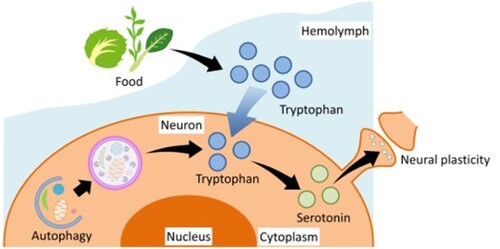
ABSTRACT
Nutritional status affects cognitive function in many types of organisms. In the pond snail Lymnaea stagnalis, 1 day of food deprivation enhances taste aversion learning ability by decreasing the serotonin (5-hydroxytryptamin; 5-HT) content in the central nervous system (CNS). On the other hand, after 5 days of food deprivation, learning ability and the CNS 5-HT concentration return to basal levels. How food deprivation leads to alterations of 5-HT levels in the CNS, however, is unknown. Here, we measured the concentration of the 5-HT precursor tryptophan in the hemolymph and CNS, and demonstrated that the CNS tryptophan concentration was higher in 5-day food-deprived snails than in non-food-deprived or 1-day food-deprived snails, whereas the hemolymph tryptophan concentration was not affected by the duration of food deprivation. This finding suggests the existence of a mediator of the CNS tryptophan concentration independent of food deprivation. To identify the mediator, we investigated autophagic flux in the CNS under different food deprivation conditions. We found that autophagic flux was significantly upregulated by inhibition of the tropomyosin receptor kinase (Trk)-Akt-mechanistic target of rapamycin complex 1 (MTORC1) pathway in the CNS of 5-day food-deprived snails. Moreover, when autophagy was inhibited, the CNS 5-HT content was significantly downregulated in 5-day food-deprived snails. Our results suggest that the hemolymph tryptophan concentration and autophagic flux in the CNS cooperatively regulate learning ability affected by different durations of food deprivation. This mechanism may underlie the selection of behaviors appropriate for animal survival depending on the degree of nutrition.
GRAPHICAL ABSTRACT
Introduction
The ability to adapt to changes in nutritional status is essential for organisms to survive [Citation1]. Organisms must make appropriate decisions for each situation to adapt to nutritional changes. Consequently, nutritional status affects cognitive function in many types of organisms [Citation2–4]; decreased intake (20%–40% reduction in total daily caloric intake) or short-term fasting (12 h–24 h fasting) improves cognitive performance across a wide range of animal species from invertebrates to vertebrates [Citation5–11]. The molecular mechanisms regulating food deprivation-induced memory enhancement, however, are not well understood.
The pond snail Lymnaea stagnalis is a good model animal for studying changes in nutritional status. The snail can learn and form conditioned taste aversion (CTA) memory [Citation12–14]. CTA is formed when an animal associates the taste of a specific food with a toxic substance, and is thought to be an adaptive survival mechanism to avoid toxic substances before they cause harm [Citation15]. In the case of snails, CTA is established by associating an appetitive stimulus (e.g. sucrose solution) with an aversive stimulus (e.g. KCl solution or electric shock). The appetitive stimulus is a conditioned stimulus (CS), and the aversive stimulus is an unconditioned stimulus (US). Repeated presentation of the CS followed by the US leads to the formation of CTA memory and suppresses CS-induced feeding behavior ((A)) [Citation16].
Figure 1. Food deprivation affects CTA learning ability in Lymnaea stagnalis (A) CTA procedure. After paired presentation of a sucrose solution (CS) and a KCl solution (US), the sucrose solution no longer induces feeding behavior. (B) Food deprivation diagram. The day of the start of fasting was defined as Day 0.
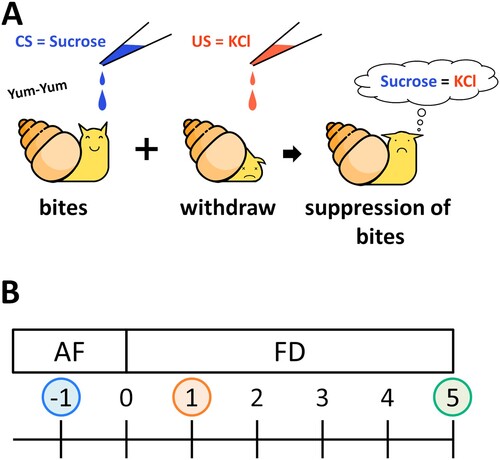
The CTA learning ability of snails changes depending on the food deprivation conditions ((B)) [Citation17,Citation18]. Snails food-deprived for 1 day (Day 1) exhibit good learning ability, whereas snails that are not food-deprived (i.e. the snails that are collected one day before food deprivation: Day −1) or are food-deprived for 5 days (Day 5) exhibited poorer learning ability [Citation19–22]. Food-deprived snails showed no observable changes in their preference for sucrose [Citation11]. The changes in the CTA learning ability reflect the CNS serotonin (5-hydroxytryptamine; 5-HT) content [Citation23,Citation24]. The CNS 5-HT content in Day 1 snails is reduced compared with that in Day −1 or Day 5 snails [Citation8]. An artificial increase in the CNS 5-HT concentration of Day 1 snails also decreases their learning ability [Citation8]. The mechanisms that regulate the CNS 5-HT content, however, are not clear.
