ABSTRACT
Neurodegenerative diseases involving pathological tau protein aggregation are collectively known as tauopathies and include Alzheimer’s disease and Pick’s disease. Recent studies show that the intake of tryptophan-tyrosine (Trp-Tyr)-related β-lactopeptides, including β-lactolin, attenuates cognitive decline in the elderly and prevents the amyloid pathology in mouse models of Alzheimer’s disease. However, the effects of Trp-Tyr-related β-lactopeptides on tau-related pathology have not been investigated. In the present study, we examined the effects of Trp-Tyr dipeptide intake on tauopathy in PS19 transgenic mice, a well-established tauopathy model. Intake of Trp-Tyr dipeptide improved the behavioral deficits observed in the open field test, prevented tau phosphorylation, and increased the dopamine turnover and synaptophysin expression in the frontal cortex. Levels of short-chain fatty acids in the cecum were lower in PS19 mice than those in wild-type mice and were increased by treatment with Trp-Tyr dipeptide. In addition, intake of Trp-Tyr dipeptide extended the lifespan of PS19 mice. These findings suggest that the intake of Trp-Tyr-related peptides improves tauopathy symptoms, resulting in improvements in behavioral deficits and longevity. Hence, the intake of Trp-Tyr-related peptides, including β-lactolin, may be beneficial for preventing dementia.
Introduction
With the rapid growth in the aging population worldwide, cognitive decline and dementia have become a growing burden on patients, families, and national healthcare systems. Due to the lack of disease-modifying therapies for dementia, increasing focus has been placed on preventive approaches. Neurodegenerative diseases involving pathological tau protein aggregation are collectively known as tauopathies and include Alzheimer’s disease, Pick’s disease, and frontotemporal dementia.
Tau, a microtubule-associated protein, is primarily expressed in the central nervous system and is responsible for the polymerization and stabilization of microtubules. However, in the case of diseases, mutated and misfolded tau can form aggregates in non-native conformations. This contributes to both a reduction in the normal physiological activity of the tau protein and a toxicity-associated dysfunction. Tau aggregates known as neurofibrillary tangles contribute to dysfunction and degeneration in age-related neurodegenerative diseases[Citation1]. Accumulating evidence shows that the deposition of phosphorylated tau induces inflammation and exacerbates neurological deficits, cognitive decline, and tauopathy[Citation2–4]. Therefore, the phosphorylation and aggregation of tau represent critical therapeutic and preventive targets for dementia.
Recent investigations indicate that the consumption of certain dairy products reduces the risk of dementia, including Alzheimer’s disease and cognitive decline in the elderly. Ozawa et al. investigated the association between dietary patterns and risk of dementia in more than 1,000 dementia-free 60- to 79-year-old Japanese participants living in a local community[Citation5,Citation6]. They observed that dietary habits that included a higher intake of milk or dairy products were associated with a lower risk of dementia. In addition, we previously demonstrated that the intake of Camembert cheese and dairy products fermented with fungi suppressed microglial activation and attenuated Alzheimer’s disease pathology in 5×FAD mice, a well-established model of Alzheimer’s disease[Citation7].
Recent studies reported that a Trp-Tyr-related β-lactopeptide rich in Camembert cheese and other cheeses fermented by Penicillium ameliorates memory impairments in a murine model of pharmacologically induced amnesia[Citation8]. Intake of β-lactopeptides increased monoamine levels in the frontal cortex and hippocampus and improved spatial working memory and attention in a mouse model of pharmacologically induced amnesia, as well as in aged mice[Citation9–11]. We previously demonstrated that supplementation with β-lactolin improves memory retrieval, attention, and executive function in the elderly[Citation12,Citation13] and promotes neural activity[Citation14] and cerebral blood flow[Citation15,Citation16]. These findings suggest that the consumption of β-lactolin is associated with the activation of the dorsolateral prefrontal cortex, a brain region critical for regulating memory retrieval and executive function. Furthermore, we demonstrated that intake of β-lactopeptides prevents Aβ-induced pathology and cognitive decline in 5×FAD mice[Citation17,Citation18]. However, the effects of β-lactopeptides on tau-associated dementia pathology remain unclear. Therefore, in the present study, we investigated the effects of Trp-Tyr dipeptide intake on tauopathy mice (PS19 mice) to examine whether Trp-Tyr dipeptide supplementation suppresses tau pathology and its associated symptoms.
