biostatistics and trial methodology. After he obtained his medical degree, he pursued his interest in clinical trials as PhD candidate at the Department of Neuromuscular Diseases under supervision of Prof. Dr. L.H. van den Berg and Prof. M.J.C. Eijkemans. In 2017, he obtained a postgraduate Master of Science degree in Epidemiology at the University of Utrecht, specializing in medical biostatistics. Since 2018, he has been working as a statistical consultant and medical statistician for the clinical trial methodology group at the Julius Center, Utrecht and The Netherlands. In 2019 he successfully defended his PhD thesis, entitled: ‘Optimizing the Design and Conduct of Clinical Trials for Amyotrophic Lateral Sclerosis’ and is currently appointed as an assistant professor at the Department of Neurology. His research focusses on addressing methodological challenges in clinical drug development to further accelerate clinical trials for neuromuscular diseases.
Leonard H. van den Berg
Leonard van den Berg obtained his PhD degree in 1995 in Utrecht. He has been a professor of Experimental Neurology since 2005 and leads a research group focused on translational research into ALS and other neuromuscular diseases. His research has been focused on the search for effective treatment for patients with motor neuron diseases and motor neuropathies by delineating the biological and molecular pathways that initiate and/or drive motor neuron degeneration. He is the principal investigator of the largest, prospective population based case–control study in ALS (PAN) to provide class I level of evidence of both environmental/lifestyle and genetic factors that determine risk and outcome of ALS and related motor neuron disorders. He is the founder and director of The Netherlands ALS Center, which aims to improve the diagnosis, treatment/care and scientific research for ALS in the Netherlands. He promotes international collaborations on ALS research as chairman of the European Network to find the Cure for ALS (ENCALS) and as the coordinator of awarded EU grants (FP7 Euro-MOTOR (systems biology)), JPND SOPHIA (biomarkers) and as the initiator of the worldwide ALS genetics research Project MinE.
Nicolette C. Notermans
Nicolette Notermans obtained her PhD degree on the clinical features and pathogenesis of Chronic Idiopathic Axonal Polyneuropathy (CIAP) and Polyneuropathy associated with Monoclonal Gammopathy of undetermined significance (MGUS) in Utrecht (1994). After her PhD, she obtained several grants (NWO, Prinses Beatrix foundation) to study the etiology of CIAP, polyneuropathy associated with MGUS and other immune-mediated neuropathies. She has initiated several studies of large, national cohorts of patients with CIAP and MGUS, in close collaboration with hematologists throughout the country. She is a member of the word-wide task force of vasculitic neuropathy and MGUS neuropathy. From 2010 to 2020 she was the director of the successful neuromuscular diseases residency program which attracts many residents from other teaching hospitals. In 2019 she became the full professor in Neuromuscular Disease and since 2020 she holds the chair of neurology at Utrecht University.
References
- Zhu Y, Minović I, Dekker LH, Eggersdorfer ML, van Zon SKR, Reijneveld SA, et al. Vitamin status and diet in elderly with low and high socioeconomic status: the Lifelines-MINUTHE study. Nutrients. 2020 Aug 31;12(9).
- Green R, Allen LH, Bjørke-Monsen A-L, Brito A, Guéant J-L, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Prim. 2017 Jun 29;3:17040.
- Bird JK, Murphy RA, Ciappio ED, McBurney MI. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017 Jun 24;9(7).
- Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994 Mar;96(3):239–46.
- Saperstein DS, Wolfe GI, Gronseth GS, Nations SP, Herbelin LL, Bryan WW, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296–301.
- Schrempf W, Eulitz M, Neumeister V, Siegert G, Koch R, Reichmann H, et al. Utility of measuring vitamin B12 and its active fraction, holotranscobalamin, in neurological vitamin B12 deficiency syndromes. J Neurol. 2011 Mar;258(3):393–401.
- Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol. 1990 Jun;34(2):99–107.
- Nardin RA, Amick ANH, Raynor EM. Vitamin B(12) and methylmalonic acid levels in patients presenting with polyneuropathy. Muscle Nerve. 2007 Oct;36(4):532–5.
- England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medi. PM R. 2009 Jan;1(1):5–13.