A well-known mechanism for regulating 5-HT levels is enhanced 5-HT metabolism, assessed on the basis of the ratio of 5-HT to its major metabolite, 5-hydroxyindole acetic acid (5-HIAA) [Citation25,Citation26]. No significant change in the 5-HIAA/5-HT ratio, however, is observed in Day 1 snails [Citation8], suggesting that the decrease in the CNS 5-HT levels in Day 1 snails is not due to 5-HT metabolism. In 5-HTergic neurons, tryptophan (Trp) serves as the precursor for 5-HT. The 5-HT metabolic pathway is initiated by the hydroxylation of Trp to the intermediate 5-hydroxytryptophan (5-HTP), which is subsequently decarboxylated to 5-HT [Citation24]. Thus, the rates of 5-HT synthesis and 5-HTergic activity are thought to be affected by the availability of Trp [Citation27,Citation28]. We therefore hypothesized that changes in the Trp availability contribute to the increased CNS 5-HT concentrations in Day 5 snails.
In addition to direct dietary intake, there is another possible pathway increasing intracellular Trp concentration through the degradation of intracellular proteins and the recycling of the resulting amino acids. Autophagy is induced by food deprivation, and it changes the intracellular amino acid concentration [Citation29,Citation30]. However, it is not clear whether long-term fasting increases the amino acid concentration in the CNS and promotes 5-HT synthesis or not. In the present study, we first measured the Trp concentrations in the hemolymph and CNS. We then explored the possible role of autophagy in the CNS in the regulation of Trp availability, 5-HT synthesis, and learning in food-deprived snails.
Materials and methods
Snails
Lymnaea stagnalis with a 20 mm –25 mm shell length obtained from our snail-rearing facility (original stocks from Vrije Universiteit Amsterdam) were used in the present study. They were fed turnip leaves (Brassica rapa var. peruviridis, known as Komatsuna in Japanese) ad libitum and kept in dechlorinated tap water under a 12 h light:12 h dark cycle at 21.0°C–22.5°C. All samples were collected in the morning.
Definition of food deprivation status
We defined the food-deprivation state of the snails as follows: (1) ‘Day 0’ = the day snails begin food deprivation; (2) ‘Day −1’ = normally fed snails; (3) ‘Day 1’ = snails are food-deprived for 1 day; and (4) ‘Day 5’ = snails food deprived for 5 days ((B)).
Tryptophan and 5-HT measurements
Tryptophan (KA1916, Abnova, Taipei, Taiwan) and 5-HT (KA2518, Abnova) concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocols. The hemolymph samples were collected as follows. After the water around the snail was wiped off with absorbent paper, the snail’s foot was poked with a needle to make it retract into the shell to expel hemolymph from the renal pore; the hemolymph sample was collected and centrifuged at 2500×g for 10 min at 4°C; and the supernatant was separated and stored at −20°C. The CNS samples were collected as follows. The dissected CNS was sonicated in the ice-cold Lymnaea saline (10 mM HEPES at pH 7.9, 50 mM NaCl, 1.6 mM KCl, 2.0 mM MgCl2 and 3.5 mM CaCl2). To avoid oxidative degradation of the 5-HT, the samples were sonicated in saline with the stabilizer provided in the ELISA kit. Because the amount of tryptophan in the CNS is very small, we combined CNS tryptophan samples from 5 snails into a single sample for measurement. After sonication, the samples were centrifuged at 18,000×g for 10 min at 4°C, and the supernatants were separated and stored at −20°C.
Autophagic flux analysis
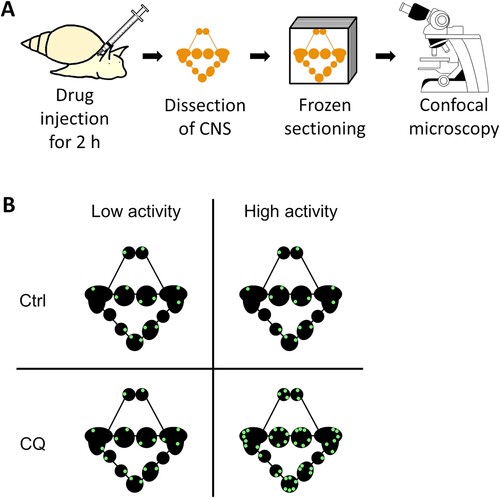
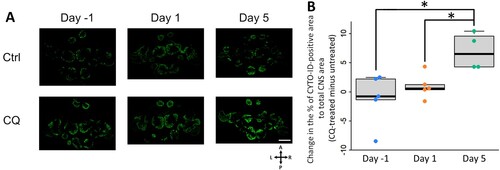
Autophagic flux in the CNS was determined using the CYTO-ID Autophagy detection kit 2.0 (ENZ-KIT175-0200, Enzo Life Sciences, Farmingdale, NY, USA). Snails anesthetized with 10% Listerine for 5 min were partially deshelled from the top of the head. An autophagosome-lysosome fusion inhibitor (60 mM chloroquine diphosphate in deionized water: CQ, CAS 50-63-5, estimated final concentration in Lymnaea = 70 μM) and CYTO-ID Green Detection Reagent 2 (0.4 μL) were gently injected adjacent to the snail CNS (total injected volume: 30 μL). As a control experiment, distilled water and CYTO-ID Green Detection Reagent 2 were injected in the same way. Two hours later the injected snails were returned to the home aquarium, and the CNS was dissected and fixed in 4% paraformaldehyde (PFA) for 30 min at room temperature. The fixed CNS were immediately frozen-sectioned into 20-μm thick sections using a cryostat and observed under a confocal laser scanning microscope (FV1000, Olympus, Tokyo, Japan; (A,B)). Slides were kept on ice when not under observation to avoid signal degradation. The area of CYTO-ID–positive signals was divided by the area of the whole CNS to quantify the accumulation of autophagosomes. The autophagic flux was determined by subtracting the signal area of the CQ-untreated sample from that of the CQ-treated sample. ImageJ (version 1.53f; https://imagej.nih.gov/ij/) was used for image processing.
