ABSTRACT
Background and objective
We recently showed that perinatal exposure to diets with unbalanced n-6:n-3 polyunsaturated fatty acid (PUFA) ratios affects the olfactory mucosa (OM) fatty acid composition. To assess the repercussions of these modifications, we investigated the impact of diets unbalanced in n-3 PUFAs on the molecular composition and functionality of the OM in young mice.
Methods
After mating, female mice were fed diets either deficient in α-linolenic acid (LOW diet) or supplemented with n-3 long-chain PUFAs (HIGH diet) during the perinatal period. Weaned male offspring were then fed ad libitum with the same experimental diets for 5 weeks. At 8 weeks of age, olfactory behavior tests were performed in young mice. The fatty acid composition of OM and olfactory cilia, as well as the expression of genes involved in different cellular pathways, were analyzed. The electroolfactograms induced by odorant stimuli were recorded to assess the impact of diets on OM functionality.
Results and conclusion
Both diets significantly modified the fatty acid profiles of OM and olfactory cilia in young mice. They also induced changes in the expression of genes involved in olfactory signaling and in olfactory neuron maturation. The electroolfactogram amplitudes were reduced in mice fed the LOW diet. Nevertheless, the LOW diet and the HIGH diet did not affect mouse olfactory behavior. Our study demonstrated that consumption of diets deficient in or supplemented with n-3 PUFAs during the perinatal and postweaning periods caused significant changes in young mouse OM. However, these modifications did not impair their olfactory capacities.
Introduction
Olfaction is a major sensory modality that provides information about the volatile chemical compounds (odorants) emitted in our environment. This sense plays a vital role in the quest for food and dietary intake, avoidance of environmental hazards, and social communication [Citation1]. There is also growing evidence that odor perception contributes to achieving and maintaining human well-being [Citation2].
Olfactory signals are induced when odorants reach the olfactory mucosa (OM) located in the nasal cavity. The OM contains several cell types, including olfactory sensory neurons (OSNs), that regenerate throughout life [Citation3]. On the apical side of the OM, OSN dendrites end with 5–20 cilia floating in the mucus. Olfactory receptors (ORs) and other signal transduction components (including olfactory G-protein subunit Gαolf, adenylate cyclase III (ACIII) and ionic transport channels) are located in these cilia. The interaction of odorants with ORs triggers the transduction cascade that converts chemical stimuli into electrical signals. The generated action potentials are then conveyed along the axons toward the olfactory bulbs where olfactory information is encoded before being carried out to the primary olfactory cortex to be further processed [Citation4,Citation5].
Endogenous and exogenous factors contribute to olfactory plasticity. In particular, evidence has shown that nutritional and metabolic status play a key role in modulating olfactory perception [Citation6]. Recent studies have shown that diets with high lipid levels can disturb rodent olfactory abilities [Citation7–9]. Whether these effects result from quantitative (i.e. an excessive amount of dietary lipids) or from qualitative (i.e. an imbalance in fatty acid (FA) composition) variations remains unknown.
Similar to the brain and retina, the rodent OM and olfactory bulbs contain high levels of polyunsaturated fatty acids (PUFAs) (up to 39% of the total FAs), mainly found in phospholipids (PLs) [Citation10,Citation11]. They are particularly rich in docosahexaenoic acid (DHA; 22:6 n-3) and arachidonic acid (AA; 20:4n-6). DHA and AA are derived from α-linolenic acid (ALA; 18:3n-3) and linoleic acid (LA; 18:2n-6), respectively, that cannot be produced by mammalian tissues and need be supplied by the diet. PUFAs are crucial for the development and functioning of mammalian neural tissues. They are involved in neurogenesis, neuronal survival, synaptic transmission, membrane biophysical properties and neuroinflammation [Citation12].
The composition of Western diets has changed dramatically over the last decades. In particular, they contain high amounts of n-6 PUFAs and small amounts of n-3 PUFAs, resulting in an n-6:n-3 ratio greater than 20:1, which contrasts sharply with the recommended ratio of 4:1 [Citation13]. The consumption of diets with a high n-6:n-3 ratio leads to reduced DHA and increased AA in many tissues. In neural tissues such as the brain and retina, these modifications can result in reduced cognitive ability, increased emotional behavior and impaired vision [Citation14,Citation15] However, it has been largely unexplored whether unbalanced PUFA diets modify the biochemical composition and functionality of olfactory tissues. A few studies performed on rats have shown that consumption of diets deficient in n-3 PUFAs for two generations significantly increased the docosapentaenoic acid (DPA n-6; 22:5n-6) level in the olfactory bulbs and impaired their olfactory discrimination capacities [Citation11,Citation16]. In a recent study, we showed that the PL molecular species profiles of OM in weaning mice were significantly modified when the maternal diets were unbalanced in n-3 PUFAs [Citation17]. The repercussions of these modifications on the properties of the peripheral olfactory system remain to be evaluated.
The present study was therefore designed to explore the effects of perinatal and postweaning diets depleted in ALA or supplemented with n-3 long-chain PUFAs on the molecular composition and functionality of OM in 8-week-old offspring. To achieve this goal, several specific targets were studied: (i) the FA composition of OM and olfactory cilia; ii) the biophysical properties of olfactory cilia; iii) the mRNA expression of genes known to play important roles in neurogenesis, olfactory signal transduction, lipid metabolism and energy balance; iv) the OM electrical potentials in response to olfactory stimuli. In addition, the olfactory capacities (detection threshold and discrimination ability) of mice were assessed.
Materials and methods
Animals and diets
The experimental procedure was conducted in conformity with the guidelines of the European Community for the use and care of laboratory animals (2010/63/EU). It was approved by the French Ministry for Research and Higher Education and the local Ethics Committee (Comité d’Ethique de l’Expérimentation Animale Grand Campus Dijon; reference APAFIS#23807-2020021909594039).
