ABSTRACT
Background
Insufficient sleep is a serious public health epidemic in modern society, impairing memory and other cognitive functions. In this study, partial sleep deprivation (SD) was used to induce cognitive impairment in mice to determine the effects of probiotics on subsequent cognitive deficits.
Methods
Lactiplantibacillus plantarum Lp-115 (Lp-115), Lacticaseibacillus paracasei Lpc-37 (Lpc-37), Bifidobacterium animalis subsp. lactis 420 (B420) and their combination were administered to mice subjected to partial SD and compared with non-SD and SD vehicle groups. Mice were administered a daily oral gavage containing either 1 × 109 colony forming units (CFU) of single-strain, 1.5 × 109 CFU of multi-strain (5 × 108 CFU/strain), or vehicle for thirty days prior to and for nine days during a behavioural test paradigm. The novel object recognition (NOR) test, spontaneous alternation Y-maze (Y-maze), and the step-through passive avoidance (STPA) task were applied to evaluate learning and memory performance following partial SD.
Results
Partial SD had a significant impact on cognitive function in vehicle mice. Intervention with Lpc-37 significantly improved recognition memory deficits in the NOR test, spatial working memory deficits in the Y-maze, and contextual long-term memory impairments in the STPA task, in mice subjected to partial SD compared to the SD vehicle group. The multi-strain significantly improved recognition memory deficits in the NOR test and spatial working memory deficits in the Y-maze in mice subjected to partial SD compared to the SD vehicle group.
Conclusions
These findings demonstrate that Lpc-37 and the multi-strain may play a role in alleviating memory impairments and improve cognitive function in partially sleep-deprived mice.
Introduction
Insufficient sleep is a pervasive and prominent public health concern within the modern 24-hour (h) ‘round-the-clock' society, and is associated with myriad of adverse physical and mental effects [Citation1]. The National Sleep Foundation recommends that adults (18–64 years) require between seven and nine h of sleep per day to maintain overall health and well-being, as well as cognitive, emotional, and physical health [Citation2]. Approximately one-third of adults in most industrialised countries report reduced sleep duration (i.e. less than seven h of daily sleep) [Citation3], and continuous sleep deprivation (SD) is linked to 7 of the 15 leading causes of death in the United States of America, related to infection, cardiovascular complications and metabolic dysfunction, among others [Citation1]. Furthermore, repeated sleep loss has severe consequences on work efficiency, public safety, overall well-being [Citation4,Citation5], and influences the development of sleep disorders including insomnia, stress and mood-related disorders, and addiction disorders [Citation6].
Numerous SD studies have unveiled various aspects of sleep architecture [Citation7], and determined the neurobehavioural consequences of sleep loss on cognitive performance including attention and working memory, and other functions such as long-term memory and decision-making [Citation8]. Cognitive enhancing interventions, such as caffeine and modafinil have been reported to reverse SD-induced cognitive impairment in animal models and humans [Citation9,Citation10], however, such interventions are associated with numerous side effects such as disorientation, habituation, and daytime fatigue [Citation11,Citation12]. Thus, there is an unmet need to investigate pharmacological interventions and dietary supplements with fewer side effects and high efficiency to counteract the cognitive deficits caused by SD.
The microbiota-gut-brain axis is a bidirectional communication pathway between the gut, including the microbiota, and the central nervous system. It presents an attractive target for the development of novel solutions related to enhancing cognitive function [Citation13]. Studies using germ-free mice have arguably provided the most compelling evidence for microbial modulation of neurocognitive development and function, since cognitive impairments are observed in germ-free models [Citation14]. Pre-clinical evidence has shown that specific Lactobacillus (sensu lato) and Bifidobacterium species/strains, either alone or in combinations improved cognitive functions in various animal model studies [Citation15–17]. Furthermore, intervention with specific Bifidobacterium strains in healthy mice selectively improved object recognition memory, decreased the number of errors in a spatial memory test, and induced better long-term learning in fear conditioning [Citation18]. Moving from pre-clinical to clinical evidence, Bifidobacterium breve A1 reversed cognitive impairment in a mouse model of Alzheimer’s disease [Citation19], while it was also found to help maintain cognitive function in elderly people with memory complaints [Citation20]. Deficits in cognitive function following SD have also previously been reported in mice and Lactobacillus plantarum MTCC 9510 was reported to be efficacious in alleviating such cognitive disruption [Citation21]. Interestingly, in healthy older adults, better sleep quality seems to associate with improved performance on cognitive tasks and higher proportions of specific gut microbial phyla, suggesting a possible relationship between sleep quality, the gut microbiome, and cognitive flexibility [Citation22]. Another study also established important links between cognition, gut microbial diversity and interleukin-6, a cytokine strongly associated with sleep physiology [Citation23].