Figure 2. Schematic diagram of in vivo autophagic flux analysis. (A) Experimental diagram. (B) Analysis method of autophagic flux. CYTO-ID-positive signals of Ctrl CNS simply indicate the amount of autophagosomes, but not the autophagosome degradation activity. Autophagic flux can be observed by comparing the signal differences between the presence and absence of lysosomal protease inhibitors (e.g. CQ). CNS, central nervous system; Ctrl, control; CQ, chloroquine.
Mrna quantification
The snail CNS was dissected, then snap-frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using ISOGEN II (311-07361, NIPPON GENE, Toyama, Japan) following the manufacturer’s protocol. cDNA was synthesized by the ReverTra Ace qPCR RT Master Mix with gDNA Remover (FSQ-301, Toyobo, Osaka, Japan). BrightGreen 5×qPCR MasterMix-ROX (MASTERMIX-5R, Applied Biological Materials, Richmond, Canada) was used to perform real-time PCR using a StepOnePlus Real-Time PCR System (4376598, Applied Biosystems, Bedford, MA, USA). The relative mRNA levels were quantified using the comparative CT method. The mRNA levels were normalized to those of heat shock protein 40 (HSP40) and β-tubulin. We performed the preliminary studies to compare the stability of several candidate reference genes for Lymnaea, and β-tubulin and HSP40 were selected as the most stable combination. The primer sequences are shown in . Efficiency values for the real-time PCR primers ranged from 90% to 110% (R2 = 0.95–0.99) and the length of the PCR product was confirmed by electrophoresis. The PCR conditions were as follows: one cycle at 95°C for 20 s, followed by 40 cycles of denaturation at 95°C for 3 s; annealing at 60°C for 30 s. Melting curve analysis was performed from 60°C to 95°C with a heating rate of 0.3°C/s.
Table 1. Primer sequences for real-time PCR amplification of Lymnaea stagnalis genes
Autophagy modification
Either an autophagy inducer, Tat-beclin-1 D11 (Tat-D11), or an autophagy inhibitor, bafilomycin A1 (BafA1), whose final concentration in the body was estimated as 20 μM or 1.5 nM, respectively, was gently injected adjacent to the CNS of the snail. The scrambled control Tat-L11S (estimated final concentration: 20 μM) was used as a control for Tat-D11, and DMSO (estimated final concentration: < 0.001%) was used as a vehicle control for BafA1. The snails were then returned to their home aquaria for 24 h, and 5-HT was measured as described in the Tryptophan and 5-HT measurements section.
Statistics
Data are expressed as box–whisker plot (middle bar = median, box limit = upper and lower quartile, whisker length = length to the maximum and minimum values within 1.5 times the quartile range), with statistical significance at α = 0.05. Welch’s t test was used to compare 2 groups. Data from multiple groups were analyzed using 1-way analysis of variance (one-way ANOVA), and Welch’s t tests with Holm's adjustment were applied as a post hoc test. The computer software used was R (version 4.1.0; https://www.r-project.org/).
Results
Food deprivation regulates the tryptophan content in the hemolymph and CNS
To explore the mechanisms underlying food deprivation-induced memory enhancement, we first examined the changes in Trp levels in the hemolymph and CNS of snails that were fed normally (Day −1) or food deprived for 1 (Day 1) or 5 days (Day 5).
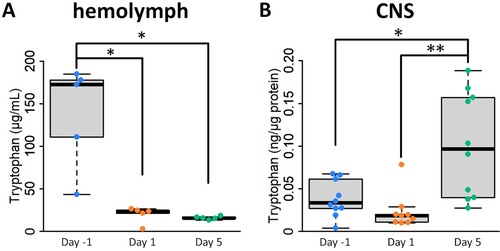
Changes in the Trp concentration associated with food deprivation differed between the CNS and hemolymph. The hemolymph Trp concentration was significantly higher in Day −1 snails than in the other snails ((A)), whereas the CNS Trp concentration was highest in Day 5 snails ((B)). The hemolymph Trp concentration decreased rapidly after 1 day of food deprivation (i.e. Day 1 snails) and did not change significantly when the food deprivation period was extended to 5 days (i.e. Day 5 snails). We interpret these data as indicating that the hemolymph Trp concentration is mainly determined by food availability. Importantly, even 1 day of food deprivation significantly reduced the hemolymph Trp concentration. On the other hand, the significant increase in the CNS Trp concentration in Day 5 snails suggests that ingested food is not the only source of Trp in the CNS.
Figure 3. Food deprivation regulates the tryptophan content in the hemolymph and CNS. (A) Changes in hemolymph 5-HT levels by food deprivation (1-way ANOVA, n = 5 each, F(2,12) = 9.41, P = 0.017, Holm’s post hoc test P = 0.032 for Day −1 vs Day 1; P = 0.032 for Day −1 vs Day 5). (B) Changes in CNS 5-HT levels by food deprivation (one-way ANOVA, n = 10 for Day −1 and Day 5, n = 9 for Day 1, F(2,26) = 6.96, P = 0.007, Holm’s post hoc test P = 0.021 for Day −1 vs Day 5; P = 0.009 for Day 1 vs Day 5). CNS, central nervous system. *P < 0.05, **P < 0.01.