The diet composition and the experimental procedure were detailed in Khoury et al. [Citation17]. Briefly, the experimental diets were manufactured by the Experimental Foods Preparation Unit (INRAE, Jouy-en-Josas, France). They were based on an AIN-93G diet formulation with 5% lipids. Three lipid blends containing various proportions of commercial high-oleic sunflower oil, sunflower oil, palm oil, rapeseed oil and fish oil were incorporated into the different diets. Compared to the control (CON) diet, the LOW diet contained a lower level of ALA, whereas the HIGH diet contained n-3 long-chain PUFAs (eicosapentaenoic acid (20:5n-3), docosapentaenoic acid (22:5n-3) and DHA) ().
Table 1. Fatty acid composition of the experimental diets (% of total fatty acids). CON: control diet; LOW: low n-3 diet; HIGH: high n-3 diet. ALA: α-linolenic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; LA: linoleic acid; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids; SFAs: saturated fatty acids.
After mating, 12-week-old nulliparous female C57BL/6 mice were fed ad libitum with one of the three experimental diets until pup weaning. Male offspring were then fed ad libitum with the same experimental diet as their dams for 5 weeks. At 8 weeks of age, mice were sacrificed by decapitation after anesthesia by i.p. injection of ketamine and xylazine (150 and 10 mg/kg bodyweight, respectively). After dissecting the nose, the OM was collected and immediately snap-frozen in liquid nitrogen. The OM was stored at − 80 °C until further processing.
Fatty acid extraction and analysis
The total lipids from OM were extracted according to the Folch method [Citation18]. The procedure was detailed in our previous study [Citation17]. After transmethylation using boron trifluoride in methanol, FA methyl esters were extracted with hexane and analyzed by gas chromatography coupled to flame ionization detection as previously described [Citation10]. The results were expressed as percentages of total FAs.
Olfactory cilia preparation
Olfactory cilia isolation was performed using the Na+ and Ca2+ shock method as described by Kuhlmann et al. [Citation19,Citation20]. Briefly, OMs from 6 mice were pooled and washed with an ice-cold solution containing 20 mM HEPES, 140 mM NaCl, 3 mM KCl, 2 mM MgSO4, 7.5 mM glucose and 5 mM EGTA (solution A; pH 7.4). They were then resuspended in solution A supplemented with 20 mM CaCl2 and 30 mM KCl. Cilia were detached by gentle stirring for 20 min at 4 °C and isolated by centrifugation for 5 min (6,600 g, 4 °C). The supernatant was collected, and the deciliation procedure was repeated five times. The supernatants were pooled and centrifuged at 45,000 g for 30 min at 4 °C. The cilia were resuspended in 500 µl of Tris-EDTA buffer and used immediately or stored at −80 °C until further analysis.
Measurement of cilia membrane fluidity
Cilia membrane fluidity was assessed by measuring fluorescence anisotropy using 1-(4-(trimethylamine) phenyl)-6-phenylhexa-1.3.5-triene (TMA-DPH) as a probe [Citation21]. Briefly, a pool of freshly prepared cilia (approximately 150 µg protein) was introduced in a 1 cm path length spectroscopic quartz cuvette placed in a stirred and thermostatically controlled (37 °C) chamber of a Fluorolog-3 spectrofluorimeter with a T-configuration (HORIBA Jobin–Yvon, Longjumeau, France). Excitation and emission wavelengths were set at 360 and 430 nm, respectively. After background noise recording, 1 µL of 1 mM TMA-DPH was added to the cuvette, and the fluorescent anisotropy (r) was measured. The r value is defined as the intensity of the linearly polarized component divided by the total light intensity [Citation19]. Fluorescence anisotropy is inversely proportional to membrane fluidity: the higher the anisotropy (r) is, the more rigid the membrane will be.
RNA extraction and qPCR
Total RNA extraction was conducted according to the manufacturer’s instructions (Nucleospin RNA plus kit, Macherey-Nagel). One microgram of total RNA was reverse transcribed with PrimeScript™ reverse transcriptase (PrimeScript™ RT reagent Kit, Takara). For quantitative PCR, 1 μL (50 ng) of cDNA template was added to the 9 μL reaction mixture containing SYBR Green Master Mix (Life Technologies) and 250 nM primers (Eurogentec; see Supplementary Table for primer sequences). qPCR was performed using a StepOne thermocycler (Thermo Fisher Scientific, Applied Biosystems) for 40 amplification cycles consisting of 3 s at 95 °C and 30 s at 60 °C. Relative quantification was achieved according to the 2-ΔΔCt method with HPRT and GAPDH as reference genes. The results are presented as the relative quantity (RQ) compared to the CON group.
Electroolfactogram (EOG) recordings
EOG recordings were performed on the OM as previously described [Citation8,Citation22]. Briefly, after sacrifice, the head was cut longitudinally, and the nasal septum was removed to expose the OM endoturbinates. A recording electrode inserted in a glass micropipette filled with a saline solution was positioned in the middle of endoturbinate III. Odorant stimulations were performed by blowing air puffs through an exchangeable Pasteur pipette enclosed in a plastic tube positioned 2 cm from the epithelial surface. A filter paper impregnated with 10 μL of an odorant solution was placed in the Pasteur pipette. Phenylethyl alcohol (PEA) and acetophenone (ACE) were used as odorants. EOG voltage signals were recorded using an Axoclamp amplifier (Axon Instruments, Molecular Devices, San Jose, USA) and digitized at a rate of 1 kHz using a Digidata 1440 (Axon Instruments). Data were analyzed using MATLAB routines to measure the peak amplitude and kinetics of the response to odorants. Dose–response curves were fitted with the Hill equation model using Prism software (GraphPad, San Diego, USA).
Behavioral experiments
Odor-induced sniffing behavior
A plethysmograph chamber (Emka Technologies, Paris, France) was used to record mouse respiratory activity during odor stimulation as previously described [Citation8]. Briefly, pressure changes induced by mouse breathing were measured by a differential pressure sensor. The respiratory signal collected from the plethysmograph was interfaced to a computer equipped with an AxoScope (Axon Instruments; sampling rate = 1 kHz). The day before the test, the mice were habituated to the plethysmograph chamber and to stimulation with distilled water. On the test day, mice were allowed to acclimate to the experimental device before starting the test. After recording breathing activity without odorant stimulation (baseline), increasing concentrations of PEA (10−9 M to 10−7 M) were applied consecutively for 30 s. The recordings were analyzed using MATLAB routines. Inspiration and expiration phases were extracted to calculate respiratory frequency and inspiration amplitude. A z-normalization was applied to integrate these parameters as a z-sniffing index [Citation23].
