ABSTRACT
Background
As the sector of the population over 65y increases, cognitive decline and dementia become a public health issue. Interventions to improve brain health and thus, quality of life for older adults are needed.
Objective
It was hypothesized that those consuming a flavonoid-rich, lyophilized wild blueberry powder would evidence improvements in cognitive performance as measured behaviorally and electrophysiologically compared to those consuming a placebo powder across a 6-month intervention period.
Design
In a double-blind, randomized placebo-controlled trial, participants experiencing cognitive issues as determined by scores on the Montreal Cognitive Assessment (MoCA) were randomized to consume either wild blueberry (n = 44) or placebo (n = 42) powder daily for 6 months. Participants who were not experiencing any cognitive issues were included as a reference group (n = 45). Participants were tested at baseline and outcome on the Cambridge Neurological Test Automated Battery (CANTAB) and in an electrophysiological paradigm known as event-related potentials (ERP).
Results
Tests of specific cognitive abilities using the CANTAB showed speed of processing not only improved in the blueberry intervention group relative to the placebo group across the 6-month intervention, but blueberries also restored speed of processing to the level of the reference group. The ERP results also showed that, relative to those consuming placebo, speed of processing improved for those in the blueberry group; this improvement was most prominent in those 75-80y.
Conclusions
Consumption of wild blueberries for six months improves cognitive aging sequelae by improving the speed of information processing in older adults.
Trial registration: ClinicalTrials.gov identifier: NCT01515098.
Introduction
The proportion of the United States population aged 65 or older will reach one in five adults by the year 2030 [Citation1]. The resulting healthcare burden to caregivers and to the country will be considerable. In a 2016 study, 39% of those over age 65 were experiencing serious cognitive issues, and 68% of those experiencing issues were unable to live independently [Citation2]. Interventions that have the potential to prevent or decrease the incidence of cognitive dysfunction in the aging population will serve as a tool to offset the impact of the aging of the American population.
Whereas cognitive aging is a typical and accepted characteristic of older life, it is not inexorable. Lifestyle measures can be employed to slow the aging of the brain and by association, the decline of cognition. Dietary factors have been studied in relation to aging and brain health with the Mediterranean diet emerging as the most healthful diet for the brain [Citation3–5]. Evidence suggests that the inclusion of fresh vegetables, fruits, legumes, fish, wine, and olive oil in the Mediterranean diet is linked to healthier brains, with the numerous polyphenols emerging as possible agents of health (for review see [Citation6]).
Members of the phytochemical class polyphenol are found in fruits, vegetables, wine, cocoa, and coffee. Polyphenol research has provided evidence that they are indeed beneficial to cognitive processes in older adults (e.g. [Citation7–20]). A premium source of polyphenols, wild blueberries are of particular interest. Preclinical research has been published in which blueberries reverse age-related neuronal decline [Citation21], improve cognition and hippocampal neurogenesis [Citation22], and reverse age-related memory decline [Citation23]. Retrospectively, in humans, fifteen years of dietary berry intake were averaged and compared to cognitive decline scores [Citation24]. Those with the highest levels of berry intake showed a delay in cognitive decline of up to 2.5 years. In the seminal clinical study, the Krikorian group showed that acute short-term (12w) wild blueberry juice consumption was related to improved cognition in a small sample (n = 9) of older adults who were experiencing cognitive issues [Citation17]. However, whereas consumption of blueberries in small samples of older adults has been shown to improve specific cognitive performances [Citation10,Citation15–17], no effect was found in other studies [Citation14,Citation25]. Of note, the Krikorian group employed fMRI during a working memory task [Citation14], and found that consumption of blueberry increased blood flow in the brain, but no commensurate improvement was seen in working memory performance. Thus, the utility of blueberry in cognitive decline shows promise, but needs further study.
We hypothesized that wild blueberries, consumed by older adults who were experiencing cognitive decline, would improve components of cognition, such as speed of processing, an element of cognition that underlies all other cognitive abilities, and that this effect would be most evident under a cognitive load [Citation26]. In addition, we designed our study to test this hypothesis in a larger (n = 133) and longer (6m) intervention (relative to the literature) in both brain and behavior by employing a brain imaging technique known as event-related potentials (ERP) and a behavioral standardized assessment of cognitive decline, the Cambridge Neurological Test Automated Battery (CANTAB).
Method
Participants
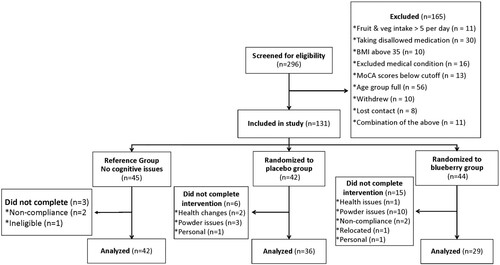
Two hundred ninety-six adults, 65–80 years of age, were recruited from a southeastern United States community. These volunteers were identified by recruiting efforts at community events, at churches, in online postings, in media coverage, and most importantly, by word of mouth. Of these, one hundred thirty-three met inclusion criteria after the completion of an in-person screening visit. Participants were deemed eligible if they consumed fewer than five daily servings of fruits and vegetables (as is typical in the U.S.); were not allergic to blueberries; were not diagnosed with dementia or Alzheimer’s disease, central nervous system disorders, psychiatric disorders, gastrointestinal or digestive issues, or diabetes; had a body mass index (BMI) less than 33 [Citation27,Citation28]; were not taking certain medications such as those with known cognitive side-effects or cerebral blood flow restrictions; and were right-handed. Ninety-seven percent of the sample self-identified as Caucasian and forty-four percent were male. provides a detailed characterization of the sample. All participants provided written consent before participating in the study. The study was conducted in accordance with the ethical standards set forth by the University of North Carolina Institutional Review Board and registered with the United States National Institutes of Health (clinicaltrials.gov; NCT01515098).