Long-term food deprivation but not short-term food deprivation enhances autophagic flux in the CNS of the pond snail
We hypothesized that the increased Trp concentration seen in the CNS of Day 5 snails is a consequence of autophagy resulting from 5 days of food deprivation. Autophagy is a major protein recycling system that degrades proteins to provide amino acids, and is known to be rapidly induced by food deprivation [Citation30]. To evaluate autophagic flux (i.e. the complete process including the amount and rate of cargo sequestration and degradation), autophagosome-lysosome fusion inhibitors are needed to measure the accumulation of autophagosomes. Because such inhibitors do not cross the blood–brain barrier, this method cannot be applied to monitor autophagic flux in the CNS of vertebrates in vivo [Citation31]. Snails, however, do not have a blood–brain barrier, making this method applicable for evaluating autophagic flux in the snail CNS in vivo.
To test our hypothesis, we first monitored the autophagic flux in the CNS of snails challenged with different durations of food deprivation using an autophagosome staining dye. We found that 5-day food deprivation led to a significant increase in the CQ-mediated accumulation of autophagosomes, suggesting that autophagic flux was activated in Day 5 snail CNS ((A,B)).
Figure 4. Long-term food deprivation but not short-term food deprivation enhances autophagic flux in the CNS of the pond snail. (A) Fluorescence imaging analysis with autophagosome staining dye, CYTO-ID, between CQ-treated and untreated CNS slices per food deprivation conditions. Scale bar = 400 μm (B) Change in the percentage of CYTO-ID-positive area to total CNS area (one-way ANOVA, n = 5 for Day −1 and Day 1, n = 4 for Day 5, F(2,11) = 6.15, P = 0.032, Holm’s post hoc test P = 0.045 for Day −1 vs Day 5; P = 0.045 for Day 1 vs Day 5). Ctrl, control; CQ, chloroquine; A, anterior; P, posterior; L, left; R, right. *P < 0.05.
Neurotrophic factor signaling activates mechanistic target of rapamycin complex 1 (MTORC1) and inhibits the formation of the autophagy initiation complex [Citation32,Citation33]. Fainzilber et al. originally identified a novel molluscan neurotrophic factor, Lymnaea cysteine-rich neurotrophic factor (CRNF), which interacts with the p75 neurotrophin receptor [Citation34]. Trophic activity through interaction with mammalian TrkB was also reported using CRNF from Aplysia, a closely related species of Lymnaea [Citation35]. Furthermore, the trophic activity of the Trk receptor family was shown to have its origin before the divergence of mollusks and vertebrates [Citation36]. With this background, we performed a real-time PCR analysis of the mRNA levels of CRNF and its receptor tyrosine kinase Trk in the central nervous system to investigate the fasting effect on the regulatory mechanism of autophagy. The Trk expression level was decreased in Day 5 snails, while the expression of CRNF was not increased ((A)). This finding suggests that suppressing MTORC1 through decreased Trk signaling mediates the autophagy activation observed in the CNS of Day 5 snails.
Figure 5. Activation of autophagy in the CNS of Day 5 snails may be induced by the repression of mTORC1 via reduced Trk signaling. (A) Normalized mRNA levels of CRNF and its receptor Trk in the CNS of snails under various food deprivation states (one-way ANOVA, n = 18 for Day −1, n = 20 for Day 1, n = 19 for Day 5, F(2,54) = 9.72, P = 0.0005, Holm’s post hoc test P = 0.002. for Day −1 vs Day 5; P = 0.019 for Day 1 vs Day 5). (B) Normalized mRNA levels of autophagy-related genes in the CNS of snails under various food deprivation states (one-way ANOVA, n = 16 each, F(2,45) = 12.33, P = 0.0001, Holm’s post hoc test P = 0.0001 for Day −1 vs Day 5; P = 0.0004 for Day 1 vs Day 5). CRNF, cysteine-rich neurotrophic factor; Trk, tropomyosin receptor kinase; ULK1, unc-51 like autophagy activating kinase 1; ULK4, unc-51 like autophagy activating kinase 4; SQSTM1, sequestosome 1. *P < 0.05, **P < 0.01.
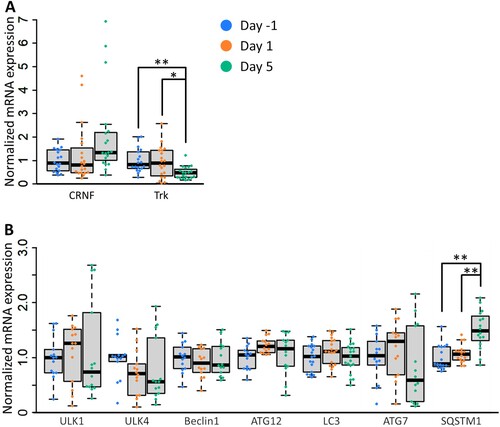
Autophagy-related genes often do not exhibit significant changes in mRNA levels when autophagy is induced [Citation37], but sometimes the induction of autophagy is accompanied by an increase in the mRNA levels of specific autophagy genes [Citation38]. Autophagy-related genes are widely conserved across species [Citation39]. We deduced the autophagy-related genes in Lymnaea by referring to the genetic information of Crassostrea gigas [Citation40]. Then, we investigated the transcriptional levels of autophagy-related genes in the snail CNS. We found that 5-day food deprivation led to the transcriptional upregulation of sequestome 1 (SQSTM1), which is involved in cargo sequestration, while food deprivation did not affect the expression of the genes involved in the induction, nucleation, and elongation of phagophores ((B)). SQSTM1 is an autophagy receptor that links ubiquitinated proteins to autophagosomes. Prolonged food deprivation increases SQSTM1 mRNA levels to restore SQSTM1 proteins that have been reduced by autophagic degradation [Citation41]. These results suggest that 5-day food deprivation regulates autophagy at the protein phosphorylation level via the Trk-Akt-MTORC1 axis in the pond snail.