Considering the important linkages between the microbiota-gut-brain axis, sleep physiology and cognition, it is reasonable to hypothesise that modulating the gut microbiota composition or influencing the mucosal physiology directly with bacterial intervention has the potential to ameliorate cognitive dysfunction in mice subjected to partial SD. To examine this hypothesis, a paradigm of repeated partial SD amongst three behavioural tests of cognition was established in mice to investigate whether three individual bacterial strains (Lactiplantibacillus plantarum Lp-115 (Lp-115), Lacticaseibacillus paracasei Lpc-37 (Lpc-37), Bifidobacterium animalis subsp. lactis 420 (B420)) and their combination could alleviate recognition memory, spatial working memory, and contextual long-term memory impairments.
Materials and methods
Animals
Five-week-old male Swiss mice weighing 30–35 g were purchased from JANVIER (Saint Berthevin, France). Mice were group housed in a temperature and humidity-controlled animal facility with a 12-hour light/dark cycle (lights off at 7pm). The SD experiment was performed when mice reached the age of 11 weeks. Mice were housed six per cage with access to food (SAFE A04C, SAFE, Route de Saint Bris, 89290 AUGY, France) and water ad libitum and all mice in a cage receiving the same intervention, either vehicle or bacterial single-strain or multi-strain. The study was conducted at Amylgen’s animal facility (Approval #A-34-169-002) in accordance with the recommendations of Directive 2010 /63/UE, European Union commission. The protocol #02441 was approved by the Occitanie-Mediterranée Ethics Committee in January 2015. The procedure used for SD was not approved at the time the study was conducted but was subsequently approved as part of protocol #28733 by the Occitanie-Mediterranée Ethics Committee in July 2021. Routinely, the overall health (hair and eyes) and activity/condition (locomotor activity and posture) of the mice were visually monitored daily. Weight measurements were recorded three times per week.
Experimental design and bacterial interventions
Mice were randomly assigned to six groups (n = 12 per group): two vehicle groups, one not exposed to SD (no SD/Veh), and the other of which was (SD/Veh), and four SD groups supplemented with one of four bacterial interventions; Lp-115 (SD/Lp-115; American Type Culture Collection (ATCC) ATCC SD5209; commercially available as HOWARU Lp-115™), Lpc-37 (SD/Lpc-37; ATCC PTA-4798; commercially available as HOWARU Lpc-37™), B420 (SD/B420; ATCC SD6685; commercially available as HOWARU B420™) or multi-strain (Lpc-37 + Lp-115 + B420; SD/multi-strain). The experimenter administering the interventions and involved in the care of the mice was blind to the experimental intervention groups and a number was assigned to each intervention to maintain the blinded condition. Mice in the bacterial intervention groups were perorally gavaged with either 1 × 109 colony forming units (CFU) of single-strain or 1.5 × 109 CFU of multi-strain (Lpc-37 at 5 × 108 CFU, Lp-115 at 5 × 108 CFU and B420 at 5 × 108 CFU). The treatments were performed every day between 9:00am and 11:00am. Freeze-dried bacterial cultures were produced by Danisco USA Inc. (IFF Health & Biosciences; Madison, WI) and freshly diluted into 100 µl of saline (0.9% NaCl in double-deionized water) daily to achieve the correct doses. Vehicle mice were perorally gavaged with 100 µl daily of saline solution only. A detailed description of the study material can be found in Table S1. Bacterial interventions continued daily for thirty days prior to, and for nine days during a behavioural test paradigm, until the last day of behavioural assessments. On day ten, the day after the last behavioural test, all mice (n = 72) were anesthetised with 4% isoflurane and sacrificed by cervical dislocation. The scheduling of procedures is captured in .
Figure 1. Schematic diagram representing the experimental schedule and timeline of procedures during the thirty-nine days of probiotic intervention. Three individual probiotic strains and their combination were tested in mice subjected to partial SD and compared with non-SD and SD vehicle groups. Mice were administered a daily oral gavage containing either 1 × 109 colony forming units (CFU) of bacterial single-strain, 1.5 × 109 CFU of bacterial combination or vehicle for thirty days prior to and for nine days during a behavioural test paradigm. Behavioural tests were applied to evaluate learning and memory performances following a five-hour SD period before the onset of the activity phase. The behavioural tests used included the novel object recognition test (NOR; days one to three), the Y-maze spontaneous alternation test (Y-maze; days five and six) and the step-through passive avoidance task (STPA; days eight and nine), with two days of rest on days four and seven. All mice were sacrificed on day ten.