Table 1. Participant characteristics
Study design
A 6-month randomized, double-blind, placebo-controlled trial was conducted at the University of North Carolina at Chapel Hill Nutrition Research Institute (Kannapolis, NC, USA). Sample size was determined by a power analysis based on data from unpublished pilot studies. The results indicated a sample size of 34 per group would provide the power (.95) to detect a medium effect size (Cohen’s f = 0.25). Eighty-six participants were classified as experiencing mild cognitive decline based on the Montreal Cognitive Assessment (MoCA) score at screening (scores < 26)[Citation29]. These participants were assigned by the study coordinator to consume 35 g per day of either a lyophilized wild blueberry powder (n = 44) or a calorie-matched placebo (n = 42) using a simple randomization scheme based on a list generated from a random number generator (random.org). Participants who were not experiencing any age-related cognitive issues (n = 45) were assigned to a reference group. The reference group participated in the protocol wholly with the exception of the intervention. In addition, participants were age- and gender-matched within age groups: 65-69y, 70-74y, and 75-80y. All groups were required to complete nine visits to the lab across six months for cognitive and behavioral assessments, diet data collection, blood and urine collection, and various health and physical activity questionnaires.
Freeze-dried wild blueberries (Vaccinium augustifolium) were supplied by the Wild Blueberry Association of North America (Old Town, ME, USA). The fruit was cleaned and frozen with 24 h of harvest. It was then freeze-dried and shipped to FutureCeuticals (Momence, IL, USA) where it was milled into a powder. FutureCeuticals manufactured the placebo powder from a formula provided by the US Highbush Blueberry Council (Folsom, CA, USA). The placebo was composed of maltodextrin and fructose with artificial and natural flavors and colors added. We-Pack-It-All (Duarte, CA, USA) packaged the powders in polyfoil bags with 17.5 g of powder per packet and implemented the blind (coded packets D, E, F, or G). The blind was preserved in a sealed envelope and kept by an administrative staff person outside the lab. Nutrient content was analyzed semi-annually by Medallion Labs (Minneapolis, MN, USA). Nutrient analysis for the blueberry and placebo powders can be found in . Blueberry and placebo powders were also analyzed to confirm polyphenolic content ().
Table 2. Average daily calories and nutrients obtained from consumption of blueberry or placebo powder across the 6 months.
Table 3. Polyphenol content of blueberry and placebo powders.
Intervention participants were provided powder packets in insulated lunch bags that contained a month’s worth of packets each labeled with the date to be consumed (two packets a day) and organized in sequential order such that all the participant need do is pull the front two packets with the day’s date on them every day. Unused packets were returned for compliance counts. Compliance among those who completed the study was 96.9% with no difference between the two intervention groups (Blueberry 96.3%, Placebo 97.6%; ns). As noted in the recruitment flowchart (), those randomized to consume the blueberry powder were more likely to drop from the study due to issues with the powders, such as nausea or inability to tolerate (n = 10) compared to the placebo group (n = 3). Thus, we completed an intent-to-treat compliance analysis. Including participants who dropped, compliance reached 86.7% across the study with compliance being lower in the blueberry group (78.5%) compared to the placebo group (95.0%).
Background and lifestyle
Participants completed a medical history and demographics form at baseline. Any changes in health or circumstances were noted at each session. Life stress assessment was completed using the Stressful Life Events Scale adapted from Bieliauskas et al. [Citation30]. Physical activity was tracked using the Physical Activity Scale for the Elderly (PASE) [Citation31]. These variables were assessed as potential covariates as described in Results.
Montreal cognitive assessment (MoCA)
The MoCA was employed as a categorization tool: participants were assigned to intervention or reference groups based on their MoCA scores. The MoCA was developed to test for mild cognitive impairment (MCI) and Alzheimer’s disease (AD) quickly and reliably [Citation29]. It has been validated as a sensitive assessment tool for MCI and AD in the aging adult population [Citation32], and it consists of eight sections that test recall memory, executive function, attention, orientation, abstraction, visuospatial skills, and naming. Sections are weighted and were scored according to the procedures established by Nasreddine and colleagues [Citation29]. All MoCAs were scored by two research assistants who achieved a reliability score of 90%.
Wechsler adult intelligence scale, fourth edition (WAIS-IV)
The WAIS-IV (Pearson; San Antonio, Texas) is used to measure intelligence and cognition in persons 16–90 years of age. It is comprised of four index scales, each consisting of several core and supplemental subtests. Research assistants administered the ten core subtests to provide a a Full-Scale IQ score, as calculated using the WAIS-IV manual. The test took approximately 90 min to complete. Participants were tested at baseline to establish an IQ score for use as a covariate, if needed.
Cambridge neurological test automated battery (Cantab)
The CANTAB (Cambridge Cognition, Cambridge, UK) is a computerized battery of standardized cognitive assessments. The battery is designed to assess many cognitive functions such as working memory, processing speed, executive function, and sustained attention in one testing session with minimal equipment. The CANTAB is a useful testing instrument in that it saves the data as it is being collected and offers customizable reports that can be imported directly into a database, making database entry quick and reliable. Language ability does not skew results, and test administration requires minimal reading and instructions.