Olfactory habituation and dishabituation test
We used the protocol of Yang and Crawley to assess the olfactory discrimination capacities of mice [Citation24]. Briefly, the day before the test, mice were placed in individual cages for 30 min. On the test day, sequential presentations of mineral oil, PEA and ACE were performed under red light. Odorants were diluted in mineral oil to an approximate gas phase partial pressure of 1 Pa [Citation25]. They were deposited on filter paper placed in a cassette fixed to the lid of the cage. Each odorant was presented during three consecutive trials for two minutes. Each trial was separated by a 1-min interval. The sniffing duration of each visit was recorded.
Statistical analyses
Statistical tests were performed with Prism software (GraphPad, San Diego, USA). FA, anisotropy and gene expression data were analyzed using the nonparametric Mann–Whitney U test. A p value <0.05 was considered statistically significant. EOG recordings and sniffing behavior data were analyzed using two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test. Data obtained in the habituation and dishabituation tests were analyzed using two-way repeated-measures ANOVA followed by Fischer’s LSD post hoc test.
Results
Effects of diet on FA composition of olfactory mucosa and olfactory cilia
Compared to the CON group, the level of total dimethyl acetals (DMAs) in offspring OM was significantly lower (- 6%) in the HIGH group (). The levels of total saturated FAs (SFAs), monounsaturated FAs (MUFAs) and PUFAs were not affected by the LOW and HIGH diets. Nevertheless, both diets significantly modified the proportions of n-6 and n-3 PUFAs in offspring OM. Compared to the CON group, the n-6:n-3 ratio was significantly enhanced (x 3.8) in the LOW group. This effect was due to a substantial increase in n-6 PUFAs (+ 50%) and an important reduction in n-3 PUFAs (- 60%) compared to the CON group (). The OM of the LOW group contained significantly higher levels of AA (+ 35%), 22:4n-6 (+ 94%) and DPA n-6 (x 11) than the OM of the CON group. Conversely, the levels of eicosapentaenoic acid (EPA; 20:5n-3), DPA n-3 and DHA were strongly reduced (−82%, −73% and −57%, respectively). Opposite effects were noticed in OM of the HIGH group. Compared to the CON group, the level of total n-3 PUFAs was increased by approximately +10%, whereas the level of total n-6 PUFAs was decreased by −20%, which led to a significant reduction in the n-6:n-3 ratio (- 30%; ). OM from the HIGH offspring had significantly higher EPA and DPA n-3 levels than the CON group (x2.6 and x1.7, respectively). In contrast, the levels of AA, 22:4n-6 and DPA n-6 were greatly reduced (−27%, −44% and −72%, respectively).
Table 2. Fatty acid composition of the olfactory mucosa and olfactory cilia (% of total fatty acids). Cilia from 6 mice were pooled into one sample. Data are expressed as the mean ± s.e.m. (for the OM, n = 5 mice/group; for the cilia, n = 3 samples in the CON and LOW groups and n = 2 samples in the HIGH group). * Values are significantly different from the CON group (Mann–Whitney U test, p < 0.05). # Sum of the percent unsaturated fatty acids multiplied by their number of double bonds. SFAs: saturated fatty acids; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids; DMAs: dimethyl acetals. n.d.: not detected.
Because OSN cilia are essential for odorant detection and signal transduction, we more specifically analyzed the FA composition of this cellular compartment. First, the data revealed that the FA profile of olfactory cilia differed noticeably from that of OM (). Compared to the OM of the CON group, the olfactory cilia of the CON group contained higher levels of SFAs (x1.5) and MUFAs (x1.3) and lower levels of PUFAs (−70%) and DMAs (−49%). The n-6 and n-3 PUFAs accounted for 6.54% and 3.44% of the total FAs in the cilia, respectively.
When comparing the cilia FA composition of the LOW and HIGH groups to that of the CON group, the data showed that the HIGH diet provoked a significant decrease in n-6 PUFAs (−16%) in cilia (). This diet also tended to increase the EPA and DPA n-3 levels (x1.3 and x1.5, respectively) and to reduce DHA levels. Regarding cilia from the LOW group, a nonsignificant increase in the n-6 PUFA level (x1.8) and a nonsignificant decrease in the n-3 PUFA level (−27%) were noted. Compared to the CON group, the unsaturation index was enhanced in the LOW group (+25%).
Influence of diet on membrane fluidity of olfactory cilia
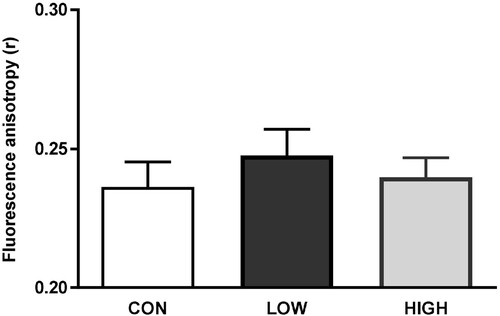
The impacts of diets unbalanced in n-3 PUFAs on the biophysical properties of olfactory cilia membranes were evaluated by fluorescence anisotropy using the TMA-DPH probe. Compared to the CON group, a slight increase in fluorescence anisotropy value (i.e. increase in membrane rigidity) was detected in the LOW group but was not significant ().
Figure 1. Assessment of cilia membrane fluidity by fluorescence anisotropy (r) using TMA-DPH as a probe. Cilia from 6 mice were pooled into one sample. Data are expressed as the mean ± s.e.m. (CON, n = 4 samples; LOW, n = 2 samples; HIGH, n = 3 samples). No significant difference was observed between the CON group and the LOW or HIGH groups (Mann–Whitney U test, p < 0.05).
Effects of diet on gene expression in the olfactory mucosa
Compared to the CON diet, the LOW diet significantly reduced the mRNA expression of RTP2 (- 34%), a protein involved in the cell surface expression of ORs, and of PDE1C (−11%), an enzyme involved in the termination of olfactory signaling, in offspring OM (A and C). mRNA expression of OMP, a marker of mature OSNs, was also reduced (−24%) in the LOW group, but no modification of mRNA expression of GAP43, a specific marker of immature OSNs, was detected (D).