Materials and methods
Materials
The Trp-Tyr dipeptide (purity > 98%) was purchased from Bachem (Bubendorf, Switzerland).
Animals
Tauopathy model mice, B6;C3-Tg mice (Prnp-MAPT*P301S)PS19Vle/J (https://www.jax.org/strain/008169)[Citation19], hereafter referred to as PS19 mice, were used in the present study. PS19 mice overexpress the T34 isoform of microtubule-associated protein tau with one N-terminal insert and four microtubule-binding repeats (1N4R) encoding the human P301S mutation. Non-transgenic wild-type littermates were used as controls in this study. All experiments were approved by the Animal Care and Use Committee of the Graduate School of Agricultural and Life Sciences, the University of Tokyo on July, 2017. Experiments were conducted from July 2017 to November 2018 in strict accordance with institutional guidelines (Approval No.: P17-020). All efforts were taken to minimize animal suffering. Mice were maintained at room temperature (23°C ± 1°C) under constant 12-h light/dark cycles (lights on from 8:00 am to 8:00 pm). Mice aged <3 months were fed a standard purified rodent growth diet (AIN-93G; Oriental Yeast, Tokyo, Japan), and mice aged ≥3 months were fed a maintenance diet (AIN-93M; Oriental Yeast). No significant differences were observed in body weight among the different mouse groups. Transgenic 2.5-month-old PS19 and wild-type male and female mice were fed a diet that either contained or did not contain 0.05% w/w Trp-Tyr dipeptide (for experiments using male wild-type mice, n = 11; wild-type mice fed Trp-Tyr peptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr peptide, n = 10; for experiments using female wild-type mice, n = 18; transgenic control mice, n = 13; transgenic mice fed Trp-Tyr peptide, n = 12). The amount of 0.05% w/w Trp-Tyr was the same as that used in previous demonstrations[Citation17,Citation18]. After a behavioral evaluation, male mice were euthanized at 9 months of age. Female mice were euthanized at 54 weeks of age. The animals were monitored daily regarding their condition and survival, and the body weight of each animal was recorded daily. Brains were removed as described in the following sections.
Open field test
To evaluate the general activity in a novel environment, mice were subjected to an open field test. Mice were placed in an open chamber (40 cm × 40 cm × 40 cm) made of gray polyvinyl chloride for 5 min. The activity was monitored using SMART video-tracking software (PanLab Harvard Apparatus, Holliston, MA, USA).
Quantification of tau and synaptophysin using ELISAs
Homogenate samples of the left hippocampus and cortex were prepared as described previously[Citation20]. Hippocampal and cortical tissues were homogenized in a Tris-buffered solution containing a protease inhibitor cocktail (BioVision, Milpitas, CA, USA) using a multi-bead shocker (Yasui Kikai, Osaka, Japan). The supernatant was collected after centrifugation at 50,000 × g for 20 min. The total protein concentration of each supernatant was measured using a BCA Protein Assay Kit (Thermo Scientific, Yokohama, Japan). The supernatant was used to quantify pTau (pS199, KHB7041, ThermoFisher Scientific, Yokohama, Japan), tTau (KHB0041, ThermoFisher Scientific), and synaptophysin (LS-F6345, LSBio, Seattle, WA, USA) using ELISAs.
Immunohistochemistry
Right brain hemispheres were fixed in 10% formalin solution (Wako, Osaka, Japan), paraffin-embedded, and cut into 5-µm serial sections to evaluate pTau deposition and neuronal marker loss. The hippocampus and cerebral cortex (2.30 mm posterior to bregma) were analyzed. After dewaxing and rehydration, the sections used for NeuN measurements were autoclaved at 121°C for 10 min in 0.2% citrate buffer (pH 6.0) for antigen retrieval. The sections were then incubated with blocking solution (8% w/v skim milk) for 30 min after inactivation of endogenous peroxidases with 3% H2O2 (Wako) in methanol for 5 min. Subsequently, the sections were incubated overnight at room temperature with primary antibodies (anti-NeuN, MAB377, 1:100 dilution, Millipore, MA, USA). After incubation with each primary antibody at 4°C overnight, immunolabeled antigens were visualized using the Dako EnVision+ System (Dako, Glostrup, Denmark) with 0.02% 3,3’-diaminobenzidine and 0.01% hydrogen peroxide as the chromogen. For the NeuN analysis, positively stained cells in each defined whole area of the hippocampus and entorhinal cortex were counted. Two sections for each sample area were prepared for analysis.