Participants completed five tests in the same order at baseline and outcome. The tests were part of the recommended battery for AD and have been shown to be sensitive to differences between MCI and AD (Egerhazi, Berecz, Bartok, & Degrell, 2006). The CANTAB battery was administered on a Sahara Slate i400 series tablet (TabletKiosk, Sand Dune Ventures, Inc), placed 30” from the edge of the table. The accompanying press pad was placed such that the bottom button was 15” from the edge, as indicated by a mark on the desktop. Participants were seated at a comfortable height within 30” of the screen and given instructions on proper hand placement. Individual tests are described in the order of administration below:
Motor Screening Task (MOT): This task is completed as a baseline to insure participants are able to properly interact with the screen. Participants are instructed to touch the flashing Xs as they appear on the screen.
Reaction Time (RTI): The Reaction Time task (RTI) is a measure of reaction time, movement time, and response accuracy for a condition in which the stimulus is predictable (simple reaction time) and for a condition in which the stimulus is unpredictable (5-choice reaction time). It familiarizes the participant with the press-pad and provides simple and choice reaction as well as movement times. The participant first holds down a button on the press pad. A yellow dot appears inside a circle on the screen. The participant releases the button as quickly as possible and touches the spot where the yellow dot appeared. The second section shows five circles on the screen, and the yellow dot could appear in any of them. The participant again releases the button as quickly as possible and presses the spot where the yellow dot appeared.
Spatial Working Memory (SWM): Spatial Working Memory is a working memory and planning task that incorporates an heuristic strategy. The participant sees gray boxes on the screen – some of which contain a hidden blue token that are revealed with a touch. The objective is to find and collect the blue tokens. The participant must remember which boxes have already been searched, as there is a penalty for opening the same box twice. The test starts with four boxes and increases incrementally to eight boxes.
Paired Associates Learning (PAL): Paired Associates Learning, a sensitive assessment of episodic memory, is a visual memory and learning task. It requires participants to identify where they have seen patterns on the screen. The participant is first shown a screen with 6 boxes. The boxes open sequentially in random order to reveal a series of abstract patterns. Then, each pattern, in turn, is shown in the middle of the screen, and the participants choose the spatial location where they recall that pattern being previously. The number of patterns to remember increases from 2 to 8 with each successful trial.
Rapid Visual Information Processing (RVP): Rapid Visual Information Processing measures the ability to sustain attention over a period of time, which requires both working memory and selective attention, and is a sensitive measure of frontal-parietal function. In this task, a white box appears in the center of the screen, and single digits appear inside the box in pseudo-random order. Participants are instructed to watch the digits change and press the button when a 3-digit target sequence appears. The task is presented in two parts. The practice involves one 3-digit target sequence. The test stage involves three 3-digit sequences. Increases in latency to respond to the target sequences and the participant’s ability to successfully identify the target have been negatively correlated with cognitive function [Citation33].
Data Reduction CANTAB outcome variables were generated internally by the software. To reduce the number of comparisons, one variable was chosen for each individual test based on type and previous findings in the literature [Citation35]; examination of further variables if needed would occur in posthoc analyses (e.g. a significant difference in errors would warrant further analyses of type of error). Variables used include SWM Total Errors, PAL Mean Trials to Success, and RVP Total Hits. To test our hypothesis regarding speed of processing, RVP Mean Latency was also tested as RVP was completed at the end of a long session, and because at this point, the participant was most likely to have been pushed past her or his tolerance levels (cognitive load capacity), it was the outcome variable most likely to be related to cognitive issues. To test the hypothesis that blueberry intervention would result in a change in cognitive abilities across the 6-month period, all variables were collapsed across the intervention period into delta scores for outcome minus baseline.
Electrophysiology procedures
Electrophysiological data were collected in a paradigm known as event-related potentials (ERP; electroencephalogram aka EEG time-locked to stimuli presentation). During a task designed to test recognition memory and speed of processing, the EEG was time-locked to the stimuli and recorded. To enable recording, the participant was fitted with a 128-sensor Geodesic Sensor Net (GSN; Electrical Geodesic, Inc., Eugene. OR); data were recorded from 128 sensors. Measurements were obtained of the participants’ head to insure the proper size net was used, but also to insure proper placement of the vertex, mastoid, and other landmark sensors. Application of the net required approximately 15 min and was well tolerated. Impedances were checked and corrected if necessary to below 50 kΩ, and the net was connected to a NetAmps 300 [Electrical Geodesic, Inc. (EGI), Eugene, OR]. The participant was seated at a desk 45 cm from a 19” Dell monitor in the participant room (separate from the control room). A Sony DCR-HC52 camera, positioned under the monitor and timelocked to the data being acquired, recorded the participant’s face during presentation of the stimuli. Stimuli were presented by EPrime 2.0 software (Psychology Software Tools, Inc., Sharpsburg, PA). Data were digitized at 250 Hz and stored by NetStation 4.4.2 (Electrical Geodesic, Inc., Eugene, OR) on a Mac computer. During recording the data were referenced to a single point at the vertex. The EGI system used a single-clock to timelock the presentation of the stimuli with the EEG continuous recording and the video capture. See Data Reduction for parameters used to generate the event-related potential (ERP).
Stimuli Abstract pictures purchased from Graphic River (graphicriver.net) were presented on the screen. They occupied the middle 50% of an 18 cm square on a white background with a 10 cm black border and were presented at a viewing angle of 68 degrees. All participants were shown the same picture 12 times for 1500 ms each with an inter-trial interval (ITI) of 500 ms to familiarize them with the standard picture. The familiarization period was followed by the presentation of 80 pictures randomly ordered by EPrime and including the standard, familiar image (50%) and trial-unique novel images (50%) for 1500 ms separated by inter-stimulus intervals of randomly varied length (500–1400 ms). Overhead fluorescent lights were off during the procedure with a small lamp burning for low ambient light. Prior research has shown that the recorded brain activity will differ during the viewing of familiar versus novel stimuli [Citation36] thereby affording the assumption of recognition memory for the familiar stimulus.