Figure 2. Gene expression in offspring olfactory mucosa. mRNA levels of genes involved in olfactory signaling pathways (A, B and C), neurogenesis (D), cellular transport (E), inflammation and resolution (F) and lipid metabolism (G) were assessed. The results are expressed as the relative quantity (RQ) compared to the CON group. Data are presented as the mean ± s.e.m. (CON, n = 8 mice; LOW, n = 8 mice and HIGH, n = 7 mice). * Values are significantly different from the CON group (Mann–Whitney U test, p<0.05).
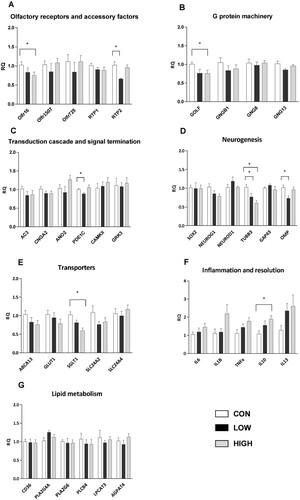
The HIGH diet significantly decreased the mRNA expression of the olfactory receptor olfr16 (−25%), the protein G subunit Gαolf (−25%) and SGLT1 (−41%), a sodium-dependent glucose transporter, in offspring OM (A, B and E). Interestingly, mRNA expression of the anti-inflammatory cytokine IL10 was substantially enhanced in the HIGH group (+74%) (F).
Notably, the mRNA expression of Tubb3, a marker of neuronal differentiation, was significantly reduced in OM from animals fed the LOW and HIGH diets (−23% and −40%, respectively) (D). Both diets had no impact on the mRNA expression of enzymes involved in lipid metabolism (G).
Effects of diet on olfactory mucosa functionality
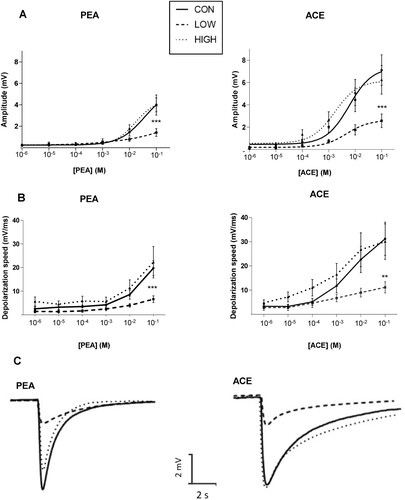
EOG recordings showed that, compared to the CON group, the amplitudes of the responses elicited by the highest concentration of PEA and ACE (10−1 M) were significantly lower in the LOW group (approximately −60%) (A). The amplitudes of EOG in the HIGH group did not differ from those recorded in the CON group. In addition, the depolarizing speeds of EOG responses elicited by the high concentrations of PEA and ACE were significantly lower in the LOW group than those in the CON group (−67% and −64%, respectively) (B). Typical EOG traces obtained after stimulation of OM by PEA or ACE (10−1 M) are shown in C.
Figure 3. Analysis of electrophysiological responses of olfactory mucosa to odorants using EOG recordings on endoturbinate III. (A) Amplitude of EOG responses after stimulation by increasing concentrations of phenylethyl alcohol (PEA) and acetophenone (ACE); (B) Depolarization speed of EOG responses after stimulation by increasing concentrations of PEA and ACE; (C) Representative EOG traces recorded on OM of the CON, LOW and HIGH groups after stimulations by PEA or ACE (10−1 M). Data are expressed as the mean ± s.e.m. (CON, n = 13 mice; LOW, n = 6 mice; HIGH, n = 9 mice). ** p<0.01 and *** p<0.005 after a two-way repeated-measures ANOVA followed by Bonferroni post hoc test.
Effects of diet on mouse olfactory abilities
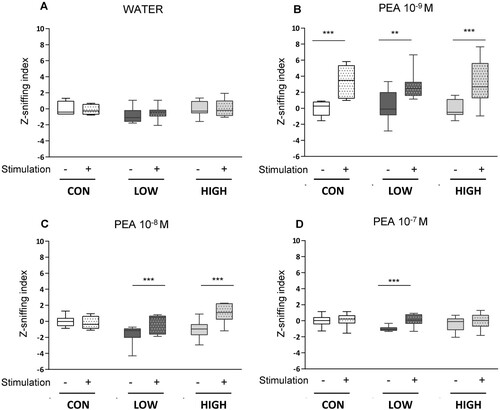
The odor detection behavior of the mice was monitored using a plethysmograph. Basal breathing frequency and amplitude were similar in all groups when mice were tested with water alone (A). When mice were stimulated with an odorant solution containing 10−9 M PEA, the Z sniffing indexes increased significantly in the CON, LOW and HIGH groups, indicating that the three groups detected odorants (B). When a higher concentration of PEA was used (10−8 M), mice fed the LOW and HIGH diets exhibited a significant increase in the Z index (C), while mice fed the CON diet did not respond. The third stimulation with 10−7 M PEA elicited a response only in the LOW group (D).
Figure 4. Odor induced sniffing behavior in response to phenylethyl alcohol (PEA) stimulation. Water (A) followed by increasing concentrations of PEA (10−9 M (B), 10−8 M (C) and 10−7 M (D)) were applied consecutively for 30 s. The respiratory frequency and inspiration amplitude were measured and integrated as a z-sniffing index. Boxes represent data included between quartile 1 and quartile 3, separated by the median; whiskers show minimum and maximum values (CON, n = 6 mice, LOW, n = 8 mice, HIGH, n = 9 mice). ** p<0.01 and ***p<0.001 after a two-way repeated-measures ANOVA followed by Bonferroni post hoc test.
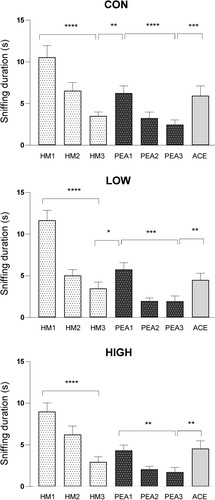
Olfactory discrimination capacities of mice were assessed using a habituation/dishabituation test (). Mice from the CON, LOW and HIGH groups showed no deficit in odor discrimination abilities during the different stages of this behavioral test. The significant main effects for the trials (F6.36 = 54.34, p <0.0001) were detected for time spent sniffing odorants. Post hoc analysis showed that CON, LOW and HIGH mice were able to discriminate the presence of ACE.