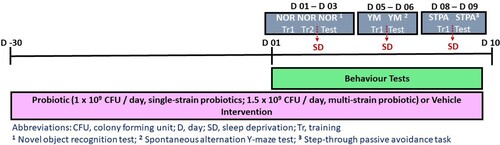
Behavioural test paradigm
Following thirty days of bacterial or vehicle intervention, all mice underwent a behavioural test paradigm to measure cognitive function (learning and memory) following SD (). The novel object recognition (NOR) test was used to measure long-term nonspatial recognition memory [Citation15]; the spontaneous alternation Y-maze (Y-maze) was used to measure spatial working memory of short-term work [Citation24], and the step-through passive avoidance (STPA) task was used to measure contextual long-term memory [Citation24]. A detailed description of the methods for each test can be found in Supplementary Methods.
Behavioural assessments were performed over nine consecutive days: day 1) NOR (habituation), day 2) NOR (same object session), day 3) NOR (novel object session), day 4) rest, day 5) Y-maze (first session), day 6) Y-maze (second session), day 7) rest, day 8) STPA (training session) and day 9) STPA (testing session). On days two, five and eight, immediately after the training sessions of each behavioural test, mice were subjected to SD for five hours before the onset of the activity phase (7pm). In this context, to determine the specific effects of probiotic administration on cognitive impairments in partially sleep-deprived mice, statistical tests were applied to analyse day 2) NOR (same object session) versus day 3) NOR (novel object session); day 5) Y-maze (first session) versus day 6) Y-maze (second session) and day 8) STPA (training session) versus day 9) STPA (testing session).
All behavioural tests were performed in specially equipped rooms within the animal facility. To minimise stress, mice were habituated to the testing room by placing home-cages there for at least 30 min. prior to testing. The same mice were assessed across all behavioural tests. A researcher who was blinded to the group designations remained in the testing room during each behavioural test. In addition, all outputs were measured by an experimenter blinded to the experimental groups.
Sleep deprivation procedure
Partial SD was induced using the ‘gentle handling' method by a fully trained technician who was familiar to the mice prior to the procedure. The ‘gentle handling' method consisted of keeping mice that were randomly allocated to the SD groups awake by gentle manipulations whenever behavioural signs of sleep were observed (i.e. drowsiness or attempts to engage in a sleeping posture) [Citation9]. Mice were sleep-deprived for five h in their home-cages, before the onset of the activity phase (7pm), immediately after the training sessions of each behavioural test on days two, five and eight ().
Statistical analyses
All values, except passive avoidance latencies, are expressed as mean ± standard error of the mean (SEM). All data were first checked for normality using the Shapiro–Wilk test and then analysed using one-way ANOVA followed by Dunnett's multiple comparisons test, if the data were normally distributed. Non-normally distributed data (i.e. passive avoidance latencies) were analysed using the non-parametric Kruskal–Wallis test, followed by Dunn’s test for multiple comparisons. All data were analysed using GraphPad Prism software v.6 (GraphPad Software Inc., La Jolla, CA, USA). A p-value lower than 0.05 was considered statistically significant.
Results
Lpc-37 and multi-strain alleviated partial SD-induced spatial recognition memory deficits in the NOR test
During the habituation phase, none of the bacterial intervention groups were statistically significantly different to the vehicle groups for time spent in the inner (Figure S1A) or outer (Figure S1B) zones of the open field (OF) arena. Furthermore, there was no statistically significant effect of the bacterial interventions on locomotor activity (Figure S1C), locomotion in the centre (inner zone; Figure S1D) or on rearing/grooming behaviour related to stereotypic activity (Figure S1E), compared to the vehicle groups.
During the second day of the NOR test and prior to SD, there was no statistically significant effect of the bacterial interventions on the object interaction time ((A)) with the two identical objects (i.e. preference index of 50% represents identical exploration of both objects).
Figure 2. Effect of probiotic intervention on recognition memory deficits, induced by partial SD. (A) Preference Index (PI) expressed as the time of interaction with object in position two (% of total time of interaction) during the same object session on training day (Day 2). (B) PI for the novel object expressed as interaction time (%) during the novel object session on test day (Day 3). (C) Discrimination index (DI) calculated from the time of interaction with novel object on Day 3. All data were analysed using the one-way ANOVA and Dunnett’s test for multiple comparisons. Data are expressed as mean ± SEM. ***, p < 0.0001 vs. No SD/vehicle group.