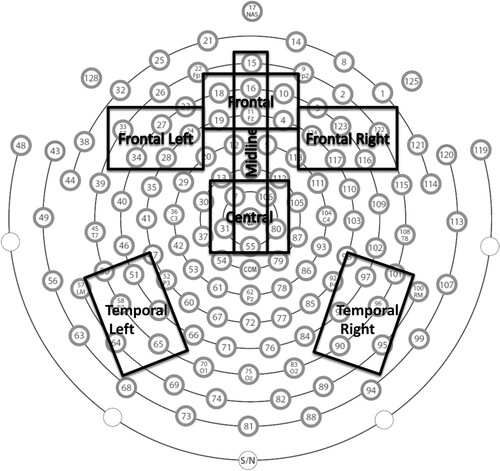
Data Reduction Recordings were filtered using lowpass (30 Hz) and highpass (0.3 Hz) filters and were segmented into individual segments corresponding to stimulus presentation and trial length. Bad channels were detected by the software using moving averages and a threshold of 250 µV. Segments that included more than 12 (>10%) bad channels were rejected. Remaining segments, in sync with session video recording, were visually examined for artifacts (eye blinks, movement, inattention, etc.), and further segments were rejected. All participants with greater than 12 good segments in each condition were included in further analyses. Next, individual channels within retained segments were replaced as necessary using spherical spline interpolation. Data were then re-referenced to the average and baseline corrected using the mean voltage during the 100 ms that preceded the stimulus presentation. Finally, trials were averaged within the condition (i.e. novel unique and familiar) and grand average waveforms were generated. The resulting waveforms were visually examined, and the windows of interest were determined. Individual statistics were generated by the software for peak amplitude and latency to peak amplitude within the negative deflection window (0-400 ms). Based on visual inspection of similarities of waveforms at individual sensors and a review of the relevant literature, sensor clusters of interest were chosen, and data were averaged across the sensors within a cluster. See for sensor clusters on the EGI 128-sensor net. Data were further reduced to test the hypothesis that blueberry consumption would result in change across the 6-month intervention period by computing a delta score of familiar minus novel for each outcome variable across each of the clusters, and then, subtracting outcome from baseline. To test the speed of processing hypothesis, the analyses were focused on the negative deflection (N200) that is related to attention processes. Recognition memory was measured in the same negative deflection: memory was assumed when processing as indicated by the negative deflection was more extensive for novel stimuli relative to familiar stimuli.
Statistics
All data were double-entered into Microsoft Excel, and these data sets were compared for differences. Any differences in entry were resolved by the staff. After the cleaning and verification process, databases were locked. Data were visually examined for outliers; none were found. All analyses were conducted using IBM SPSS Statistics (Version 27). Data were also assessed for adherence to the assumptions for linear analyses and were found to be normally distributed with homogenous variances and no multicollinearity.
To control for multiple tests of significance, outcome variables were entered into multivariate analyses of variance for ERP and CANTAB, in turn. Significant results were followed up with reduced models and posthoc analyes when three groups were analyzed. Significance was based on p < 0.05.
Results
Demographics and lifestyle variables were compared among groups using one-way analyses of variance (ANOVA). Means and standard deviations are shown in . Variables were analyzed for their appropriateness as covariates. Education and IQ were found to differ among groups when all three groups (Blueberry, Placebo, and Reference) were entered, F (2, 126) = 5.42, p = .006; F (2, 125) = 18.61, p = .000, respectively. Because the two variables are related, IQ was chosen for use as a covariate given that one might have a relatively high IQ without the benefit of a formal education, especially in this generation. A posthoc Tukey test revealed no difference in Education and IQ between the Blueberry and Placebo groups (p = .95, p = .58, respectively). Thus, IQ was only used as a covariate when all three groups were considered. By design, the score on the MoCA at screening was also found to differ between the intervention groups and the reference group.
CANTAB Analyses To control for multiple comparisons, change scores across the 6-month intervention period for the CANTAB variables of interest – SWM Total Errors, PAL Mean Trials to Success, RVP Total Hits, and RVP Mean Latency – were entered into a MANOVA with Group as the between-subjects factor. Analyses were run twice: once with all three groups controlling for IQ to determine the effect when compared to those with no cognitive decline and once with only the intervention groups to directly compare treatments.
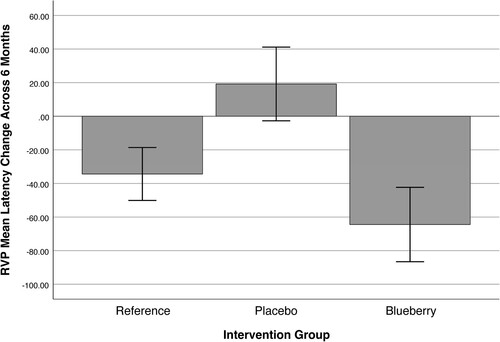
The results of the multivariate analyses of the three groups controlling for IQ scores revealed a between-subjects effect of group in RVP Mean Latency, F (2, 104) = 4.59, p = .012. None of the other dependent variables showed significance between groups. The multivariate model was not significant. The association is depicted in : the blueberry group’s latency (speed of processing) improved across the 6-month intervention period and was not significantly different than the change in latency for the reference group. When the intervention groups were analyzed without the reference group, the pattern of results was unchanged with the RVP Mean Latency now showing a greater between-subjects effect of group, F (1,64) = 7.01, p = .01.
Figure 3. Multivariate analyses of the change in rapid visual processing (RVP) latency scores across the 6-month intervention period by group. Negative numbers indicate an improvement in processing speed. Error bars indicate ± 2 SD.