Long-term food deprivation-induced autophagy increases CNS 5-HT levels
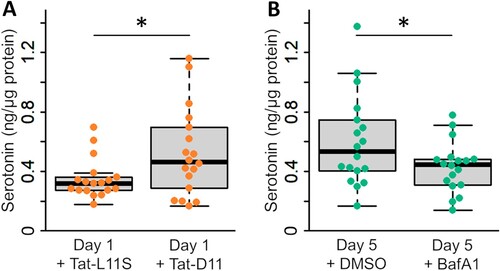
To test the hypothesis that autophagy directly regulates the CNS 5-HT content, we examined the effects of the autophagy inducer Tat-D11 and the autophagy inhibitor BafA1 on the 5-HT content in the CNS. Treatment with Tat-D11 for 24 h increased the CNS 5-HT content in Day 1 snails compared with Tat-L11S-treated (a scrambled peptide of Tat-D11) Day 1 snails ((A)). In addition, treatment with BafA1 for 24 h decreased the CNS 5-HT content in Day 5 snails compared with DMSO-treated (vehicle) Day 5 snails ((B)). Taken together, these results indicate that the CNS 5-HT content is affected by the supply of amino acids via autophagy activity.
Figure 6. Long-term food deprivation-induced autophagy increases the CNS 5-HT concentration. (A) 5-HT concentrations in the CNS of Tat-D11– and Tat-L11S–treated Day 1 snails (Welch’s t-test, n = 18 each, t(34) = −2.28, P = 0.032). (B) 5-HT levels in the CNS of BafA1- and DMSO-treated Day 5 snails (Welch’s t-test, n = 18 each, t(34) = 2.08, P = 0.047). Tat-L11S, Tat-Becline-1 L11 scrambled control; Tat-D11, Tat-becline-1 D11; DMSO, dimethyl sulfoxide; BafA1, bafilomycin A1. *P < 0.05.
We do not need the dat of Trp concentration in the CNS. There are two reasons: The first reason is that our primary interest is the concentration of 5-HT in the CNS. 5-HT regulates the ability to learn conditioned taste aversion, and Trp is its precursor. The second reason is that the autophagic flux was modified for only 24 h in our experiment. Because Trp in cells is rapidly metabolized, a short autophagic regulation cannot result in sufficient accumulation of Trp. Therefore, it appears that the autophagy does not change the Trp concentration. In addition, because the intracellular Trp concentration is affected by both the rate of Trp supply and that of metabolism, it is impossible to predict the amount of 5-HT from the amount of Trp. From these reasons, we avoided showing the Trp concentration after modification of autophagic flux. In Day 5 snails, the Trp concentration in the CNS may have increased because autophagy was enhanced for a sufficient period.
Discussion
Summary of main findings
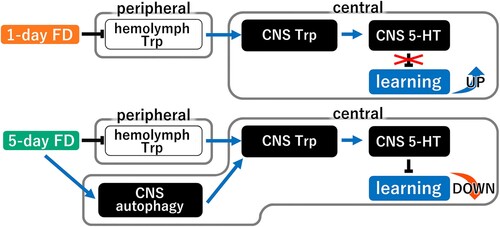
Here, we provide direct evidence of a novel mechanism regulating the 5-HT content in the snail CNS. The CNS 5-HT content in the snail CNS directly correlates with alterations in learning ability according to the food deprivation status. Thus, Day 1 snails (i.e. 1 day of food deprivation) have better learning ability and a lower CNS 5-HT content compared with Day −1 and Day 5 snails [Citation8]. Moreover, Day 5 snails (i.e. 5 days of food deprivation) exhibited the lowest learning ability and a higher CNS 5-HT content than Day 1 snails, which exhibited the highest learning and memory. The CNS 5-HT content of the Day 5 snails was comparable to that of non-food deprived snails (e.g. Day −1 snails) and both groups exhibited poorer learning and memory compared with Day 1 snails [Citation24]. We demonstrated that the CNS 5-HT content is decreased in Day 1 snails due to the decreased supply of amino acids as a result of food deprivation. The decreased concentration of Trp in the hemolymph caused by food deprivation decreases the availability of Trp in the CNS and thus inhibits 5-HT synthesis. On the other hand, the activation of autophagic flux restores the CNS 5-HT content in Day 5 snails. The small difference in 5-HT content between Day 1 and Day 5 snails compared to the previous study may be due to the difference in sensitivity between ELISA and HPLC and that in standardization methods. The enhanced autophagic flux supplies amino acids to the neurons, thereby promoting 5-HT synthesis in the CNS ().
Figure 7. A working model for food deprivation-induced learning regulation. In this model, short-term food deprivation decreases Trp in the hemolymph and decreases both Trp and 5-HT in the CNS, which enhances the CTA learning ability. When food deprivation is prolonged, however, autophagy in the CNS is enhanced to provide amino acids (Trp) by degrading proteins in the neuron, thereby ameliorating the lack of CNS Trp. As a result, CNS 5-HT concentrations are increased and the enhanced CTA learning ability is reversed. FD, food deprivation; Trp, tryptophan; 5-HT, 5-hydroxytryptamin.