Consecutive sections were stained using immunohistochemical techniques. After deparaffinization and rehydration, antigen retrieval was performed via heating to detect pTau. To deactivate endogenous peroxidases, sections were immersed in 1% hydrogen peroxide in methanol for 5 min. To avoid nonspecific antibody binding, sections were immersed in 8% skim milk in a Tris-buffered solution. As the primary antibody, mouse anti-hp-tau antibody (clone AT8, MN1020, 1:500, Thermo Scientific, Rockford, IL, USA) was used. After incubation with the primary antibody at 4°C overnight, immunolabeled antigens were visualized using the Dako EnVision+ System (Dako) with 0.02% 3,3’-diaminobenzidine and 0.01% hydrogen peroxide as the chromogen. For tau analysis, the size of the positive region per area was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). We took the pictures of each defined whole brain region and quantified the whole positive area that included the neuronal bodies, dendrites, and glial or other types of cells.
Analysis of dopamine levels
To evaluate the levels of dopamine and its metabolites in the brain, tissues were homogenized in 0.2 M perchloric acid (Wako) containing 100 μM EDTA•2Na (Sigma-Aldrich, St. Louis, MO, USA). After centrifugation, the supernatant was analyzed with HPLC using EICOMPAK SC-5ODS and PREPAK columns (Eicom, Kyoto, Japan) with an ECD unit. The mobile phase consisted of 83% 0.1 M acetic acid in citric acid buffer (pH 3.5), 17% methanol (Wako), 190 mg/mL of sodium 1-octane sulfonate (Wako), and 5 mg/mL of EDTA•2Na. For ECD, the applied voltage was 750 mV versus an Ag/AgCl reference electrode.
Analysis of gut metabolites
To evaluate the levels of short-chain fatty acids (SCFAs) in the cecum, an aliquot of the sample was diluted with 4 volumes of distilled water to prepare the homogenate. Acetic acid, propionic acid, n-butyric acid, iso-butyric acid, caproic acid, n-valeric acid, and iso-valeric acid in the homogenate were derivatized with 2-nitrophenylhydrazine hydrochloride in the presence of 1-ethyl-3-(3(dimethylamino)propyl)carbodiimide hydrochloride and were measured by the HPLC system according to a previous report[Citation21].
Statistical analysis
Data are presented as the mean ± standard error of the mean. Data were analyzed using the two-way ANOVA followed by the Tukey–Kramer or Student’s t-tests, as described in the figure legends. Survival rate data were analyzed using the log-rank test. All statistical analyses were performed using the Ekuseru-Toukei 2012 software (Social Survey Research Information, Tokyo, Japan). Differences were considered statistically significant at p < 0.05.
Results
Effects of Trp-Tyr Intake on open field activity in PS19 mice
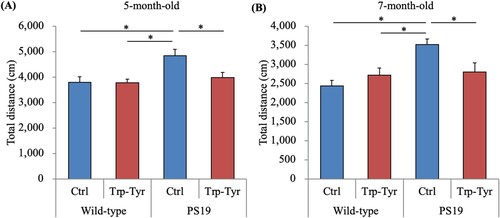
PS19 mice were subjected to an open field test to evaluate the effects of Trp-Tyr dipeptide intake on locomotor activity. The total distance traveled in the open field test was significantly higher in control PS19 mice than in wild-type mice and was significantly lower in PS19 mice fed Trp-Tyr than in control PS19 mice at 5 and 7 months of age (A and B).