ERP Analyses Following pre-processing (see Data Reduction), the delta scores for change across the 6-month intervention period were entered into a multivariate regression to predict, in turn, the latency and peak amplitude detected and averaged within the clusters () related to the negative deflection in the first 400 ms after the stimulus was presented on the screen.
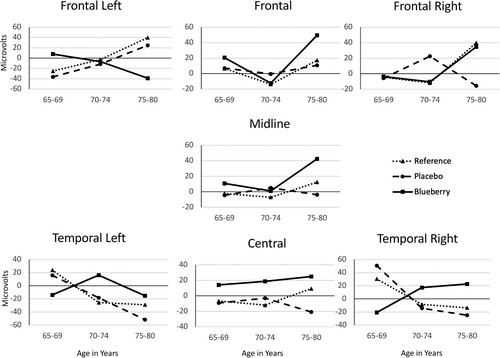
For latency to peak processing, there were no significant differences among the groups. However, the participants were matched by age and sex. Given the known decline of neuronal function as we age, the analyses were repeated with age group (65-69, 70-74, 75-80) as an additional factor. The results revealed a significant Group X Age Group interaction, V = .558; F (36, 332) = 1.495, p = .038, partial η2 = .14. Between-subjects analyses showed a significant corrected model effect at the frontal left and temporal left clusters, F (8, 88) = 2.35, p = .024, partial η2 = .18 and F (8, 88) = 2.14, p = .04, partial η2 = .16, respectively. Further, there was a main effect of age at the frontal and temporal left clusters, F (2, 88) = 3.23, p = .044, partial η2 = .07 and F (2, 88) = 3.46, p = .036, partial η2 = .07, respectively. There was also a Group X Age interaction at the frontal left cluster, F (4, 88) = 2.84, p = .029, partial η2 = .11. Posthoc analyses (Fisher’s LSD) of the main effect of age showed that 75-80y-olds performed worse than the 70-74y-olds as measured at the frontal cluster (p = .02). At the temporal left cluster, the 65-69y-olds outperformed the 75-80y-olds (p = .002). The Group X Age Group analyses are illustrated in .
Figure 4. Speed of processing (latency to peak processing) as measured electrophysiologically and analyzed by cluster (see ) across the scalp from front to back as Group X Age Group. Analyses revealed an overall significant Group X Age Group interaction effect (p = .038, partial η2 = .14). Posthoc analyses revealed the interaction to be significant at the frontal left and temporal left clusters: Frontal Left panel: p = .024, partial η2 = .18; Temporal Left panel: p = .04, partial η2 = .16; data at other clusters, ns. There was also a main effect of age: Frontal panel, (p = .044, partial η2 = .07); Temporal Left panel, (p = .036, partial η2 = .07); data at other clusters, ns.
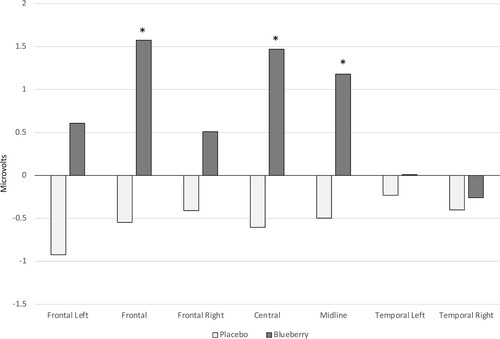
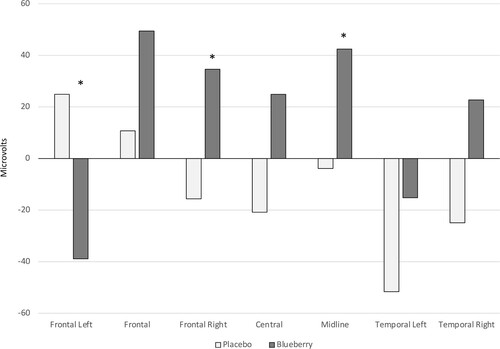
To further probe the interaction, a reduced model was employed analyzing only the 75- to 80-year-olds in the intervention groups. There was no significance in the multivariate tests. However, the between-subjects corrected model showed a significant main effect of group at the frontal left, frontal right, and midline clusters, F (1, 19) = 5.25, p = .034, partial η2 = .22; F (1, 19) = 5.34, p = .032, partial η2 = .22; and F (1, 19) = 5.23, p = .034, partial η2 = .22, respectively. The data are illustrated in .
Figure 5. Speed of processing (latency) performance as measured electrophysiologically from the stimuli onset to the peak amplitude of the negative deflection in the blueberry group compared to the placebo group in only the 75- to 80-year-olds. * p < .05
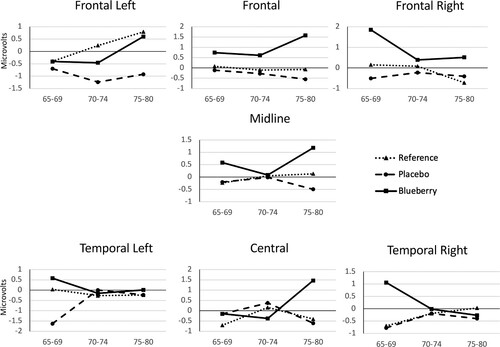
Multivariate tests of peak amplitude in the early window (0-250 ms) nearly reached significance, V = .285; F (18, 174) = 1.606, p = .063, partial η2 = .14. A main effect of group trended toward significance in the frontal and frontal right clusters, F (2, 94) = 2.67, p = .075, partial η2 = .06 and F (2, 94) = 2.28, p = .10, partial η2 = .05, respectively. Posthoc analyses revealed a trend in the frontal cluster toward the blueberry intervention participants outperforming the placebo participants (p = .07, 95% CI = −2.61 – .08). In analyses with the additional age group factor included, no significance was found in the multivariate statistics. However, similar trends were revealed: main effect of group neared significance at the frontal, frontal left, and frontal right clusters, F (2, 88) = 2.57, p = .083, partial η2 = .06; F (2, 88) = 2.54, p = .08, partial η2 = .06; and F (2, 88) = 2.62, p = .08, partial η2 = .06, respectively. Posthoc analyses revealed a trend in the frontal cluster toward the blueberry intervention participants outperforming the placebo participants (p = .08; 95% CI = −2.64 – .12). The Group X Age Group analyses are illustrated in .