- Richtlijn: Polyneuropathie (Clinical practice guideline: Polyneuropathy) [Internet]. Nederlandse vereniging van Neurologie. 2005 [cited 2020 Dec 3]. Available from: https://richtlijnendatabase.nl/richtlijn/polyneuropathie/startpagina_-_polyneuropathie.html
- Heuß D. et al. Diagnostik bei Polyneuropathien, S1-Leitlinie, 2019, in: Deutsche Gesellschaft für Neurologie (Hrsg.) [Internet]. www.dgn.org/leitlinien. 2019 [cited 2020 Dec 3]. Available from: https://dgn.org/wp-content/uploads/2012/12/030-067l_S1_Polyneuropathien_Diagnostik_2012_verlaengert.pdf
- HAS. Haute Autorité de Santé. Prise en charge diagnostique des neuropathies périphériques (polyneuropathies et mononeuropathies multiples). Saint-Denis La Plaine [Internet]. 2007 [cited 2020 Nov 23]. Available from: https://www.has-sante.fr/jcms/c_598221/prise-en-charge-diagnostique-des-neuropathies-peripheriques-polyneuropathies-et-mononeuropathies-multiples
- Aparicio-Ugarriza R, Palacios G, Alder M, González-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med. 2015 Jan 1;53(8):1149–59.
- Solomon LR. Vitamin B12-responsive neuropathies: A case series. Nutr Neurosci. 2016 May;19(4):162–8.
- Stabler SP. Vitamin B 12 deficiency. N Engl J Med. 2013 Jan;368(2):149–60.
- Gadoth N, Figlin E, Chetrit A, Sela B-A, Seligsohn U. The neurology of cobalamin deficiency in an elderly population in Israel. J Neurol. 2006 Jan;253(1):45–50.
- Ven Jvd. Leeftijds- en geslachtsafhankelijke referentiewaarden voor de CKD-EPI eGFR. Ned Tijdschr Klin Chem Labgeneesk. 2016;41:211–3.
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005 Jan 25;64(2):199–207.
- Visser NA, Vrancken AF, van der Schouw YT, van den Berg LH, Notermans NC. Chronic idiopathic axonal polyneuropathy is associated with the metabolic syndrome. Diabetes Care. 2013;36(4):817–22.
- Wiersma T, Woutersen-Koch H. NHG-Standaard Diagnostiek van vitamine-B12-deficiëntie [Internet]. Vol. 57, huisarts & Wetenschap. 2014 [cited 2020 Dec 3]. p. 472. Available from: https://richtlijnen.nhg.org/files/2020-05/nhg-standpunt_vitamine_b12-2.pdf
- Nygård O, Refsum H, Ueland PM, Vollset SE. Major lifestyle determinants of plasma total homocysteine distribution: The Hordaland Homocysteine study. Am J Clin Nutr. 1998;67(2):263–70.
- Koehler KM, Baumgartner RN, Garry PJ, Allen RH, Stabler SP, Rimm EB. Association of folate intake and serum homocysteine in elderly persons according to vitamin supplementation and alcohol use. Am J Clin Nutr. 2001;73(3):628–37.
- Riphagen IJ, Minović I, Groothof D, Post A, Eggersdorfer ML, Kootstra-Ros JE, et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: results from the prospective Lifelines-MINUTHE study. BMC Med. 2020;18(1):380.
- Hannibal L, Lysne V, Bjørke-Monsen A-L, Behringer S, Grünert SC, Spiekerkoetter U, et al. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front Mol Biosci. 2016;3:27.
- Solomon LR. Cobalamin-responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood [Internet]. 2005 Feb 1;105(3):978–85. author reply 1137. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15466926.
- Vodicka E, Kim K, Devine EB, Gnanasakthy A, Scoggins JF, Patrick DL. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007-2013). Contemp Clin Trials [Internet]. 2015 Jul;43:1–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25896116.
- Sanz-Cuesta T, Escortell-Mayor E, Cura-Gonzalez I, Martin-Fernandez J, Riesgo-Fuertes R, Garrido-Elustondo S, et al. Oral versus intramuscular administration of vitamin B12 for vitamin B12 deficiency in primary care: a pragmatic, randomised, non-inferiority clinical trial (OB12). BMJ Open [Internet]. 2020;10(8):e033687), Available from: http://www.ncbi.nlm.nih.gov/pubmed/32819927.
- Andrès E, Serraj K, Zhu J, Vermorken AJM. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013 Jun;106(6):505–15.