PS19 and wild-type male mice (2.5 months of age) were fed a diet containing 0 or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide. At 5 and 7 months of age, mice were subjected to the open field test. The total distance (cm) traveled in the open field was measured. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using the two-way ANOVA followed by the Tukey–Kramer test. *p < 0.05. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
Effects of Trp-Tyr Intake on neuronal and synaptic loss in PS19 mice
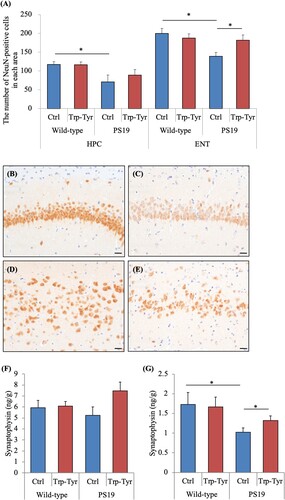
To evaluate the effects of Trp-Tyr dipeptide intake on neuronal marker loss in PS19 mice, the number of the NeuN-positive cells per area was analyzed immunohistochemically. The number of the NeuN-positive cells per area in the hippocampus was significantly lower in control PS19 mice than in control wild-type mice (A- C), whereas no significant difference was observed between PS19 mice fed Trp-Tyr dipeptide and control PS19 mice (A). The number of the NeuN-positive cells per area in the entorhinal cortex was significantly lower in control PS19 mice than in control wild-type mice (A, D, and E). Conversely, the number of the NeuN-positive cells per area in the entorhinal cortex was significantly higher in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice (A). The protein levels of synaptophysin in the hippocampus and cortex were measured using ELISAs. No significant between-group differences were observed in hippocampal synaptophysin levels (F(3, 39) = 1.87, p > 0.05) (F). In contrast, synaptophysin levels in the cortex were significantly lower in control PS19 mice than in control wild-type mice and were significantly higher in PS19 mice fed Trp-Tyr dipeptide compared to control PS19 mice (F(3, 39) = 3.65, p < 0.05, two-way ANOVA; p = 0.031 and 0.043, respectively, designated comparison, Tukey–Kramer post-hoc test) (G).
PS19 and wild-type male mice (2.5 months of age) were fed a diet containing 0 or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide for 6.5 months. (A) The NeuN-positive area was measured immunohistochemically, and NeuN-positive cells in the hippocampus (HPC) and entorhinal cortex (ENT) were counted. (B-E) Representative images of the HPC of control wild-type mice and control PS19 mice (B and C, respectively), and ENT of control wild-type mice and control PS19 mice (D and E, respectively). (F) and (G) Levels of synaptophysin in the HPC and cortex, respectively. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using the two-way ANOVA followed by the Tukey–Kramer test. *p < 0.05. Scale bars are 20 µm. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
Effects of Trp-Tyr Intake on dopamine metabolite levels in PS19 mice
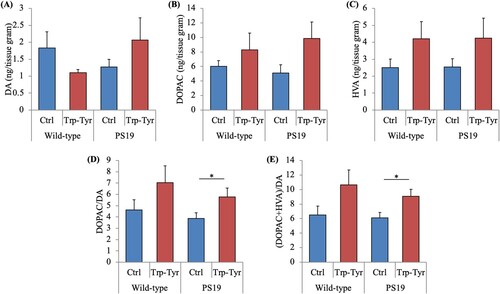
To evaluate the effects of Trp-Tyr dipeptide intake on dopamine release in the frontal cortex, levels of dopamine and its metabolites were measured. No significant between-group differences were observed in the levels of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) (A-C, respectively). Dopamine turnover, indicated by DOPAC/dopamine ratio (F(3, 39) = 3.96, p < 0.05, two-way ANOVA; p = 0.045, designated comparison, Tukey–Kramer post-hoc test) and (DOPAC + HVA)/dopamine ratios (F(3, 39) = 5.15, p < 0.05, two-way ANOVA; p = 0.040, designated comparison, Tukey–Kramer post-hoc test) (D and E, respectively), was significantly higher in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice.
PS19 and wild-type male mice (2.5 months of age) were fed a diet containing 0 or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide for 6.5 months. (A)-(C) Levels of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) per tissue gram, respectively, in the cortex. Metabolite levels were measured using a high-performance liquid chromatography-electrochemical detection system. (D) and (E) Ratios of DOPAC/DA and (DOPAC + HVA)/DA, respectively. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using the two-way ANOVA followed by the Tukey–Kramer test. *p < 0.05. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
Effects of Trp-Tyr Intake on tau phosphorylation in PS19 mice
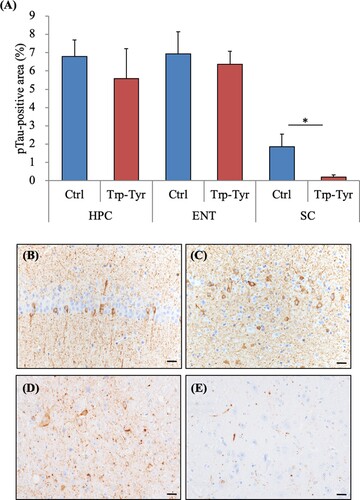
To evaluate the effects of Trp-Tyr dipeptide intake on tau phosphorylation, levels of pTau in PS19 mice were analyzed. The immunohistochemical analyses of the hippocampus, entorhinal cortex, and spinal cord (B-E) revealed that the size of the pTau-positive region per area in the spinal cord was significantly smaller in PS19 mice fed Trp-Tyr dipeptide mice than in control PS19 mice (A). No significant between-group differences were observed in the examined brain regions. Based on semiquantitative ELISA analyses, the hippocampal pTau levels were significantly lower in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice (A). The tTau levels as well as the ratios of pTau to tTau in the hippocampus were not significantly different between the groups (A). In the cortex, the ratio of pTau to tTau was significantly lower in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice (B). Levels of pTau in the cortex exhibited a trend to be lower in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice, but this did not reach statistical significance (B). The cortical tTau levels did not differ between the groups (B).