Figure 6. Recognition memory performance (peak amplitude) as measured electrophysiologically and analyzed by cluster (see ) across the scalp from front to back as Group X Age Group. A main effect of group neared significance: Frontal panel, p = .083, partial η2 = .06; Frontal Left panel, p = .08, partial η2 = .06; and Frontal Right p = .08, partial η2 = .06. Data at other clusters, p > .10.
After examination of the graphs in and in consideration of the findings from the reduced model in the latency analyses, a reduced model was also run on the peak amplitude data for the 75- to80y-olds. There was no significance in the multivariate tests. However, the between-subjects corrected model showed a significant main effect of group at the frontal, central, and midline clusters, F (1, 19) = 4.39, p = .05, partial η2 = .19; F (1, 19) = 6.67, p = .018, partial η2 = .26; and F (1, 19) = 4.89, p = .04, partial η2 = .20, respectively. The blueberry group outperformed the placebo group in all instances ().
Discussion
In the randomized, placebo-controlled clinical trial reported here, we tested the hypothesis that consuming a flavonoid-rich, lyophilized wild blueberry powder across a 6-month period would improve components of cognition in those who had been experiencing self-reported cognitive decline relative to those experiencing self-reported cognitive decline and consuming a placebo powder. Relative to the placebo group, the results revealed an improvement in speed of processing in those consuming blueberries when tested in electrophysiological and behavioral protocols. Importantly, the effect was most robust in those 75–80 years of age.
Processing speed is a basic component that underlies all cognitive abilities. At its most basic cellular definition, processing speed is the rapidity with which the neural structures are able to move bits of chemical or electrical representations of information from one neuron to the next. At the behavioral level, processing speed is defined as the time required to complete a mental task, from which we can infer the differences in neuronal processing. In this study, there is evidence of improvements in processing speed by both the neural (ERP) and behavioral (CANTAB) definitions. These improvements may have a direct effect on one of the hallmarks of aging - cognitive slowing. As we age, the brain begins to operate at a slower pace (as does the body as a whole), and with that slowed pace comes a marked increase in the time required to complete a mental task [Citation37]. The improvement in processing speed shown in the data suggests a potential explanation for how the consumption of polyphenols was able to effectively stave off cognitive decline for approximately 2.5 years as shown in the Devore retrospective study [Citation24]. That is, consumption of a diet rich in polyphenols may support the integrity of the neural structures thereby supporting speed of processing and delaying the cognitive slowing that typically accompanies aging.
Importantly, the results also indicate that blueberry consumption is protective against cognitive fatigue. The CANTAB results showed a significant difference between the groups in the RVP latency data. In cognitive science, it is known that cognitive performance decreases as demand on mental faculties increases [e.g. Citation38]. Moreover, when employing test batteries such as the CANTAB, it is common practice to arrange the embedded tests as we did: the task difficulty increases as the participant tires. Thus, we assume that fatigue was an issue as the participants reached the end of a 3-hour session and were met with the most difficult of the CANTAB assessments – the RVP test. Those participants who had consumed blueberries for 6 months not only continued to perform well under cognitively fatiguing circumstances, but their performance matched that of the participants who had never experienced cognitive decline (reference group). Thus, the consumption of blueberries restored the performance of participants who had been experiencing cognitive issues to the level of the cognitively-intact, age-matched peers when in a cognitively fatigued state.
When measured electrophysiologically in an ERP paradigm, the improvement in cognitive abilities (processing speed and recognition memory) in the blueberry group was most evident in the 75–80 age group ( and ). High cognitive load can be considered from two perspectives. First, as is generally the case, it can be defined from the bottom-up when the large magnitude or complexity of the task at hand creates a cognitive load. Alternatively, cognitive load can be viewed from the top-down when the suboptimal status of the neural structures is such that the task at hand is not manageable. From both perspectives, there exists a mismatch between the demand presented by the environment and the integrity of the neural structures asked to meet that demand. In the case of the 75- to 80-year-olds, the integrity of the brain could be assumed to be more compromised by aging than in the younger ages. Much like the utility of the blueberry protocol to support the brain during the cognitive fatigue experienced at the end of a long testing session, blueberry consumption supported the brain and counteracted the characteristics of aging. Indeed, Gratton and colleagues found cocoa flavanols only improved cognitive performance when information processing demands were high in young, healthy adults [Citation26]. It is evidenced in the electrophysiological and behavioral data reported here that a diet high in polyphenols supports the brain when it is in a fatigued state and when it is in an aged state. It can further be extrapolated from these data that as the magnitude and complexity of a manageable cognitive load decreases with brain aging, the support provided by the consumption of blueberries will be increasingly evident.
Conversely, the blueberry protocol did not have an effect on those who were in their 60s and were already experiencing cognitive decline. Presumably, these participants were not experiencing what we would consider typical aging, but rather were showing the early signs of disease-state sequelae. Thus, it is possible that polyphenols support the typically-aging brain, but are not able to remediate the brain that is on a disease pathway. However, it is also possible that polyphenol consumption could be useful as a prophylactic against cognitive issues if started earlier in life. More research is needed on the prevention of cognitive decline, and the interventions should begin in middle age.
