ABSTRACT
Objectives: The metabolic syndrome is associated with cardiovascular diseases and cognitive decline. The egg protein hydrolysate NWT-03 has shown to improve cardiovascular risk factors in humans. This study investigated whether NWT-03 also has an effect on cognitive function.
Methods: Men and women with the metabolic syndrome (n = 76) with a mean age of 60 ± 10 years participated in this randomized, double-blind, placebo-controlled, cross-over trial with an intervention (5 g/day NWT-03) and control period (5 g/day maltodextrin) of 4 weeks separated by a wash-out period of 2–8 weeks. Cognitive function was assessed with the anti-cue reaction time test (impulse control) and psychomotor vigilance test (sustained attention) at day 0, 2, and 27 of both periods. Serum brain-derived neurotrophic factor (BDNF) concentrations were measured at the start and end of both periods.
Results: NWT-03 consumption significantly improved the change (day 27 – day 0) in response times of the anti-cue reaction time test compared with the control period (P < 0.001), but not of the psychomotor vigilance test (P = 0.487). Serum BDNF concentrations of all subjects did not significantly change (P = 0.241).
Conclusion: NWT-03 has the ability to improve cognitive function within the executive function domain. The underlying mechanism warrants further research and could either be indirect via inhibition of dipeptidyl peptidase 4 (DPP4) or direct via passage of small peptides over the blood–brain barrier inducing local effects.
Trial registration: ClinicalTrials.gov identifier: NCT02561663.
Introduction
The metabolic syndrome is a condition in which characteristics of dyslipidemia, obesity, hyperglycemia, and hypertension occur simultaneously [Citation1]. It has been shown that this condition increases the risk of developing several pathologies, amongst others resulting in cardiovascular and neurodegenerative diseases [Citation2]. Regarding neurodegenerative disease development, not only the individual components are associated with cognitive decline, but particularly the totality of the metabolic syndrome condition [Citation3]. Fortunately, there are clear indications that these pathophysiological consequences of the metabolic syndrome are reversible [Citation4]. Moreover, to delay or even prevent the transition from metabolic syndrome into type 2 diabetes, cardiovascular diseases, or dementia, the beneficial effects of a healthy lifestyle including a healthy diet have been acknowledged [Citation5].
The egg protein hydrolysate NWT-03 is considered a functional food ingredient, which could contribute to cardiovascular health [Citation6]. The hydrolysate, which is obtained by enzymatic hydrolysis of the protein lysozyme isolated from hen egg whites, consists of di- and tri-peptides together with free amino acids. Because it has the capacity to inhibit the activity of both angiotensin-converting enzyme (ACE) as well as dipeptidyl peptidase 4 (DPP4), it has the potential to beneficially influence several cardiovascular risk factors [Citation7]. ACE inhibition is known to lower blood pressure [Citation8], while inhibition of DPP4 influences glucagon-like peptide 1 (GLP-1) formation, which contributes to an improvement of an impaired glucose metabolism and dyslipidemia [Citation9]. For NWT-03, Wang et al. showed in Zucker diabetic fatty rats that daily intake of 1 g/kg NWT-03 for 15 weeks attenuated renovascular damage [Citation7]. Moreover, Plat et al. have shown that consuming 2 g NWT-03 per day lowered blood pressure in subjects with mild hypertension [Citation6]. Finally, there are also indications that consumption of 5 g NWT-03 per day by overweight and obese subjects with impaired glucose tolerance or type 2 diabetes improved vascular function measured as carotid-to-radial pulse wave velocity and augmentation index, as well as several metabolic parameters such as triacylglycerol (TAG), glucose, insulin, and high-density lipoprotein (HDL) cholesterol concentrations [Citation10].
Since it has been postulated that peripheral vascular function reflects the condition of brain vascular function [Citation11], we here questioned whether NWT-03 consumption could potentially improve not only peripheral cardiovascular risk factors, but also central risk factors. Besides a possible indirect effect via the above-mentioned metabolic improvements, it might also be that NWT-03 affects brain vasculature directly since small peptides can cross the blood–brain barrier [Citation12]. Therefore, we here examined the effects of NWT-03 consumption on parameters reflecting cognitive function in subjects with the metabolic syndrome.