Table 1. Phosphorylation of tau in PS19 mice.
PS19 and wild-type mice (2.5 months of age) were fed a diet containing 0 (Ctrl) or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide for 6.5 months. Data describe total tau (tTau), phosphorylated tau (pTau), and the ratio of pTau to tTau in the hippocampus (A) and cortex (B) of PS19 mice. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using Student’s t-test. *p < 0.05. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
PS19 and wild-type mice (2.5 months of age) were fed a diet containing 0 or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide for 6.5 months. (A) The ratios of phosphorylated tau (pTau) in the hippocampus (HPC), entorhinal cortex (ENT), pontine nucleus (PN), and spinal cord (SC) in PS19 mice were measured immunohistochemically. (B)-(D) The representative pTau images of the HPC, ENT, and SC in PS19 mice, respectively. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using Student’s t-test. *p < 0.05. Scale bars are 20 μm. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
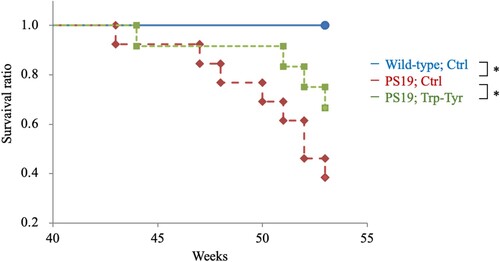
Effects of Trp-Tyr dipeptide intake on longevity in PS19 mice
To evaluate the effects of Trp-Tyr dipeptide intake on longevity of PS19 mice, the survival ratio was assessed. At 43 weeks of age, control PS19 mice exhibited a lower survival ratio compared to wild-type mice. At 53 weeks of age, the survival ratio was 1.0 in wild-type mice, 0.38 in control PS19 mice, and 0.67 in PS19 mice fed Trp-Tyr dipeptide (). Compared to wild-type mice, PS19 mice exhibited a significantly lower survival ratio. (p = 0.0018, Wild-type; Ctrl vs PS19; Trp-Tyr, p = 0.031, Wild-type; Ctrl vs PS19; Trp-Tyr) In contrast, the survival ratio of PS19 mice fed Trp-Tyr dipeptide was significantly higher than that of control PS19 mice. (p = 0.046, PS19; Ctrl vs PS19; Trp-Tyr)
PS19 and wild-type mice (2.5 months of age) were fed a diet containing 0 (Ctrl) or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide until 53 weeks of age (wild-type mice, n = 18; transgenic control mice, n = 13; transgenic mice fed Trp-Tyr peptide, n = 12). Kaplan–Meier survival curves of mice. p-values were determined using the log-rank test. *p < 0.05. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
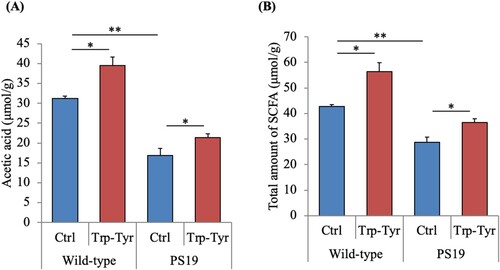
Effects of Trp-Tyr dipeptide intake on the gut metabolites in PS19 mice
To evaluate the effects of Trp-Tyr dipeptide intake on gut microbiota, fatty acid levels in the cecum were analyzed. The levels of acetic acid and the total amount of SCFAs in the cecum were significantly lower in PS19 mice than in wild-type mice (A and B). However, the levels of acetic acid and the total amount of SCFAs were significantly higher in PS19 mice fed Trp-Tyr dipeptide than in control PS19 mice (A and B).