Figure 5. Assessment of olfactory discrimination capacities of mice using a habituation/dishabituation test. Animals were sequentially exposed three times to mineral oil (HM, acclimatization), three times to phenylethyl alcohol (PEA, habituation) and finally to acetophenone (ACE, dishabituation). The duration of sniffing in response to each odorant presentation was measured. Data are presented as the mean ± s.e.m. (CON, n = 24 mice; LOW, n = 21 mice; HIGH, n = 24 mice). * p<0.05, ** p<0.005; *** p<0.001, **** p<0.0001 after a two-way repeated-measures ANOVA followed by Fisher’s LSD post hoc test.
Discussion
The present study aimed to investigate the impact of diets deficient in ALA (LOW diet) or supplemented with n-3 long-chain PUFAs (HIGH diet), administered to females from conception until the end of lactation and then to their pups for 5 weeks, on olfactory peripheral system properties and olfactory behavior of the offspring. Our results showed that consumption of the LOW diet induced significant molecular and functional changes in OM. In contrast, molecular modifications elicited by the HIGH diet had no repercussions on OM functionality.
The FA profiles of OM and olfactory cilia were modified by n-3 PUFA unbalanced diets
FA analyses showed that unbalanced n-3 PUFA diets induced significant changes in the PUFA levels of offspring OM. Diets deficient in n-3 PUFAs provoked a substantial increase in n-6 PUFAs (mainly 22:4n-6 and DPA n-6) as well as a strong decrease in n-3 PUFAs (EPA, DPA n-3 and DHA). The diet supplemented with n-3 long-chain PUFAs induced opposite effects, i.e. an increase in n-3 PUFAs (EPA and DPA n-3) and a decrease in n-6 PUFAs (mainly 22:4n-6 and DPA n-6), but to a lesser extent. These observations are in accordance with previous studies showing that PUFA-rich regions of the brain are highly affected by n-3-deficient diets, whereas n-3-supplemented diets have more moderate effects in young adult rodents [Citation26–30]. Our results also showed that the reciprocal replacement of DHA by DPA n-6 in OM was not complete in 8-week-old mice fed an n-3 PUFA-deficient diet. AA and 22:4n-6 also contributed to replacing DHA in OM from LOW young mice. The incomplete replacement of DHA by DPA n-6 in OM during the developmental period might impair the neuronal membrane architecture and homeostasis of OM cells.
In addition, we specifically analyzed the FA pattern of olfactory cilia that emanate from the apical dendrites of OSNs. These structures play an important role in olfactory signaling because they contain ORs and other signal transduction elements. There is growing evidence that signaling components are associated within microdomains along the cilia [Citation31–33] probably in lipid raft domains [Citation34,Citation35]. Rafts are characterized by a high content of cholesterol and complex lipids rich in SFAs and by a reduced content of PUFAs. We observed that mouse olfactory cilia contained high levels of SFAs (60–65% of total FAs) and low amounts of PUFAs (9–14% of total FAs). Our findings are consistent with previous works reporting the presence of complex sphingolipids containing SFAs in ciliary membranes, including mammalian olfactory cilia [Citation36,Citation37].
Although the results should be considered with caution due to the small number of analyzed samples, the FA analyses in olfactory cilia indicated that the LOW diet tended to increase the n-6 PUFA level, causing an enhancement in PUFAs at the expense of SFAs. A decrease in n-3 PUFAs (including DHA) was also detected. Recent studies suggest that PUFAs incorporated into PLs influence raft formation [Citation38–40]. Although not particularly abundant in lipid rafts, DHA would have a significant impact on the structure of these microdomains, due in part to its effects on cholesterol distribution [Citation38,Citation41]. An increase in the FA unsaturation index of olfactory cilia from the LOW group was also observed, suggesting that membrane fluidity could be reduced in these cilia. The slight increase in fluorescence anisotropy observed in LOW cilia reinforces this assumption. Overall, changes in cilia FA composition might impact OSN membrane organization, thereby affecting olfactory cell signaling.
OSN maturation was affected by n-3 PUFA unbalanced diets
It is well known that OM has the capacity to generate new OSNs throughout life. The OM contains two kinds of neurocompetent stem cells: globose basal cells (GBCs) and horizontal basal cells (HBCs). GBCs generally give rise to OSNs and other cell types, whereas HBCs are quiescent stem cells that are activated in cases of severe injury [Citation42]. Both n-3 PUFA unbalanced diets did not modify the expression of progenitor markers (SOX2, neurogenin 1 and neuroD1), indicating that neurogenesis was not disrupted by these diets. Nevertheless, a decrease in the expression of TUBB3, a specific marker of neuronal axon cytoskeleton [Citation43], was observed in the LOW and HIGH OM, thus revealing an impact on OSN maturation. The LOW diet also reduced the expression of OMP, a specific marker of mature OSNs. These findings suggest that n-3 unbalanced diets can affect OSN maturation.
An n-3 PUFA-deficient diet altered peripheral olfactory signaling
EOG recordings revealed that the n-3 PUFA-deficient diet provoked a reduction in the sensitivity of OSNs to odorant stimuli. Both EOG amplitude and kinetics were modulated by this diet, indicating that OSN transduction pathways were probably affected. In fact, a reduction in the expression of genes involved in olfactory signaling, namely, OMP, RTP2 and PDE1C, was observed in OM from the LOW group. Recent studies have demonstrated that OMP is involved in the olfactory transduction cascade [Citation44]. OMP modulates the kinetics of cAMP, the second messenger of olfactory transduction [Citation45]. OSNs lacking OMP exhibit odorant responses with lower amplitude and lower kinetics than OSNs expressing OMP [Citation45–47]. These effects are in line with our observations. RTP2 is a chaperone protein responsible for transporting most ORs to the OSN plasma membrane [Citation48]. A lack of RTP2 in the mouse OM affects OR trafficking and provokes a reduction in the number of mature OSNs and in OSN electrophysiological responses [Citation49]. Reductions in immature OSNs and OM sensitivity in LOW mice might therefore be associated, at least in part, with the reduction in RTP2 expression. How PDE1C reduction affects OM sensitivity is not clear. Indeed, this phosphodiesterase greatly contributes to cAMP degradation in cilia, but other mechanisms may account for the termination of OSN responses. It has been suggested that PDE1C also plays a role in regulating OSN sensitivity and adaptation [Citation50]. These properties might be altered in OM from the LOW group.