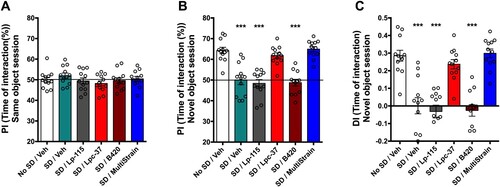
Partial SD, immediately following the acquisition of the memory (familiar objects learned on day two of the NOR test) induced a statistically significant disruption in the tendency to interact with the novel object (i.e. preference index) on day three of the NOR test. While the no SD/Veh group had a high interaction time (64.3% ± 1.55) with the novel object that was statistically significant (p < 0.0001), the SD/Veh group showed no preference between the novel and familiar objects (50.1% ± 2.29) ((B)).
Following intervention with Lpc-37, and despite the session of partial SD, the SD/Lpc-37 group demonstrated a preference for the novel object (61.85% ± 1.31; (B)), without any statistical difference with the no SD/Veh group. Similarly, the SD/multi-strain group restored a preference for the novel object (64.95% ± 1.24; (B)), similar to that observed in the no SD/Veh group. The SD/Lp-115 and SD/B420 groups showed no preference for the novel object (48.41% ± 1.8 and 48.73% ± 1.63, respectively), with a statistically significant difference (p < 0.0001) with the no SD/Veh group ((B)).
The SD/Veh group had a statistically significantly lower discrimination index for time of interaction compared to the no SD/Veh group (p < 0.0001; (C)). The SD/Lpc-37 group (p < 0.0001) and the SD/multi-strain group (p < 0.0001) showed a statistically significantly greater tendency to discriminate between the novel and familiar objects, even after partial SD, reflected in the discrimination index for time of interaction, compared to the SD/Veh group ((C)). Furthermore, the SD/Lpc-37 and SD/multi-strain groups did not statistically significantly differ from the no SD/Veh group ((C)). The SD/Lp-115 and SD/B420 groups showed no effects on the tendency to discriminate between novel and familiar objects in this index compared to the SD/Veh group ((C)).
Lpc-37 and multi-strain alleviate partial SD-induced short-term spatial working memory deficits in the Y-maze
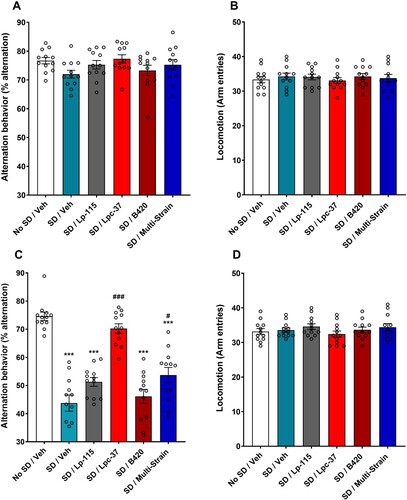
On day five of the behavioural test paradigm, during the first session of the spontaneous alternation Y-maze and prior to SD, there was no statistically significant effect of the bacterial interventions on spontaneous alteration behaviour, compared to the vehicle groups ((A)). Furthermore, there was no statistically significant effect of the bacterial interventions on locomotion or arm entries, compared to the vehicle groups ((B)). Partial SD, prior to the second session of the spontaneous alternation Y-maze induced a statistically significant disruption to spatial working memory in the Y-maze on day six of the behavioural test paradigm. During the second session, the SD/Veh group exhibited a statistically significant reduction in spontaneous alternation behaviour, compared to the no SD/Veh group (p < 0.0001; (C)). Following intervention with Lpc-37, and despite the second session of partial SD, the SD/Lpc-37 group maintained spontaneous alternation behaviour, compared to the SD/Veh group (p < 0.0001; (C)). Furthermore, the SD/Lpc-37 group did not statistically significantly differ from the no SD/Veh group ((C)). A partial maintenance of spontaneous alternation behaviour was observed after intervention with the bacterial multi-strain, compared to the SD/Veh group (p = 0.0104; (C)). The SD/Lp-115 and SD/B420 groups showed reductions in spontaneous alternation behaviour, compared to the SD/Veh group ((C)). There was no statistically significant effect of the bacterial interventions on locomotion or arm entries, compared to the vehicle groups ((D)).
Figure 3. Effect of probiotic intervention on spatial working memory deficits, induced by partial SD. (A and B) Alternation behaviour (%) and locomotion (arm entries) on test day 1 (Day 5), respectively. (C and D) Alternation behaviour (%) and locomotion (arm entries) on test day 2 (Day 6), respectively. (A–D) All data were analysed using the one-way ANOVA and Dunnett’s test for multiple comparisons. n = 12 for all groups. Data are expressed as mean ± SEM. ***p < 0.001 vs. No SD/vehicle group; #p < 0.05 vs. SD/vehicle group; ###p < 0.001 vs. SD/vehicle group (C).