Methods
Subjects
Seventy-nine men and women aged 18–75 years with metabolic syndrome were recruited. Metabolic syndrome was defined by having three of the five following risk factors: body mass index (BMI) > 30 kg/m2 or waist circumference >94 cm (men) or >80 cm (women); TAG >1.7 mmol/L; HDL cholesterol <1.03 mmol/L (men) or <1.29 mmol/L (women); fasting plasma glucose >5.6 mmol/L; systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, according to the IDF criteria [Citation13]. These criteria were assessed on a first screening visit at the research facilities in the Metabolic Research Unit Maastricht after a 10-hours overnight fast. For this, a fasting blood sample was taken and anthropometrics and blood pressure were measured. Another fasting blood sample was taken on a second screening visit for analysis of the same biochemical parameters. The TAG, HDL cholesterol, and glucose concentrations of both screening visits were averaged to decide about eligibility. Besides a stable body weight (≤5% change in the past 3 months), eligibility was further evaluated according to the following exclusion criteria: pregnancy, smoker, significant acute or chronic illness such as diabetes or cardiovascular disease, medication targeting blood pressure or inflammation, drug use, and consumption >21 alcoholic beverages/week (men) or >14 alcoholic beverages/week (women). Informed consent was signed by all volunteers before screening. Study procedures were in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethical Committee of the University Hospital Maastricht/Maastricht University (METC153021). The study was registered in September 2015 at ClinicalTrials.gov (NCT02561663).
Study design
The study had a double-blind, placebo-controlled, cross-over design. The total study duration was 10–16 weeks with an experimental and control period of both 4 weeks, separated by a wash-out period varying from 2 to 8 weeks. Three visits were scheduled in each period, i.e. at day 0, 2, and 27, for anthropometrics, cognitive measurements, and blood sampling. Subjects were randomly allocated to start with either the placebo or NWT-03 intervention period based on a computer-generated randomization scheme created by an independent researcher.
Subjects were asked to withdraw from any strenuous physical activity or alcohol consumption two days before each measurement. In addition, they were provided with a standard meal which they had to consume on the evening preceding each measurement to minimize the meal effect. Subjects were instructed to travel to the research facilities by car or public transport in a fasted state (≥10 h). During the entire study period subjects were instructed to follow their usual dietary and exercise routine.
Study product
Subjects received sachets with 5 g dry powder (provided by Newtricious R&D, ‘s-Hertogenbosch, The Netherlands) containing either the active study product NWT-03 or a matching placebo (maltodextrin), which they had to consume daily, always at the same time before breakfast. The powders had to be dissolved in 200 mL water. The components of the active sachet were spray-dried egg protein hydrolysate (NWT-03; NIZO Food Research, Ede, The Netherlands), citric acid, flavoring, acesulfame K, sucralose, and quinine HCL. The components of the matched placebo sachet were maltodextrin, flavoring, citric acid, cloudifier, tartaric acid, malic acid, acesulfame K, sucralose, caramel E150a, and quinine HCL. Both subject and researcher were blinded since the active and placebo powders were similar in color and taste. At the start of each period, subjects were provided with a box containing 30 sachets that were labeled according to GMP guidelines. They were asked to return the used and unused sachets in order to monitor compliance.
Anthropometrics, blood pressure, and cognitive function
During every visit, first a fasting blood sample was taken and body weight and waist circumference were measured. Next, subjects rested for 5 min preceding the blood pressure measurements. Systolic and diastolic blood pressure, plus heart rate were measured at least 4 times using an automated blood pressure monitoring device (Omron M7 IntelliTMsense, Omron, Hoofddorp, The Netherlands). The first measurement was discarded and the average was calculated based on three final stable measurements.