PS19 and wild-type mice (2.5 months of age) were fed a diet containing 0 or 0.05% (w/w) tryptophan-tyrosine (Trp-Tyr) dipeptide for 6.5 months. The levels of acetic acid (A) and total short-chain fatty acids (B) in the cecum were measured. Data are presented as the mean ± standard error of the mean (wild-type mice, n = 11; wild-type mice fed Trp-Tyr dipeptide, n = 10; transgenic control mice, n = 11; transgenic mice fed Trp-Tyr dipeptide, n = 10). p-values shown in the graph were calculated using the two-way ANOVA followed by the Tukey–Kramer test. *p < 0.05, **p < 0.01. Ctrl, control mice; Trp-Tyr, tryptophan-tyrosine-fed mice.
Discussion
The current study demonstrated that the intake of Trp-Tyr dipeptide, which constitutes the core sequence of β-lactopeptides and β-lactolin, improved tauopathy-related pathology, increased enteric SCFA levels, and prolonged the lifespan in a mouse model of tauopathy. Previous studies have reported that the orally administered Trp-Tyr dipeptide and β-lactolin were smoothly absorbed into the blood and delivered to each brain region, including the hippocampus and cortex and increased the dopamine levels via inhibition of monoamine oxidase B, thereby improving memory impairments in a mouse model of scopolamine-induced amnesia, as well as in aged mice[Citation8,Citation11,Citation17]. In addition, the intake of Trp-Tyr dipeptide and β-lactolin suppressed Aβ pathology and microglial activation and ameliorated memory impairments in 5×FAD mice, a well-established Alzheimer’s disease model[Citation18]. We recently demonstrated that supplementation with the Trp-Tyr-related peptide β-lactolin improved frontal cortex-associated cognitive function in elderly individuals with subjective cognitive decline[Citation13]. The current study is the first to demonstrate the effects of Trp-Tyr dipeptide intake on tauopathy and gut metabolites.
In the current study, Trp-Tyr dipeptide intake suppressed tau phosphorylation in the cortex and hippocampus measured by ELISA and neurofibrillary tangle levels in the spinal cord but not in the hippocampus and cortex. This suggests that Trp-Tyr suppressed tau phosphorylation but did not affect deposited and aggregated tau. Furthermore, Trp-Tyr dipeptide intake attenuated the loss of synapses. This was indicated by using neuronal markers in the hippocampus and cortex; synaptophysin (a presynaptic marker) was assessed using ELISAs and NeuN (a neuronal marker) using immunohistochemical staining. Tau is associated with synaptic dysfunction and binds to synaptic vehicles via its N-terminal domain, thereby interfering with presynaptic function[Citation22]. Previous studies have shown that 7-month-old male PS19 mice display a reduction in synaptophysin and Nissl-positive neurons in the cortex and hippocampus. Neuronal loss was also observed in 6-month-old PS19 mice[Citation19,Citation23]. These findings are consistent with the results of the current study. Trp-Tyr dipeptide intake suppressed the reduction in presynaptic and neuronal markers, which may explain the behavioral differences in PS19 mice in the open field test. PS19 mice at 7 months of age exhibit higher locomotor activity in the open field test[Citation24], which is consistent with the results of the current study. PS19 mice at 5–7 months of age showed higher locomotor activity than wild-type mice; therefore, it was difficult to evaluate the cognitive function using behavioral evaluation, including the Y-maze test and object recognition test.
We performed the open field test to evaluate the general activity level, including locomotor activity, in a novel environment. The PS19 mice in the current study traveled a higher total distance in the open field test compared to wild-type mice. This locomotor hyperactivity, a behavioral deficit, is considered to be caused by disinhibition of exploratory behavior rather than a motor disturbance, which is observed as an early disease phenotype in PS19 mice[Citation25]. According to our study results, the locomotor hyperactivity in PS19 mice was improved by Trp-Tyr intake.
Our previous randomized controlled clinical trial demonstrated that compared to placebo, supplementation with the Trp-Tyr-related peptide β-lactolin improved inhibition in older adults. Inhibition is one of the executive functions among the cognitive function associated with the activity of the prefrontal cortex[Citation26]. The protective effects of Trp-Tyr dipeptide intake on synapses and dopamine metabolism may at least partly underpin improvements in inhibition. Our study findings suggest that the changes in dopamine turnover by Trp-Tyr dipeptide improved tau-induced behavioral deficits and synapse loss, but the exact mechanisms need to be investigated in further studies. A previous study reported that intake of Trp-Tyr dipeptide and the Trp-Tyr-related peptide β-lactolin suppresses microglial activation and reduces inflammation in the brain in aged mice and 5×FAD mice. Microglial activation is associated with tau pathology[Citation27,Citation28], and depletion of reactive microglia suppresses tau pathology in tauopathy mice[Citation29,Citation30]. Thus, microglia may be associated with the Trp-Tyr-induced reduction in tau phosphorylation.