Lpc-37 alleviates partial SD-induced contextual long-term memory deficits in the STPA task
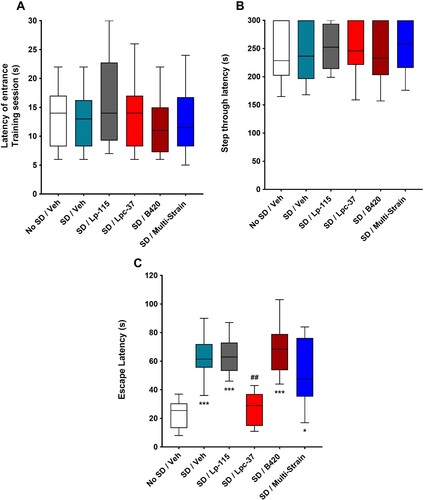
On day eight of the behavioural test paradigm, during the training session of the STPA task and prior to SD, there was no statistically significant effect of the bacterial interventions on step-through/entrance latency time, compared to the vehicle groups ((A)). Partial SD, immediately following the acquisition of the memory (association of a foot shock within the dark compartment), induced a statistically significant disruption to contextual long-term memory in the STPA task on day nine of the behavioural test paradigm, but only reflected in the escape latency measure. During the testing session, step through latency time was similar between the vehicle groups ((B)), and there were no statistically significant effects of the bacterial interventions on the step through latency time, compared to the SD/Veh group ((B)). All mice, whether sleep-deprived or not, resisted entry to the dark chamber and used the maximum allowed time of 300s before being manually moved into the dark compartment. Once inside the dark compartment, the SD/Veh group showed a statistically significant increase in escape latency or were statistically significantly slower to exit the dark compartment, compared to the no SD/Veh group (p = 0.0002; (C)). The SD/Lpc-37 group was the only bacterial intervention group that had a statistically significant reduction in escape latency time or were statistically significantly faster to exit the dark compartment, compared to the SD/Veh group (p = 0.0022; (C)). Furthermore, the SD/Lpc-37 group was the only bacterial intervention group that was not statistically significantly different to the no SD/Veh group for escape latency time, indicating that intervention with Lpc-37 seemingly normalised the behaviour to that of the no SD/Veh group.
Figure 4. Effect of probiotic intervention on contextual long-term memory deficits, induced by partial SD. (A) Latency of entrance (s) on training day (Day 8). (B) Step-through latency (s) on test day (Day 9). (C) Escape latency (s) on test day (Day 9). (A–C) All data were analysed using the Kruskal–Wallis non-parametric ANOVA and Dunn’s test for multiple comparisons. n = 12 for all groups. Data are expressed as mean ± SEM. *p < 0.05 vs. No SD/vehicle group; ***p < 0.001 vs. No SD/vehicle group; ##p < 0.01 vs. SD/vehicle group (C).
Discussion
The microbiota-gut-brain axis has emerged as a relevant pathway which has a significant influence on cognition. Considerable evidence demonstrates links between sleep loss and a decline in cognitive performance and memory deficits, as well as alterations in the gut microbiome [Citation8,Citation22,Citation23]. Intriguingly, probiotic interventions with different Lactobacillus (sensu lato) and/or Bifidobacterium species/strains have been shown to improve memory and cognition in mice and humans [Citation13,Citation17]. Therefore, a link between sleep, cognition, probiotics and communication along the microbiota-gut-brain axis appears to exist.
We previously demonstrated in a pilot in vivo study that partial SD of six h prior to the onset of the activity phase was sufficient to induce cognitive behavioural deficits in mice (unpublished data). The purpose of this current study was to investigate whether intervention with individual bacterial strains or their combination could improve cognitive behavioural measures in partially sleep-deprived mice. Three behavioural tests, the NOR test, the Y-maze and the STPA task, were selected based on their use in numerous studies to investigate the effects of SD on learning and memory behaviours [Citation25–27]. The pre-clinical model of SD selected in this study employed the ‘gentle handling' technique, which has proven successful in previous studies to achieve sleep restriction and disruption of normal cognitive function in rodents [Citation9]. In the present study, a five h SD period before the onset of the activity phase was selected since it has been reported that SD during the dark (activity) phase does not cause memory deficits [Citation28]. The findings presented in this study suggest that intervention with Lpc-37 and with the combination of Lpc-37, Lp-115 and B420 were effective towards improving cognitive behavioural measures in partially SD mice. Lpc-37 was the most efficacious strain compared with the bacterial combination for improving recognition memory, spatial working memory, and contextual long-term memory impairments in mice that had their sleep partially disrupted. In addition, the bacterial combination significantly improved recognition memory and spatial working memory deficits in partially sleep-deprived mice.