Directly after consumption of the designated study product, subjects performed two cognitive tests in succession. The first test was the anti-cue reaction time test [Citation14], which is a measure for impulse control and part of the executive function domain. In short, an informative cue appeared before the target stimulus with a variable interval ranging from 100 to 850 ms. In this 4-choice reaction time task, subjects had to respond as fast as possible to the location of the target stimulus by pressing 1 of 4 keys using their index and middle finger of both hands. In the uncued condition, the cue appeared at all four target locations, whereas in the cued condition, the cue appeared on the opposite site of the target location. Subjects first received 20 practice trials, followed by 200 experimental trials divided in four blocks of 50 trials with a 30 s break in between. The total duration of the anti-cue task was 20 min. Response times and response errors (pressing the wrong response key) were measured. Mean response times (only of correct trials) were calculated for each subject as a function of preparation interval (5 categories: 100, 150, 250, 450, 850 ms) and cue type (cued, uncued). Response errors occurred on 1.06% of all trials. Prior to calculating mean response times, we removed trials with outliers. Trials were excluded from analyses when the response time was below 150 ms (anticipation) or above 3000 ms (delayed response), or when the response time deviated more than 3 standard deviations (SD) from the subject’s overall mean response time. Using these outlier criteria, 1.36% of the trials were removed. The second test was the psychomotor vigilance test, which is a measure for sustained attention and part of the attention domain. Here, subjects had to respond as fast as possible when a stimulus was presented by pressing the space bar (a simple 1-choice reaction time test). A new stimulus was presented after each response with a variable interval ranging from 1–10 s. In total 80 stimuli were presented. The total duration of this task was 8 min. After removal of outliers (0.42% of all trials), we calculated the average response times for each subject as a function of preparation interval (5 categories with each 16 trials: 1–2 s, 3–4 s, 5–6 s, 7–8 s, 9–10 s). Note that no response errors could occur because there was only one possible response.
Biochemical analyses
Blood was collected in serum STT-II advance tubes (Becton Dickinson). After a minimum of 30 min clotting time at room temperature, tubes were centrifuged at 1300 g for 10 min at 21°C to obtain serum. In addition, on day 0, blood was collected in sodium fluoride (NaF) tubes (Becton Dickinson). Directly after sampling, these NaF tubes were centrifuged at 1300 g for 10 min at 4 °C to obtain plasma. Subsequently, serum and plasma were portioned into aliquots, frozen in liquid nitrogen, and stored at −80°C until analyses.
Brain-derived neurotrophic factor (BDNF) was measured in serum samples of day 0 and 27 of each period by an enzyme-linked immunosorbent assay (Duo Kit ELISA, R & D Systems, Minneapolis, MN, United States) according to manufacturer’s instructions. Fasting TAG (GPO Trinder, Sigma-Aldrich Corp., St. Louis, MO, United States), total cholesterol (CHOD-PAP method; Roche Diagnostic Systems), and HDL cholesterol concentrations (precipitation method; Roche Diagnostics, Mannheim, Germany) were measured in serum samples at baseline. LDL cholesterol was calculated using the Friedewald equation [Citation15]. Fasting glucose concentrations (Glucose HK CP, Horiba ABX, Montpellier, France) were measured in NaF plasma samples at baseline.
Statistical analysis
Data are presented as mean ± SD for numerical variables and number of subjects (%) for categorical variables. For both cognitive parameters as well as serum BDNF concentrations, baseline values at the start of the placebo and intervention periods were compared using paired-samples t-test. In order to examine changes in cognitive performance, differences in response times (day 27 minus day 0; under the same conditions, i.e. same preparation interval and cue type) were analyzed with linear mixed models. For this, the changes in response times were used as dependent variable with treatment, period, preparation interval, cue type, and all 2-, 3-, and 4-way interactions [treatment*period*preparation interval*cue type] as fixed factors. A top-down approach was used to simplify this model as much as possible, in which the highest order term was tested first. In case not significant, it was removed from the model and the procedure was repeated for the remaining variables until only significant variables were left in the model or until the model only included treatment (which should remain in the model to answer the research question). Lower-order terms of a significant interaction term were not tested (so-called hierarchical principle). This approach was applied to both the anti-cue reaction time test and the psychomotor vigilance test, but cue type was not part of the latter model as it was not part of the test. The same linear mixed model was used to examine changes in serum BDNF concentrations, but only with treatment, period, and its interaction [treatment*period] as fixed factors. Associations between changes in cognitive parameters and changes in serum BDNF concentrations were evaluated with Pearson correlation analysis. For all tests, two-sided p-values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corporation, Armonk, NY, United States).