The current study also demonstrated that the levels of acetic acid and SCFAs in the cecum were lower in PS19 mice than in wild-type mice. Furthermore, the levels of acetic acid and SCFAs were higher in wild-type mice and PS19 mice fed Trp-Tyr-related peptide than in control mice. Previous studies have highlighted an association between Aβ and SCFAs. In this regard, it is suggested that SCFAs suppress Aβ aggregation and microglial activation[Citation31,Citation32]. Fecal microbiota transplantation ameliorates cognitive impairments and reduces Aβ deposition[Citation33,Citation34]. SCFAs from the gut play a critical role in brain-gut axis communication[Citation35]. SCFAs can activate FFAR2 and FFAR3 on enteroendocrine cells, thereby stimulating the secretion of glucagon-like peptide 1 and peptide YY. These peptides activate the vagal nerve connecting to the brainstem, which improves the affective state and cognitive function. SCFAs increase neurotrophic factors such as the brain-derived neurotrophic factor and glial cell-derived neurotrophic factor in the brain, which are associated with neuronal proliferation. Furthermore, the ketogenic diet has been reported to modulate gut metabolites and SCFAs, which are associated with markers of Alzheimer's disease in subjects with mild cognitive impairment[Citation36]. Collectively, these findings suggest that the increase in SCFA levels following Trp-Tyr dipeptide intake may have suppressed neuronal marker loss, synaptic dysfunction, and behavioral deficits in the PS19 mice observed herein.
The current study has several limitations. We evaluated the locomotor activity and conducted histopathological and biochemical analysis in male mice but not in female mice. It has been reported that PS19 mice show sexual dimorphism behavior[Citation37]; hence, a further study needs to evaluate the effects of Trp-Tyr dipeptide on female PS19 mice to make a generalized conclusion. In the current study, Trp-Tyr dipeptide intake improved the phosphorylation of tau and the behavioral deficits in PS19 mice but did not alter neurofibrillary tangles in the hippocampus and entorhinal cortex. Further studies are needed to investigate these effects in detail. Although we provided evidence for the neuroprotective effects of Trp-Tyr dipeptide intake in a mouse model of tauopathy, we did not elucidate the underlying mechanisms and the target molecules of the Trp-Tyr dipeptide. Further studies are warranted to investigate the relationships among Trp-Tyr dipeptide intake, tauopathy, and gut metabolites. Furthermore, we measured fatty acids in the cecum but did not examine the bacterial flora; hence, we were unable to determine whether Trp-Tyr dipeptide intake directly improved SCFA levels or whether the effects were mediated via modification of the bacterial flora.
In conclusion, neurodegenerative diseases with tau pathology represent a societal issue of growing concern that is in urgent need of effective therapeutic approaches. Preventive lifestyle approaches have garnered increasing attention, and nutritional substances have excellent safety profiles without long-term side effects. Here, we demonstrated that Trp-Tyr dipeptide intake prolonged the lifespan in PS19 mice, highlighting the potential of supplementation with Trp-Tyr-related peptides as a disease-modifying treatment for tau-related symptoms. Furthermore, our findings suggest that the intake of Trp-Tyr dipeptide and β-lactolin, which are found in high quantities in fermented dairy products and are safe and easy to consume, may prevent tau-associated impairment of brain function.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol 2013;70(3):304-10.
- Katsumoto A, Takeuchi H, Takahashi K, Tanaka F. Microglia in Alzheimer's Disease: Risk Factors and Inflammation. Front Neurol 2018;9:978.
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575-90.
- Lee DC, Rizer J, Selenica ML, Reid P, Kraft C, Johnson A, et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation 2010;7:56.
- Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr 2013;97(5):1076-82.
- Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, Mukai N, et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: the Hisayama Study. J Am Geriatr Soc 2014;62(7):1224-30.
- Ano Y, Ozawa M, Kutsukake T, Sugiyama S, Uchida K, Yoshida A, et al. Preventive effects of a fermented dairy product against Alzheimer's disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS One 2015;10(3):e0118512.