5-HT synthesis is regulated by 2 key enzymes: tryptophan hydroxylase (TH) and decarboxylase of aromatic amino acids (DAA). The TH activity is considered a main rate-limiting factor in 5-HT synthesis. Recent data suggest that DAA activity controlling the 5-HTP/5-HT ratio is regulated by various factors [Citation42]. Under a condition of Trp deficiency, however, the role of both enzymes in 5-HT accumulation may become secondary. Indeed, in our present study performed using food-deprived snails, we demonstrated how changes in the Trp level alone affects the CNS 5-HT content. We did not, however, analyze the changes in the activity of TH and DAA (or in the level of expression of their respective genes). Therefore, we cannot exclude the possibility that food deprivation and/or autophagy also influence the enzymatic activity. Nevertheless, our results reveal a clear link among the availability of Trp, autophagy, and the 5-HT content.
The above-described mechanism may underlie nutrition-induced changes in the content of 5-HT and 5-HT-related behaviors, explaining, in part, how food availability alters CTA learning and memory, i.e. why Day 1 snails have better CTA memory than either Day −1 or Day 5 snails. The enhancement of CTA learning and memory formation resulting from a decreased CNS 5-HT content following short-term food deprivation may enhance behavioral adaptability and facilitate the discovery of novel food items. For example, short-term food deprivation increases the activity of neurons that control locomotor activity [Citation42–44], and is assumed to induce exploratory behavior. In contrast, if these strategies fail and food deprivation is prolonged, autophagy activation in the CNS enhances survival by restoring amino acid deficiency through proteolysis and preventing extra energy consumption by returning learning ability to basal levels.
Our findings also revealed that the snail CNS is nutritionally protected, similar to other organisms. Autophagy is induced in cultured cells within 30 min of nutrient deprivation, and occurs in muscles and internal organs in vivo within 12–24 h [Citation30,Citation45,Citation46]. Such a rapid response, however, is not observed in the CNS [Citation30]. In some cases, autophagy in the CNS may even be suppressed by nutritional deficiency [Citation32]. In snails, autophagy was not induced by 1-day of food deprivation, indicating that the CNS is nutritionally protected, as in other organisms.
How and why the present finding agrees with existing literature
In the brains of rodents subjected to dietary restriction (DR: reduction of total daily caloric intake or restriction of feeding time) for weeks to months, 5-HTergic activity is suppressed due to reduced Trp intake [Citation9,Citation47,Citation48]. In those animals, however, the amount of 5-HT in the brain does not decrease, which is not consistent with the suppression of 5-HTergic activity [Citation9,Citation47]. Our results suggest that long-term DR induces autophagy and increases the amount of Trp and 5-HT in the CNS. This idea is consistent with previous findings showing that monthly DR induces neural autophagy [Citation49]. It is also consistent with previous studies showing that the Trp intake levels were almost the same between a DR group and non-DR group; DR increases Trp and 5-HT levels in the brain [Citation50]. Thus, the present findings indicate that the effect of DR on the 5-HT levels in the brain is time-dependent. The DR effects vary between short-term and long-term food restriction. Further investigation of the interactions among DR, brain autophagy, and brain 5-HT will broaden our understanding of the involvement of nutritional regulation in neural plasticity.
Previous studies from our group demonstrated that injecting insulin into Day 5 snails reduces the amount of 5-HT in the CNS and improves learning performance [Citation8]. Our results raise the possibility that autophagy may also be involved in this insulin-induced reduction of 5-HT levels in the CNS. Insulin inhibits autophagy through the activation of MTORC1 [Citation51–53]. Administering insulin to Day 5 snails inhibits 5-HT synthesis by suppressing autophagy, thereby reducing the amount of 5-HT in the CNS. The decrease in 5-HT induced by insulin injection was only observed 1 h after the injection [Citation8], suggesting that in addition to 5-HT synthesis inhibition, 5-HT degradation is also accelerated by insulin injection.
Strengths and limitations of the present study
The present study revealed a new connection among 4 factors: nutrition, autophagy, 5-HT, and cognition. We were able to directly observe autophagy flux in the CNS in vivo by administering an autophagy-lysosome fusion inhibitor without surgical procedures such as intracerebroventricular injection. How autophagy affects learning in food-deprived snails, and the morphologic and electrophysiologic changes of the neurons remain to be investigated. The downstream pathway of Trk should also be investigated at the phosphorylation level. Further studies are needed to clarify how autophagy induction affects neural plasticity and cognitive function, and the protein(s) altered at the phosphorylation level by long-term food deprivation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data may be made available upon request.
Additional information
Funding
Notes on contributors
Yuki Totani
Yuki Totani is a PhD candidate in the Integrative Bioscience and Biomedical Engineering Program at Waseda University, Tokyo, Japan.
Junko Nakai
Junko Nakai is a PhD candidate in the Integrative Bioscience and Biomedical Engineering Program at Waseda University, Tokyo, Japan.
Dai Hatakeyama
Dai Hatakeyama is an Associate Professor at the Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Tokushima, Japan.
Varvara E. Dyakonova
Varvara E. Dyakonova is a Leading Scientist at the Koltzov Institute of Developmental Biology, Russian Academy of Sciences, Moscow, Russia.
Ken Lukowiak
Ken Lukowiak is a Professor at the Hotchkiss Brain Institute, University of Calgary, AB, Canada.