The NOR test is a commonly used behavioural assay for investigating the neurobiology of non-spatial hippocampal-dependent memory in rodents [Citation29]. In the present study, partial SD immediately following the acquisition of the memory of the familiar object from the training session led to significant neurocognitive deficits in the recognition memory ability of mice, demonstrated through an inability of sleep-deprived mice to discriminate between the novel and familiar objects during the testing session. Intervention with Lpc-37 and the multi-strain prevented this deficit to hippocampal dependent non-spatial recognition memory. Since increased exploration of a novel object over a familiar object has been associated with better recognition, and thus improved memory, our data indicate that Lpc-37 and the multi-strain may have improved memory processes. This result is in agreement with a study conducted by Stenman et al. which showed that Lpc-37 prevented stress-associated deficits in recognition memory from developing in the same NOR test in a mouse model of chronic restraint stress [Citation15]. Similarly, another study demonstrated the ability of a probiotic combination of Lacticaseibacillus rhamnosus R0011 and Lactobacillus helveticus R0052 to improve memory processes impaired by stress and infection in the NOR test [Citation30], in addition to other animal model studies highlighting an improvement in memory of objects and object location following probiotic intervention, reviewed in [Citation17]. In humans, multiple aspects of memory performance and cognition were improved in a cohort of stressed but otherwise healthy adults following a 12-week intervention with L. plantarum DR7 [Citation31].
Impaired spontaneous alternation behaviour in the Y-maze following partial SD was demonstrated through an inability of mice to remember the previously visited arms of the Y-maze. Intervention with Lpc-37 and with the multi-strain alleviated this deficit to short-term spatial working memory. This finding suggests an improvement in hippocampal-prefrontal dynamics related to spatial working memory performances (i.e. working memory for location), thus broadening the effects on Lpc-37 and the multi-strain on cognitive function within different brain regions. These findings are in agreement with other in vivo studies reporting the ability of Lactobacillus (sensu lato) strains to ameliorate spatial memory impairments [Citation17].
In the hippocampal dependent STPA task, the aim was to assess the effects of the single strains and/or multi-strain on partial SD-induced contextual long-term memory impairments. A disruption of passive avoidance memory following SD is well documented in a number of pre-clinical studies, for example [Citation32]. The results of the STPA task revealed that partial SD had no impact on step-through latency and did not disrupt the memory of the association between moving to the dark chamber and receiving a foot shock. However, once inside the dark chamber, mice in the Lpc-37 group showed a significantly improved memory consolidation in the STPA task by reducing escape latency time from the dark compartment. Therefore, once inside the dark chamber, mice in the Lpc-37 group recalled the memory of the foot shock faster than those in the SD/Veh group. Similarly, L. plantarum MTCC 9510 seemed to protect against SD-induced cognitive behaviour deficits in the passive avoidance test in mice [Citation21].
To date, the exact mechanisms by which probiotics may influence behaviour and cognitive function are not well-established. Probiotics elicit differential species- and strain-dependent effects on the microbiota composition [Citation15], and thus likely confer differing effects on the immune system, vagus nerve and on the synthesis and metabolism of metabolites and neurotransmitters. Taken together, the mechanisms of probiotics appear to be very complex, thus warranting deeper mechanistic studies to fully determine the effect of specific probiotics on brain physiology, function and behaviour.
Finally, since stress and anxiety are known to impair cognition and as insufficient sleep may result in an increased risk of anxiety and stress, it is reasonable to hypothesise in this study that the improvements in memory observed could be related to the hypothalamic pituitary adrenal (HPA) axis response to stress following intervention with Lpc-37. Indeed, probiotics have been shown to influence the HPA axis response to stress [Citation33,Citation34] and in this context, we previously demonstrated that Lpc-37 prevented stress-related behaviours from developing in chronically stressed mice, concomitantly improving cognition [Citation15]. A subsequent clinical study was conducted to further investigate the clinical stress-related effects of probiotics and confirmed Lpc-37 as a safe and effective probiotic to reduce perceived stress in the healthy adults, compared to placebo [Citation35]. Furthermore, in support of this hypothesis, Dhaliwal et al. showed that serum corticosterone concentration of mice subjected to SD was approximately four-fold higher than the control group, whereas L. plantarum MTCC 9510 supplementation improved spatial and working memory deficits, and attenuated the stress-induced levels of corticosterone [Citation21]. Thus, it would be interesting to test this hypothesis in future mechanistic studies and to determine whether the effects of Lpc-37 and multi-strain on cognitive behaviour may be related to changes in the amygdala, hippocampus and/or prefrontal cortex brain regions involved in the stress response or the HPA axis response to stress. Further to this, the hippocampus, a region critical for learning and memory, is particularly sensitive to SD, and its impairment has been reported to result in abnormalities in gastrointestinal motility, a key regulator of microbiota composition [Citation36]. It would also be highly interesting to investigate alterations in gut motility during acute SD, and correlate to potential alterations in both hippocampal function and gut microbiota composition.