An n-3 PUFA-deficient diet disrupted olfactory mucosa sensitivity but not olfactory behavior
The use of a plethysmograph to record mouse breathing showed that animals from the LOW and CON groups increased their sniffing behavior when exposed to the lowest PEA concentration tested, revealing a similar odor sensitivity threshold. However, a difference in PEA sensitivity between these groups was not excluded. Mice from the CON group only reacted to the first PEA stimulation, whereas the LOW mice reacted to all stimulations. We hypothesize that mice from the LOW group need more odor sampling to analyze the olfactory cues, that is, to determine the odor quality, familiarity, relevance and pleasantness. Alteration of OM sensitivity (revealed by the EOG recordings) in these mice might result in a lower signal input in the olfactory bulbs and piriform cortex, making them unable to fully process the olfactory information during the first odorant stimulation. Nevertheless, despite this weakness, our results indicated that olfactory circuits ensured a sense of smell continuity in mice from the LOW group. This is in agreement with previous studies showing that mice with damaged OM are still able to identify 45% of tested odorants [Citation51]. The habituation/dishabituation behavioral test showed no impact of the LOW diet on olfactory discrimination ability, in contrast to previous studies that reported olfactory discrimination deficits in rats fed n-3 altered diets for two generations [Citation11,Citation16]. This discrepancy might be due to differences in the duration of dietary intervention (two generations versus one generation in this study), the diet composition (higher levels of fat and LA than in our diet) and the species (rat instead of mouse).
Changes induced by diet supplemented with n-3 long-chain PUFAs had no impact on OM functionality and olfactory behavior
The HIGH diet induced a significant decrease in olfr16 and Gαolf mRNA levels in offspring OM, but no other factor involved in the transduction cascade was affected by this diet. These results suggest that the first steps of the canonical olfactory transduction cascade were slightly affected by this diet. Otherwise, the HIGH diet significantly decreased the expression of SGLT1, a membrane glucose transporter. SGLT1 is known to be mainly expressed in key structures of the brain to ensure optimal neuronal functions [Citation52]. The role and precise localization of SGLT1 in the OM remain to be elucidated. Alteration of glucose transport might affect cell homeostasis and hence the functionality of OSNs and/or other OM cells. Nevertheless, consumption of the n-3-supplemented diet did not modify the OM sensitivity or olfactory capacities of mice, suggesting that the observed molecular modifications would have no repercussions on olfactory signal processing in our experimental conditions.
Furthermore, a significant enhancement of the anti-inflammatory marker IL10 in OM from mice fed the HIGH diet was observed. This finding is consistent with the well-documented effects of n-3 PUFAs, specifically EPA and DHA, on inflammation resolution [Citation53,Citation54]. In OM, inflammatory mediators can be produced in response to external factors such as viral and bacterial infections or toxins [Citation55,Citation56]. It would be of great interest to assess whether dietary supplementation with n-3 PUFAs might reduce inflammatory processes in this tissue.
In conclusion, our study showed that consumption of n-3 PUFA unbalanced diets during the perinatal and postweaning periods affected the FA composition of mouse OM and modulated the expression of a number of genes involved in signal transduction and cellular metabolism. In addition, a diet deficient in n-3 PUFAs was found to impair the electrophysiological properties of OM. However, despite these changes at the peripheral level, mouse olfactory abilities were maintained. These outcomes are in good agreement with earlier studies indicating that rodents can detect odors even though their OM is severely damaged [Citation57,Citation58]. Our study supports the view that olfaction is an extremely resilient chemosensory system.
Author contributions
A.M.L.B., V.S., D.J. N.A., L.B. and X.G. conceived and designed the study; V.S. supervised the animal experiment procedures. V.S., L.M., D.J. and S.G. performed analyses; V.S., L.M., D.J. and A.M.L.B. analyzed and interpreted the data; V.S and A.M.L.B. wrote the paper. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (525.8 KB)Acknowledgements
The authors acknowledge the staff of the animal facility of the Centre des Sciences du Goût et de l’Alimentation (Dijon, France) for animal care. The authors also thank Pascale Winckler and Jean-Marie Perrier-Cornet from the Dimacell Plateform (Agrosup Dijon, INRAE, Univ. Bourgogne Franche-Comté, F-21000 Dijon, France) for their help in performing the anisotropy analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Vanessa Soubeyre
Vanessa Soubeyre was hired by the National Center of Scientific Research (CNRS) as an engineer in 2002. In 2013, she joined the Centre for Taste and Feeding Behavior (CSGA) in Dijon, France, and she obtained her Ph.D. from the University of Bourgogne-Franche Comté in 2021. Her Ph.D. research project dealt with the impact of diets unbalanced in n-3 fatty acids on olfactory mucosa physiology. She is now working at the Institute of Functional Genomics in Montpellier, France. Her research interests are neuronal circuits involved in the physiopathology of somatosensation.
Laetitia Merle
Laetitia Merle obtained her Ph.D. at the Center for Taste and Feeding Behavior (CSGA) in Dijon, France, in 2018. Her Ph.D. research project dealt with the impact of a maternal high fat high sucrose diet on olfactory abilities in the progeny. She then worked as a postdoctoral researcher at the University of Colorado, USA, for 2 years, investigating the potential link between olfaction, viral infection and neurodegenerative diseases. Since 2022, she is a postdoctoral researcher at the French National Institute for Agronomy (INRAE), focusing her research on Covid-19-related olfactory loss.