Etsuro Ito
Etsuro Ito is a Professor at the Department of Biology, Waseda University, Tokyo, Japan, and a Visiting Professor at the Graduate Institute of Medicine, School of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
References
- Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. doi:10.1016/j.arr.2014.12.011
- Prasad C. We are what we eat. Nutr Neurosci. 1998;1:1–1. doi:10.1080/1028415X.1998.11747207
- Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A, et al. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):81–94. doi:10.1038/nrn.2017.156
- Gudden J, Vasquez AA, Bloemendaal M. The effects of intermittent fasting on brain and cognitive function. Nutrients. 2021;13(9):3166. doi:10.3390/nu13093166
- Xia SZ, Liu L, Feng CH, GUO A, et al. Nutritional effects on operant visual learning in Drosophila melanogaster. Physiol Behav. 1997;62(2):263–271. doi:10.1016/S0031-9384(97)00113-3
- Gailliot MT, Baumeister RF. The physiology of willpower: linking blood glucose to self-control. Personal Soc Psychol Rev. 2007;11(4):303–327. doi:10.1177/1088868307303030
- Qi Y, Yang Y. Hunger states control the directions of synaptic plasticity via switching cell type-specific subunits of NMDA receptors. J Neurosci. 2015;35(38):13171–13182. doi:10.1523/JNEUROSCI.0855-15.2015
- Aonuma H, Totani Y, Kaneda M, Nakamura R, Watanabe T, Hatakeyama D, et al. Effects of 5-HT and insulin on learning and memory formation in food-deprived snails. Neurobiol Learn Mem. 2018;148:20–29. doi:10.1016/j.nlm.2017.12.010
- Teng LL, Lu GL, Chiou LC, Lin W-S, Cheng Y-Y, Hsueh T-E, et al. Serotonin receptor HTR6-mediated mTORC1 signaling regulates dietary restriction–induced memory enhancement. PLoS Biol. 2019;17(3):e2007097. doi:10.1371/journal.pbio.2007097
- Brünner B, Saumweber J, Samur M, Schumann I, Mahishi D, Rohwedder A, Thum AS, et al. Food restriction reconfigures naïve and learned choice behavior in Drosophila larvae. J Neurogenet. 2020;34(1):123–132. doi:10.1080/01677063.2020.1714612
- Totani Y, Nakai J, Dyakonova VE, Lukowiak K, Sakakibara M, Ito E, et al. Induction of LTM following an insulin injection. eNeuro. 2020;7:2), ENEURO.0088-20.2020. doi:10.1523/ENEURO.0088-20.2020
- Sunada H, Totani Y, Nakamura R, Sakakibara M, Lukowiak K, Ito E, et al. Two strains of Lymnaea stagnalis and the progeny from their mating display differential memory-forming ability on associative learning tasks. Front Behav Neurosci. 2017;11:161. doi:10.3389/fnbeh.2017.00161
- Nakai J, Totani Y, Kojima S, Sakakibara M, Ito E, et al. Features of behavioral changes underlying conditioned taste aversion in the pond snail Lymnaea stagnalis. Invert Neurosci. 2020;20(2):8. doi:10.1007/s10158-020-00241-7
- Totani Y, Kotani S, Odai K, Ito E, Sakakibara M, et al. Real-time analysis of animal feeding behavior with a low-calculation-power CPU. IEEE Trans Biomed Eng. 2020;67(4):1197–1205. doi:10.1109/TBME.2019.2933243
- Bernstein IL. Taste aversion learning: A contemporary perspective. Nutrition. 1999;15(3):229–234. doi:10.1016/S0899-9007(98)00192-0
- Kojima S, Yamanaka M, Fujito Y, Kojima S, Yamanaka M, Fujito Y, Ito E, et al. Differential neuroethological effects of aversive and appetitive reinforcing stimuli on associative learning in Lymnaea stagnalis. Zool Sci. 1996;13(6):803–812. doi:10.2108/zsj.13.803
- Nakai J, Totani Y, Hatakeyama D, Dyakonova VE, Ito E. Another example of conditioned taste aversion: Case of snails. Biology. 2020;9(12):422.
- Totani Y, Nakai J, Hatakeyama D, Ito E, et al. Memory-enhancing effects of short-term fasting. Eur Zool J. 2020;87(1):597–602. doi:10.1080/24750263.2020.1827053
- Sugai R, Azami S, Shiga H, Watanabe T, Sadamoto H, Kobayashi S, et al. One-trial conditioned taste aversion in lymnaea: good and poor performers in long-term memory acquisition. J Exp Biol. 2007;210(7):1225–1237. doi:10.1242/jeb.02735
- Mita K, Okuta A, Okada R, Hatakeyama D, Otsuka E, Yamagishi M, Morikawa M, et al. What are the elements of motivation for acquisition of conditioned taste aversion? Neurobiol Learn Mem. 2014;107:1–12. doi:10.1016/j.nlm.2013.10.013
- Mita K, Yamagishi M, Fujito Y, Lukowiak K, et al. An increase in insulin is important for the acquisition conditioned taste aversion in lymnaea. Neurobiol Learn Mem. 2014;116:132–138. doi:10.1016/j.nlm.2014.10.006
- Ito E, Yamagishi M, Hatakeyama D, Watanabe T, Fujito Y, Dyakonova V, Lukowiak K, et al. Memory block: a consequence of conflict resolution. J Exp Biol. 2015;218(11):1699–1704.