Other protective factors against cognitive decline have been established [Citation39–41] and were evident in the reference group whose members had not experienced significant cognitive issues. Those who had never experienced age-related cognitive decline differed from those who were experiencing cognitive decline on factors that are protective for brain health: IQ (p < 0.000), education (p = 0.004), and exercise (p = 0.07). That is, those with higher IQs who attained a higher level of education and were more physically active were more likely to maintain their brain health and thus, cognitive abilities across the years. This confirmatory analysis speaks to the influence of the environment and lifestyle on the aging brain and its ability to maintain structural integrity and thus, cognitive capacity across the lifespan given the proper environment and lifestyle.
Strengths and Limitations This randomized, placebo-controlled clinical trial is one of the longer and the largest to date. Since the inception of this research study, several other trials with smaller samples and/or shorter durations have been reported [Citation10,Citation11,Citation14,Citation16,Citation25]. A particular strength of the trial reported here is that we included behavioral testing as well as brain imaging and as such, were able to validate the findings both behaviorally and electrophysiologically. In addition, the inclusion of a reference group enabled us to compare those with healthful neural aging to those with declining brain health. In this study, background diet was not restricted before or during the study period. Thus, we cannot exclude the possibility that participation in the study may have prompted some participants to eat more healthfully. A major limitation exists in generalizability: this sample of volunteers consisted mostly of Caucasians and as such the results may not be generalizable to a more diverse population.
Conclusion and Future Directions The daily consumption of wild blueberries is shown here to improve speed of processing, especially in those 75–80 years of age. Wild blueberries have high levels of polyphenols that may be responsible for this effect. Preclinical work [e.g. Citation22,Citation42] informed the initial clinical work. Now, the results of this and other clinical work will enable the development of an animal model with the potential to determine mechanisms through which the consumption of blueberries supports neural functioning. In addition, as is the case with least squares statistical analyses, participants who did not respond to the treatment were lost in the analyses of the mean. Individual differences analyses with an eye to precision nutrition hypotheses will be employed to determine the factors, such as background diet, genetics, or lifestyle that would predispose one to not respond to the beneficial bioactives in the wild blueberry. The healthful properties of the wild blueberry are undeniably important to the human brain: characterizing the participants in a precision nutrition paradigm will enable us to document the sector of the population that will most benefit from these properties.
Abbreviations:
AD, Alzheimer’s disease; CANTAB, Cambridge Neurological Test Automated Battery; ERP, event-related potentials; fMRI, functional magnetic resonance imaging; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment
This trial is registered at clinicaltrials.gov #NCT01515098.
Acknowledgements
The authors thank the participants for their time and dedication to this project. In addition to the participants, the project had community support in the form of focus groups and participation on the BERRY Advisory Board, which were instrumental in finalizing the protocol and recruiting plans. We thank these community members for their time. It was a herculean effort by all. In addition, we thank Kim Adams, Christa Thomas, Andrea Armer, and a myriad of interns for their tireless work in support of this project. We also would like to thank Tondra Blevins and the other staff in the clinical suite at the UNC-CH Nutrition Research Institute for their support with sample collection. The UNC-CH Nutrition Research Institute deserves a special thank you for providing space and equipment for the project. Of course, we thank the Wild Blueberry Association of North America for providing the fruit.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Conflict of interest statement
No authors have any conflict of interests to disclose.
Data availability statement
Data described in the manuscript, the code book, and the analytic code will be made available upon request pending approval by the authors and signed agreements.
Additional information
Funding
Notes on contributors
Carol L. Cheatham
Carol L. Cheatham is a developmental cognitive neuroscientist with a joint appointment in the University of North Carolina at Chapel Hill's Department of Psychology & Neuroscience and the University of North Carolina at Chapel Hill's Nutrition Research Institute. Her research focuses on the effects of nutrition on brain development and function.
L. Grant Canipe
L. Grant Canipe III is an Assistant Professor at the Chicago School of Professional Psychology (Dallas campus), where he leads the scientific and research competency education for the PsyD in Clinical Psychology program. His research interests include cognitive aging and the creation of outreach programs to bridge the gap between the clinical and research communities.
Grace Millsap
Grace Millsap served as the project coordinator on this study. She is passionate about research with a specific interest in the effects of yoga on the function of the brain.
Julie M. Stegall
Julie M. Stegall After receiving her MSW at UNC Chapel Hill, Julie worked in many different settings with families, children, and individuals of all ages before eventually landing in research. Julie currently coordinates research studies at the UNC Nutrition Research Institute where she has worked since 2009.
Sheau Ching Chai
Sheau Ching Chai, PhD, RD is an Associate Professor at the University of Delaware. Dr. Chai's primary research includes the investigation of functional foods and chronic disease prevention as well as the development of interventions to improve nutrition-related health outcomes for older adults and postmenopausal women.
Kelly W. Sheppard
Kelly W. Sheppard graduated from the University of North Carolina at Chapel Hill in 2016 with a Ph.D. in Psychology. She investigated the role of omega-6 and omega-3 fatty acids in cognitive abilities in school-age children. She currently works on translational drug development research contracts.
Mary Ann Lila
Mary Ann Lila is Director of the Plants for Human Health Institute, North Carolina State University, North Carolina Research Campus. She holds the David H. Murdock Distinguished Professorship, and is a Professor in the Department of Food, Bioprocessing, and Nutrition Sciences. Lila has ongoing research projects in Australia, New Zealand, and various countries in Europe and Africa, and is Vice President of the Global Institute for BioExploration (GIBEX).
References
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060: Population estimates and projections. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf: U.S. Government Publishing Office; 2015.