- Ano Y, Ayabe T, Kutsukake T, Ohya R, Takaichi Y, Uchida S, et al. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol Aging 2018;72:23-31.
- Ayabe T, Ano Y, Ohya R, Kitaoka S, Furuyashiki T. The Lacto-Tetrapeptide Gly-Thr-Trp-Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus. Nutrients 2019;11(10):2469.
- Ayabe T, Ohya R, Ano Y. β-lactolin, a whey-derived glycine-threonine-tryptophan-tyrosine lactotetrapeptide, improves prefrontal cortex-associated reversal learning in mice. Biosci Biotechnol Biochem 2020;84(5):1039-46.
- Ano Y, Ayabe T, Ohya R, Kondo K, Kitaoka S, Furuyashiki T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of beta-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients 2019;11(2):348.
- Kita M, Obara K, Kondo S, Umeda S, Ano Y. Effect of Supplementation of a Whey Peptide Rich in Tryptophan-Tyrosine-Related Peptides on Cognitive Performance in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018;10(7):899.
- Kita M, Kobayashi K, Obara K, Koikeda T, Umeda S, Ano Y. Supplementation With Whey Peptide Rich in beta-Lactolin Improves Cognitive Performance in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Front Neurosci 2019;13:399.
- Kanatome A, Ano Y, Shinagawa K, Ide Y, Shibata M, Umeda S. β-Lactolin Enhances Neural Activity, Indicated by Event-Related P300 Amplitude, in Healthy Adults: A Randomized Controlled Trial. J Alzheimers Dis 2021;81(2):787–796. doi: 10.3233/jad-201413.
- Ano Y, Kobayashi K, Hanyuda M, Kawashima R. β-lactolin increases cerebral blood flow in dorsolateral prefrontal cortex in healthy adults: a randomized controlled trial. Aging (Albany NY) 2020;12(18):18660-75.
- Ano Y, Kita M, Kobayashi K, Koikeda T, Kawashima R. Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial. J Clin Med 2021;10(3):480.
- Ano Y, Yoshino Y, Kutsukake T, Ohya R, Fukuda T, Uchida K, et al. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging (Albany NY) 2019;11(10):2949-67.
- Ano Y, Ohya R, Takaichi Y, Washinuma T, Uchida K, Takashima A, et al. β-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer's Disease Pathologies and Cognitive Decline. J Alzheimers Dis 2020;73(4):1331-42.
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007;53(3):337-51.
- Ano Y, Dohata A, Taniguchi Y, Hoshi A, Uchida K, Takashima A, et al. Iso-alpha-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer's Disease. J Biol Chem 2017;292(9):3720-8.
- Miwa H, Yamamoto M. High-performance liquid chromatographic analysis of serum short-chain fatty acids by direct derivatization. J Chromatogr 1987;421(1):33-41.
- Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun 2017;8:15295.
- Jiang T, Tan L, Zhu XC, Zhou JS, Cao L, Tan MS, et al. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiol Aging 2015;36(12):3176-86.
- Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova NN, et al. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. Faseb j 2011;25(11):4063-72.
- Scattoni ML, Gasparini L, Alleva E, Goedert M, Calamandrei G, Spillantini MG. Early behavioural markers of disease in P301S tau transgenic mice. Behav Brain Res 2010;208(1):250-7.
- Diamond A. Executive functions. Annu Rev Psychol 2013;64:135-68.
- Španić E, Langer Horvat L, Hof PR, Šimić G. Role of Microglial Cells in Alzheimer's Disease Tau Propagation. Front Aging Neurosci 2019;11:271.
- Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 2015;138(Pt 6):1738-55.
- Shi Y, Manis M, Long J, Wang K, Sullivan PM, Remolina Serrano J, et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med 2019;216(11):2546-61.
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015;18(11):1584-93.
- Liu J, Li H, Gong T, Chen W, Mao S, Kong Y, et al. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer's Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-κB. J Agric Food Chem 2020;68(27):7152-61.
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 2018;18(1):83-90.
- Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 2017;7(1):2426.
- Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, et al. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry 2019;9(1):189.
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020;11:25.
- Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine 2019;47:529-42.
- Sun Y, Guo Y, Feng X, Jia M, Ai N, Dong Y, et al. The behavioural and neuropathologic sexual dimorphism and absence of MIP-3α in tau P301S mouse model of Alzheimer's disease. J Neuroinflammation 2020;17(1):72.