- Aonuma H, Totani Y, Sakakibara M, Lukowiak K, Ito E, et al. Comparison of brain monoamine content in three populations of Lymnaea that correlates with taste-aversive learning ability. Biophys Physicobiology. 2018;15:129–135. doi:10.2142/biophysico.15.0_129
- Totani Y, Aonuma H, Oike A, Watanabe T, Hatakeyama D, Sakakibara M, et al. Monoamines, insulin and the roles they play in associative learning in pond snails. Front Behav Neurosci. 2019;13:65. doi:10.3389/fnbeh.2019.00065
- Chaouloff F, Laude D, Merino D, Serrurrier B, Guezennec Y, Elghozi JL, et al. Amphetamine and α-methyl-p-tyrosine affect the exercise-induced imbalance between the availability of tryptophan and synthesis of serotonin in the brain of the rat. Neuropharmacology. 1987;26(8):1099–1106. doi:10.1016/0028-3908(87)90254-1
- Keszthelyi D, Troost FJ, Masclee AAM. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21(12):1239–1249. doi:10.1111/j.1365-2982.2009.01370.x
- Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. Br J Psychiatry. 2001;178:399–405. doi:10.1192/bjp.178.5.399
- Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front Endocrinol. 2019;10:158. doi:10.3389/fendo.2019.00158
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi:10.1016/j.cell.2011.10.026
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101–1111. doi:10.1091/mbc.e03-09-0704
- Klionsky DJ, Abdel-Aziz AK, Abdlefatah S, Abdelfatah S, Abdellatif M, Abdoli A, Abel SA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021;17(1):1–382. doi:10.1080/15548627.2020.1797280
- Nikoletopoulou V, Sidiropoulou K, Kallergi E. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 2017;26(1):230–242 e5. doi: 10.1016/j.cmet.2017.06.005.
- Zhan Z, Wu Y, Liu Z, Quan Y, Li D, Huang Y, et al. Reduced dendritic spines in the visual cortex contralateral to the optic nerve crush eye in adult mice. Invest Ophthalmol Vis Sci. 2020;61(10):55. doi:10.1167/iovs.61.10.55
- Fainzilber M, Smit AB, Syed NI, Wildering WC, Hermann PM, van der Schors RC, et al. CRNF, a molluscan neurotrophic factor that interacts with the p75 neurotrophin receptor. Science. 1996;274(5292):1540–1543. doi:10.1126/science.274.5292.1540
- Pu L, Kopec AM, Boyle HD, Carew TJ. A novel cysteine-rich neurotrophic factor in Aplysia facilitates growth, MAPK activation, and long-term synaptic facilitation. Learn Mem. 2014;21(4):215–222. doi:10.1101/lm.033662.113
- Beck G, Munno DW, Levy Z, Dissel HM, van-Minnen J, Syed NI, Fainzilber M, et al. Neurotrophic activities of trk receptors conserved over 600 million years of evolution. J Neurobiol. 2004;60(1):12–20. doi:10.1002/neu.10329
- Martinet W, Meyer GD, Andries L, De Meyer GRY, Herman AG, Kockx MM. In situ detection of starvation-induced autophagy. J Histochem Cytochem. 2016;54(1):85–96.
- Mitroulis I, Kourtzelis I, Kambas K, Rafail S, Chrysanthopoulou A, Speletas M, Ritis K, et al. Regulation of the autophagic machinery in human neutrophils. Eur J Immunol. 2010;40(5):1461–1472. doi:10.1002/eji.200940025
- Song Q, Liu H, Zhen H, Zhao B, et al. Autophagy and its role in regeneration and remodeling within invertebrate. Cell Biosci. 2020;10:111. doi:10.1186/s13578-020-00467-3
- Picot S, Faury N, Arzul I, Chollet B, Renault T, Morga B, et al. Identification of the autophagy pathway in a mollusk bivalve, Crassostrea gigas. Autophagy. 2020;16(11):2017–2035. doi:10.1080/15548627.2020.1713643
- Sahani MH, Itakura E, Mizushima N. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy. 2014;10(3):431–441. doi:10.4161/auto.27344
- Aonuma H, Mezheritskiy M, Boldyshev B, Totani Y, Vorontsov D, Zakharov I, et al. The role of serotonin in the influence of intense locomotion on the behavior under uncertainty in the mollusk Lymnaea stagnalis. Front Physiol. 2020;11:221. doi:10.3389/fphys.2020.00221
- Dyakonova V, Hernádi L, Ito E, Zakharov I, Sakharov D, et al. The activity of isolated snail neurons controlling locomotion is affected by glucose. Biophys. 2015;11:55–60. doi:10.2142/biophysics.11.55
- Dyakonova VE, Hernádi L, Ito E, Chistopolsky IA, Zakharov IS, Sakharov DA. The activity of isolated neurons and the modulatory state of an isolated nervous system represent a recent behavioural state. J Exp Biol. 2015;218(8):1151–1158.
- Gomes LC, Di BG, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13(5):589–598. doi:10.1038/ncb2220
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi:10.4161/auto.4600
- Jahng JW, Kim JG, Kim HJ, Kang D-W, Lee J-H, et al. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007;1150:100–107. doi:10.1016/j.brainres.2007.02.080
- Cui R, Fan J, Ge T, Tang L, Li B, et al. The mechanism of acute fasting-induced antidepressant-like effects in mice. J Cell Mol Med. 2018;22(1):223–229. doi:10.1111/jcmm.13310
- Bagherniya M, Butler AE, Barreto GE, Sahebkar A, et al. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 2018;47:183–197. doi:10.1016/j.arr.2018.08.004
- Kohsaka S, Takamatsu K, Tsukada Y. Effect of food restriction on serotonin metabolism in rat brain. Neurochem Res. 1980;5(1):69–79. doi:10.1007/BF00964461
- Naito T, Kuma A, Mizushima N. Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. J Biol Chem. 2013;288(29):21074–21081. doi:10.1074/jbc.M113.456228
- Lumeng C, Saltiel AR. Insulin htts on autophagy. Autophagy. 2006;2(3):250–253. doi:10.4161/auto.2788
- Ribeiro M, López de Figueroa P, Blanco FJ, Mendes AF, Caramés B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr Cartil. 2016;24(4):731–739. doi:10.1016/j.joca.2015.10.017