The results of the present study indicate that specific species and strains may be superior to others for ameliorating cognitive impairments in partially sleep-deprived mice. While the doses of the individual strains were lower in the multi-strain, the combination overall had a significant impact on some behavioural outcomes. However, the effects of Lpc-37 more often improved learning and memory outcomes. In some cases, intervention with Lpc-37 normalised the behavioural profile associated with memory consolidation to the levels that were comparable to the no SD/vehicle group. It could also be hypothesised that the reduced efficacy of the multi-strain combination may have been due to antagonistic intra-strain inhibition by different probiotic strains [Citation37].
The pre-clinical model used in this study indicates that a paradigm of repetitive partial SD is a suitable model for inducing specific cognitive deficits in mice. Lpc-37 significantly improved recognition memory deficits, spatial working memory deficits, and contextual long-term memory impairments in mice subjected to partial SD. The multi-strain significantly improved recognition memory deficits and spatial working memory deficits in mice subjected to partial SD. Altogether, these novel findings add to the increasing body of data indicating that probiotic consumption could have positive effects on cognition, placing Lpc-37 and the multi-strain as novel candidates for clinical studies on counteracting the cognitive effects of insufficient sleep. Future studies are required to elucidate the mechanisms responsible for improvements in the behavioural phenotypes associated with insufficient sleep. Furthermore, the pre-clinical model used in this study highlighted in strain level differences on cognitive outcomes and thus seems well applicable for screening candidate probiotics for translation to human clinical trials.
Supplemental Material
Download Zip (550.7 KB)Acknowledgements
We wish to acknowledge Rita Stiemke from IFF Health & Biosciences for the production and quality control of the freeze-dried bacterial cultures.
Disclosure statement
SMG, MJL, and EP are, or were, employees of IFF Health & Biosciences that research and manufacture probiotics.
Data availability statement
Data are available upon request from the corresponding author.
Additional information
Funding
Notes on contributors
Síle M. Griffin
Síle Griffin completed her BSc in Neuroscience at University College Cork, Ireland in 2009, MSc in Stem Cells and Regeneration from the University of Bristol, United Kingdom in 2010, and PhD in Neuroscience from Keele University, United Kingdom in 2015. Her doctoral research focused on stem cell therapies for neurodegenerative diseases, in particular the role of vitamins in brain development and repair. Following her academic studies, Síle worked at Cambridge Clinical Trials Unit, prior to joining DuPont Nutrition and Biosciences, Finland in 2018. Here, she worked as a Scientist on the Brain Health Platform, conducting preclinical and clinical trials to establish effective nutritional therapies for cognitive health.
Markus J. Lehtinen
Markus Lehtinen holds BEng in Bio and Food Technology, MSc in Biochemistry, and PhD in Medicine from the University of Helsinki, where he worked on innate immune function for 10 years. In 2011, he started at IFF Health & Biosciences, Finland, and currently works there as a Principal Scientist leading pre-clinical and clinical research on probiotics and microbiome modulators on immune function.
Johann Meunier
Johann Meunier obtained a PhD in Neuroscience at the University of Montpellier, France in 2007. He then worked at NIDA, NIH, Baltimore, USA as a postdoctoral researcher prior to joining Amylgen SAS, France as a scientist in Neurobiology.
Laura Ceolin
Laura Ceolin obtained her PhD in Neuroscience at the University of Bristol, United Kingdom in 2009. After that she worked as a postdoctoral researcher at the University of Bristol for 2 years and at the Institute of Functional Genomics (IGF), CNRS, in Montpellier for 6 years investigating the physiology, the pharmacology and the molecular mechanisms of psychiatric and neurological diseases. Since 2017, she has joined Amylgen company as a project manager to bring scientific support and to be responsible of coordination of the studies.
Francois J. Roman
François J. Roman founded FRconsulting in October 2007. As a consultant, he has been collaborating with several biotech companies and universities in France and Belgium. In October 2009, he cofounded Amylgen and in 2016, SigmaThera aiming to repurpose a compound for the treatment of neurodegenerative diseases. Previously, François J. Roman had several executive positions at Euroscreen, Pfizer, Parke-Davis, Jouveinal Laboratoires, and Laboratoires Servier. For more than 30 years, F.J. Roman has been involved in many Drug Discovery programs in a broad range of therapeutic areas. F.J. Roman holds a PhD in Biochemistry and has more than 40 publications and 35 patents.