David Jarriault
David Jarriault obtained his Ph.D. on olfactory coding at the French National Institute for Agronomy (INRAE) in 2009. He then worked as a postdoctoral researcher at the Centre for Genomic Regulation in Barcelona, Spain, and at the department of Zoology in Dunedin, New Zealand. In 2013, he was hired by INRAE as a research scientist. His research interests are olfaction and nutrition, with a focus on olfactory receptor neuron physiology.
Stéphane Grégoire
Stéphane Grégoire obtained a BTEC Higher National Diploma on biochemical and biological analyses in Dijon, France, in 1993. He was hired by the French National Institute for Agronomy (INRAE) in 1996 as an engineer assistant and was promoted as an engineer in 2020. He is responsible for the lipid analyses within the Eye and Nutrition Research Group at the Centre for Taste and Feeding Behavior (CSGA) in Dijon.
Lionel Bretillon
Lionel Bretillon obtained his Ph.D. on lipid metabolism and nutrition from the University of Bordeaux, France, in 1999. He further stayed as a postdoctoral fellow at the Karolinska Institute in Stockholm, Sweden, working on cholesterol homeostasis in Alzheimer disease. He then appointed in 2000 as a researcher at the French National Institute for Agronomy (INRAE) in Dijon. He developed his expertise in lipid metabolism and nutrition as determinants and factors of retinal function and diseases.
Niyazi Acar
Niyazi Acar obtained his Ph.D. on lipid nutrition and neuronal physiology at the University of Bourgogne, Dijon, France, in 2002. Thereafter, he stayed at the Department of Ophthalmology of the University Eye Hospital Tuebingen, Germany, as a post-doctoral fellow for 1 year, before being hired as a research scientist by the French National Institute for Agronomy (INRAE) in 2003. Since 2017, he leads the Eye and Nutrition Research Group at the Centre for Taste and Feeding Behavior (CSGA) in Dijon. The objectives of his group are to better understand the relationships between dietary lipids and eye physiology, as well as to design nutritional/pharmacological strategies to prevent age-related retinal diseases.
Xavier Grosmaitre
Xavier Grosmaitre obtained his Ph.D. in 2001 in neurophysiology from the University Paris-VI, performing his research at the French National Institute for Agronomy (INRAE). His academic career started in the USA where he first joined Pr. Gordon Shepherd’s lab in the Neurobiology Section of Yale Medical School as a postdoctoral associate, and then Pr. Minghong Ma at the Perelman School of Medicine of the University of Pennsylvania as a Research Associate. In 2008, he joined the National Center of Scientific Research (CNRS) in Dijon as a research scientist and group leader. The scientific objectives of his team are to investigate the neurobiological, behavioral and developmental properties of the olfactory system in mice.
Anne Marie Le Bon
Anne Marie Le Bon obtained her Ph.D. in food science from the University of Paris-VII, France, in 1987. She was then hired by the French National Institute for Agronomy (INRAE) as a research scientist and developed research in nutrition, toxicology and food safety. In 2010, she joined the Centre for Taste and Feeding Behavior (CSGA) in Dijon, France. Her main interest is the impact of nutrition on the properties of the peripheral olfactory system.
References
- Stevenson RJ. An initial evaluation of the functions of human olfaction. Chem Senses. 2010;35:3–20.
- Boesveldt S, Parma V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021;383:559–67.
- Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, et al. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J Comp Neurol. 2017;525:1034–54.
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–8.
- Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33:839–59.
- Palouzier-Paulignan B, Lacroix M-C, Aimé P, Baly C, Caillol M, Congar P, et al. Olfaction under metabolic influences. Chem Senses. 2012;37:769–97.
- Thiebaud N, Johnson MC, Butler JL, Bell GA, Ferguson KL, Fadool AR, et al. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci. 2014;34:6970–84.
- Merle L, Person O, Bonnet P, Grégoire S, Soubeyre V, Grosmaitre X, et al. Maternal high fat high sugar diet disrupts olfactory behavior but not mucosa sensitivity in the offspring. Psychoneuroendocrinology. 2019;104:249–58.
- Lacroix M-C, Caillol M, Durieux D, Monnerie R, Grebert D, Pellerin L, et al. Long-lasting metabolic imbalance related to obesity alters olfactory tissue homeostasis and impairs olfactory-driven behaviors. Chem Senses. 2015;40:537–56.
- Le Bon AM, Deprêtre N, Sibille E, Cabaret S, Grégoire S, Soubeyre V, et al. Comprehensive study of rodent olfactory tissue lipid composition. Prostaglandins Leukot Essent Fatty Acids. 2018;131:32–43.
- Hichami A, Datiche F, Ullah S, Liénard F, Chardigny J-M, Cattarelli M, et al. Olfactory discrimination ability and brain expression of c-fos, Gir and Glut1 mRNA are altered in n-3 fatty acid-depleted rats. Behav Brain Res. 2007;184:1–10.
- Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85.
- Anses. Apports en acides gras de la population vivant en France et comparaison aux apports nutritionnels conseillés définis en 2010. 2015. Available from: https://www.anses.fr/fr/content/les-lipides.
- Brenna JT. Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life. Matern Child Nutr. 2011;7(Suppl 2):59–79.
- de Velasco PC, Mendonça HR, Borba JMC, Andrade da Costa BLDS, Guedes RCA, Navarro DMDAF, et al. Nutritional restriction of omega-3 fatty acids alters topographical fine tuning and leads to a delay in the critical period in the rodent visual system. Exp Neurol. 2012;234:220–9.
- Greiner RS, Moriguchi T, Slotnick BM, Hutton A, Salem N. Olfactory discrimination deficits in n-3 fatty acid-deficient rats. Physiol Behav. 2001;72:379–85.
- Khoury S, Soubeyre V, Cabaret S, Merle L, Grégoire S, Deprêtre N, et al. Perinatal exposure to diets with different n-6:n-3 fatty acid ratios affects olfactory tissue fatty acid composition. Sci Rep. 2020;10:10785.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
- Kuhlmann K, Tschapek A, Wiese H, Eisenacher M, Meyer HE, Hatt HH, et al. The membrane proteome of sensory cilia to the depth of olfactory receptors. Mol Cell Proteomics. 2014;13:1828–43.