- Roberts AW, Ogunwole SW, Blakeslee L, et al. The population 65 years and older in the United States: 2016. https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf: U.S. Government Publishing Office; 2018.
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia. Epidemiology. 2013;24(4):479–89.
- Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Advances in Nutrition. 2016;7(5):889–904.
- Knight A, Bryan J, Murphy K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in western countries? New insights and future directions. Ageing Res Rev. 2016;25:85–101.
- Siervo M, Shannon OM, Llewellyn DJ, Stephan Blossom CM, Fontana L, et al. Mediterranean diet and cognitive function: from methodology to mechanisms of action. Free Radical Biol Med. 2021;176:105–117.
- Ide K, Yamada H, Takuma N, Park M, Wakamiya N, Nakase J, et al. Green tea consumption affects cognitive dysfunction in the elderly: a pilot study. Nutrients. 2014;6(10):4032–42.
- Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the cocoa, cognition, and aging (CoCoA) study—a randomized controlled trial. Am J Clin Nutr. 2015;101(3):538–48.
- Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects With mild cognitive impairment. Hypertension. 2012;60(3):794–801.
- Bowtell JL, Aboo-Bakkar Z, Conway ME, Adlam A-LR, Fulford J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl Physiol Nutr Metab. 2017;42(7):773–779.
- Bensalem J, Dudonné S, Etchamendy N, Pellay H, Amadieu C, Gaudout D, et al. Polyphenols from grape and blueberry improve episodic memory in healthy elderly with lower level of memory performance: A bicentric double-blind, randomized, placebo-controlled clinical study. The Journals of Gerontology: Series A. 2019;74(7):996–1007.
- Jackson PA, Haskell-Ramsay C, Forster J, Khan J, Veasey R, Kennedy DO, et al. Acute cognitive performance and mood effects of coffee berry and apple extracts: A randomised, double blind, placebo controlled crossover study in healthy humans. Nutr Neurosci. 2021: 1–9.
- Socci V, Tempesta D, Desideri G, et al. Enhancing human cognition with cocoa flavonoids. Front Nutr. 2017;4(19.
- Boespflug EL, Eliassen JC, Dudley JA, Shidler MD, Kalt W, Summer SS, et al. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr Neurosci. 2018;21(4):297–305.
- McNamara RK, Kalt W, Shidler MD. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging. 2018;64:147–156.
- Miller MG, Hamilton DA, Joseph JA, Shukitt-Hale B, et al. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2018;57(3):1169–1180.
- Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58(7):3996–4000.
- Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA, et al. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103(5):730–4.
- Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, Shidler MD, Sadat-Hossieny S, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem. 2012;60(23):5736–42.
- Bookheimer SY, Renner BA, Ekstrom A, et al. Pomegranate juice augments memory and FMRI activity in middle-aged and older adults with mild memory complaints. Evid Based Complement Alternat Med. 2013;946298.
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19(18):8114–21.
- Casadesus G, Shukitt-Hale B, Stellwagen HM. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004 Oct-Dec;7(5-6):309–16.
- Malin DH, Lee DR, Goyarzu P, Chang Y-H, Ennis LJ, Beckett E, et al. Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition. 2011;27(3):338–42.
- Devore EE, Kang JH, Breteler MM, et al. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135–43.
- Whyte A, Cheng N, Fromentin E, Williams C, et al. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of Low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue™) in maintenance of episodic and working memory in older adults. Nutrients. 2018;10(6.
- Gratton G, Weaver SR, Burley CV, Low KA, Maclin EL, Johns PW, et al. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci Rep. 2020;10(1):19409.
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21.
- Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90.
- Nasreddine ZS, Phillips NA, Bedirian V. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
- Bieliauskas LA, Counte MA, Blandon GL. Inventorying stressing life events as related to health change in the elderly. Stress Med. 1995;11:93–103.
- Washburn RA, Smith KW, Jette AM, Janney CA, et al. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162.
- Koski L, Xie H, Finch L, Koski L, Xie H, Finch L. Measuring cognition in a geriatric outpatient clinic: rasch analysis of the Montreal cognitive assessment. J Geriatr Psychiatry Neurol. 2009;22(3):151–60.
- Chamberlain SR, Robbins TW, Winder-Rhodes S, Müller U, Sahakian BJ, Blackwell AD, Barnett JH, et al. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011;69(12):1192–203.
- Köstering L, Stahl C, Leonhart R, Weiller C, Kaller CP. Development of planning abilities in normal aging: differential effects of specific cognitive demands. Dev Psychol. 2014;50(1):293–303.
- Kuzmickiene J, Kaubrys G. Cognitive results of CANTAB tests and their change due to the first dose of donepezil may predict treatment efficacy in Alzheimer disease. Med Sci Monit. 2015;21:3887–3899.
- Wiebe SA, Cheatham CL, Lukowski AF. Infants’ ERP responses to novel and familiar stimuli change over time: implications for novelty detection and memory. Infancy. 2006;9(1):21–44.
- Cohen RA, Marsiske MM, Smith GE. Handbook of clinical neurology. Handb Clin Neurol. 2019;167:149–180.
- Holtzer R, Shuman M, Mahoney JR, Lipton R, Verghese J. Cognitive fatigue defined in the context of attention networks. Aging, Neuropsychology, and Cognition. 2010;18(1):108–28.
- Silva MVF, Loures C, Alves LCV, de Souza LC, Borges KBG, Carvalho M, et al. Alzheimer's disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33.
- Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ. 2015;39(2):55–62.
- Stern Y, Gurland B, Tatemichi TK. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA: The Journal of the American Medical Association. 1994;271(13):1004–10.
- Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005 Jan;81–1:313S–316S.