Elaine Patterson
Elaine Patterson holds a BSc honors degree in Neuroscience and a PhD in Microbiology from University College Cork, Ireland. During her post-doctoral research at APC Microbiome Ireland, Elaine combined her knowledge and technical expertise in Neuroscience with that of Microbiology and began investigating the microbiota-gut-brain axis. Between 2017 and 2021, Elaine worked as a Senior Scientist and Technical Lead with IFF (formerly DuPont Nutrition & Biosciences), and led a platform of research and innovation projects investigating how probiotics and dietary ingredients may affect brain physiology, function and behaviour. Throughout her career, Elaine has led and contributed towards several publications in the fields of microbiome, probiotics and health.
References
- Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare. 2019;7(1):1.
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation’s updated sleep duration recommendations. Sleep Health. 2015;1(4):233–43.
- Owen JE, Veasey S. Impact of sleep disturbances on neurodegeneration: insight from studies in animal models. Neurobiol Dis. 2020;139:104820.
- Magnavita N, Garbarino S. Sleep, health and wellness at work: a scoping review. Int J Environ Res Public Health. 2017;14(11):1347.
- Simon EB, Walker MP. Sleep loss causes social withdrawal and loneliness. Nat Commun. 2018;9(1):1–9.
- Geoffroy PA, Tebeka S, Blanco C, Dubertret C, Le Strat Y. Shorter and longer durations of sleep are associated with an increased twelve-month prevalence of psychiatric and substance use disorders: findings from a nationally representative survey of US adults (NESARC-III). J Psychiatr Res. 2020;124:34–41.
- Toth LA, Bhargava P. Animal models of sleep disorders. Comp Med. 2013;63(2):91–104.
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404.
- Colavito V, Fabene PF, Zucconi G, Pifferi F, Lamberty Y, Bentivoglio M. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front Syst Neurosci. 2013;7:106.
- Wesensten NJ, Killgore WD, Balkin TJ. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res. 2005;14(3):255–66.
- Sheng P, Hou L, Wang X, Wang X, Huang C, Yu M, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. Public Libr Sci One. 2013;8(12):e81802.
- Snel J, Lorist MM. Effects of caffeine on sleep and cognition. Prog Brain Res. 2011;190:105–17.
- Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF, et al. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477–502.
- Luczynski P, McVey Neufeld K-A, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsuchopharmacolog. 2016;19(8):1–17.
- Stenman LK, Patterson E, Meunier J, Roman FJ, Lehtinen MJ. Strain specific stress-modulating effects of candidate probiotics: a systematic screening in a mouse model of chronic restraint stress. Behav Brain Res. 2020;379:112376.
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, et al. Administration of lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–77.
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013.
- Savignac H, Kiely B, Dinan T, Cryan J. B ifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26(11):1615–27.
- Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep. 2017;7(1):1–10.
- Kobayashi Y, Kuhara T, Oki M, Xiao J-Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2019;10(5):511–20.
- Dhaliwal J, Singh DP, Singh S, Pinnaka AK, Boparai RK, Bishnoi M, et al. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J Appl Microbiol. 2018;125(1):257–69.
- Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017;38:104–7.
- Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, et al. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. 2019;14(10):e0222394.
- Blivet G, Roman F, Meunier J, Ceolin L, Butterlin J-M, Touchon J. Neuroprotection afforded by a preventive CogniXtra treatment against amyloid beta Aβ25-35 peptide-induced toxicity in mice. Int J Clin Nutr Diet. 2019;5:139.
- Rahman H, Muralidharan P, Sivaraman D, Saha D. Continuous sleep deprivation for 5 days produces loss of memory in mice and may be a cause of Alzheimer’s disease. Ann Biol Res. 2010;1(4):185–93.
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee S-Y, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–9.
- Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85(3):263–71.
- Palchykova S, Winsky-Sommerer R, Tobler I. Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol Int. 2009;26(4):682–96.
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110.
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17.
- Chong H, Yusoff NAA, Hor Y-Y, Lew L-C, Jaafar MH, Choi S-B, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. 2019;10(4):355–73.
- Wang JH, van den Buuse M, Tian SW, Ma YY. Effect of paradoxical sleep deprivation and stress on passive avoidance behavior. Physiol Behav. 2003;79(4–5):591–6.
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–74.
- Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, Sanz Y, et al. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56.
- Patterson E, Griffin SM, Ibarra A, Ellsiepen E, Hellhammer J. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol Stress. 2020;13:100277.
- El Aidy S, Bolsius YG, Raven F, Havekes R. A brief period of sleep deprivation leads to subtle changes in mouse gut microbiota. J Sleep Res. 2019;29:e12920.
- McFarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med. 2018;5:124.