- Kurtenbach S, Gießl A, Strömberg S, Kremers J, Atorf J, Rasche S, et al. The BEACH protein LRBA promotes the localization of the heterotrimeric G-protein golf to olfactory cilia. Sci Rep. 2017;7:8409.
- Debbabi M, Zarrouk A, Bezine M, Meddeb W, Nury T, Badreddine A, et al. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem Phys Lipids. 2017;207:151–70.
- Lacroix M-C, Badonnel K, Meunier N, Tan F, Poupon CS-L, Durieux D, et al. Expression of insulin system in the olfactory epithelium: first approaches to its role and regulation. J Neuroendocrinol. 2008;20:1176–90.
- Guilloux J-P, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31.
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8:Unit 8.24.
- Mandairon N, Sultan S, Rey N, Kermen F, Moreno M, Busto G, et al. A computer-assisted odorized hole-board for testing olfactory perception in mice. J Neurosci Methods. 2009;180:296–303.
- Pélerin H, Jouin M, Lallemand M-S, Alessandri J-M, Cunnane SC, Langelier B, et al. Gene expression of fatty acid transport and binding proteins in the blood-brain barrier and the cerebral cortex of the rat: differences across development and with different DHA brain status. Prostaglandins Leukot Essent Fatty Acids. 2014;91:213–20.
- Joffre C, Grégoire S, De Smedt V, Acar N, Bretillon L, Nadjar A, et al. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot Essent Fatty Acids. 2016;114:1–10.
- Lacombe RJS, Chouinard-Watkins R, Bazinet RP. Brain docosahexaenoic acid uptake and metabolism. Mol Aspects Med. 2018;64:109–34.
- Schnebelen C, Viau S, Grégoire S, Joffre C, Creuzot-Garcher CP, Bron AM, et al. Nutrition for the eye: different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res. 2009;41:216–24.
- Schnebelen C, Grégoire S, Pasquis B, Joffre C, Creuzot-Garcher CP, Bron AM, et al. Dietary n-3 and n-6 PUFA enhance DHA incorporation in retinal phospholipids without affecting PGE(1) and PGE (2) levels. Lipids. 2009;44:465–70.
- Takeuchi H, Kurahashi T. Second messenger molecules have a limited spread in olfactory cilia. J Gen Physiol. 2018;150:1647–59.
- Meyer SA, Ozbay BN, Potcoava M, Salcedo E, Restrepo D, Gibson EA. Super-resolution imaging of ciliary microdomains in isolated olfactory sensory neurons using a custom two-color stimulated emission depletion microscope. J Biomed Opt. 2016;21:66017.
- Castillo K, Restrepo D, Bacigalupo J. Cellular and molecular Ca2+ microdomains in olfactory cilia support low signaling amplification of odor transduction. Eur J Neurosci. 2010;32:932–8.
- Schreiber S, Fleischer J, Breer H, Boekhoff I. A possible role for caveolin as a signaling organizer in olfactory sensory membranes. J Biol Chem. 2000;275:24115–23.
- Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, Lynch L, et al. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol Pharmacol. 2004;65:503–11.
- Lobasso S, Lopalco P, Angelini R, Baronio M, Fanizzi FP, Babudri F, et al. Lipidomic analysis of porcine olfactory epithelial membranes and cilia. Lipids. 2010;45:593–602.
- Kaiser F, Huebecker M, Wachten D. Sphingolipids controlling ciliary and microvillar function. FEBS Lett. 2020;594:3652–67.
- Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta. 2015;1848:211–9.
- Bennett WFD, Shea J-E, Tieleman DP. Phospholipid chain interactions with cholesterol drive domain formation in lipid membranes. Biophys J. 2018;114:2595–605.
- Wang C, Yu Y, Regen SL. Lipid raft formation: key role of polyunsaturated phospholipids. Angew Chem Int Ed Engl. 2017;56:1639–42.
- Wassall SR, Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim Biophys Acta. 2009;1788:24–32.
- Glezer I, Malnic B. Olfactory receptor function. Handb Clin Neurol. 2019;164:67–78.
- Roskams AJ, Cai X, Ronnett GV. Expression of neuron-specific beta-III tubulin during olfactory neurogenesis in the embryonic and adult rat. Neuroscience. 1998;83:191–200.
- Dibattista M, Al Koborssy D, Genovese F, Reisert J. The functional relevance of olfactory marker protein in the vertebrate olfactory system: a never-ending story. Cell Tissue Res. 2021;383:409–27.
- Reisert J, Yau K-W, Margolis FL. Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol. 2007;585:731–40.
- Lee AC, He J, Ma M. Olfactory marker protein is critical for functional maturation of olfactory sensory neurons and development of mother preference. J Neurosci. 2011;31:2974–82.
- Dibattista M, Reisert J. The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J Neurosci. 2016;36:2995–3006.
- Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–91.
- Sharma R, Ishimaru Y, Davison I, Ikegami K, Chien M-S, You H, et al. Olfactory receptor accessory proteins play crucial roles in receptor function and gene choice. eLife. 2017;6:e21895.
- Cygnar KD, Zhao H. Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci. 2009;12:454–62.
- Youngentob SL, Schwob JE. Odorant identification and quality perception following methyl bromide-induced lesions of the olfactory epithelium. Behav Neurosci. 2006;120:1346–55.
- Julliard A-K, Al Koborssy D, Fadool DA, Palouzier-Paulignan B. Nutrient sensing: another chemosensitivity of the olfactory system. Front Physiol. 2017;8:468.
- Joffre C, Dinel A-L, Chataigner M, Pallet V. Layé s. n-3 polyunsaturated fatty acids and their derivates reduce neuroinflammation during aging. Nutrients. 2020;12:647.
- Calder PC. n-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc Nutr Soc. 2020;79: 1–13.
- Islam Z, Harkema JR, Pestka JJ. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ Health Perspect. 2006;114:1099–107.
- Imamura F, Hasegawa-Ishii S. Environmental toxicants-induced immune responses in the olfactory mucosa. Front Immunol. 2016;7:475.
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol Behav. 1997;62:1241–52.
- Cheung MC, Jang W, Schwob JE, Wachowiak M. Functional recovery of odor representations in regenerated sensory inputs to the olfactory bulb. Front Neural Circuits. 2013;7:207.