Results
Study characteristics and subjects
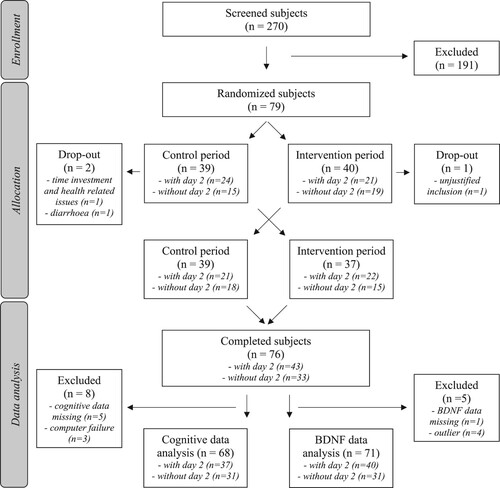
Of the 270 subjects that were screened for inclusion, 79 eligible subjects were randomized to start either with the NWT-03 or the control products (). During the first experimental period, two subjects dropped out due to study-unrelated health issues and we noticed one invalid inclusion that was removed from the study. In total, 76 subjects completed the entire study. However, 8 subjects were excluded from the cognitive data analyses because of missing data. Due to logistic reasons, we were only able to perform the measurements at day 2 in approximately half of the subjects, while we performed the measurements at day 0 and 27 in all subjects. This change from the initial protocol was random and based on subjects’ availability and logistics in our study planning. Only data from the 37 subjects that completed all measurements (day 0, 2, and 27) as initially planned in the approved study protocol were used for statistical analyses of the cognitive function tests. The cognitive data of the remaining 31 subjects that did not complete the cognitive measurements at day 2 were also statistically analyzed but reported separately in the supplemental data. The reason to not combine the two groups with and without cognitive measurements at day 2 lies in the fact that multiple testing of cognitive performance results in a learning curve [Citation16], and, hence, including the data collected at day 2 could potentially influence the results at day 27. As this learning effect is not an issue for serum BDNF concentrations, BDNF data were reported for the total study population. However, to be complete and able to link BDNF concentrations to cognitive performance, BDNF concentrations were also reported in the two subgroups according to the cognitive tests. BDNF data was missing from 1 subject and data from 4 other subjects were excluded due to outliers, which means that data from 71 subjects were used for BDNF analyses. The mean age (± SD) of the study population that completed all measurements (day 0, 2, and 27) was 60 ± 10 years with a mean BMI of 31.9 ± 3.6 kg/m2. All baseline subject characteristics are presented in . Baseline characteristics of the subjects that did not complete the cognitive measurements on day 2 can be found in Supplemental Table 1. Baseline characteristics did not significantly differ between these two subgroups indicating that there was no selection bias for specific subgroups.
Table 1. Baseline subject characteristics.
Anti-cue reaction time test
The response times at the start of both periods were not significantly different (P = 0.390). The final statistical model to evaluate the effect of NWT-03 versus placebo contained treatment and period, but not the interaction term, indicating that the treatment effect did not significantly depend on period. NWT-03 consumption had a significant effect on response time (P < 0.001; ). After the intervention with NWT-03, the mean decrease in response time (−17.8 ± 51.4 ms) was significantly larger as compared to the mean decrease observed during the control period (−3.8 ± 56.5 ms). The results of the subjects that did not complete the cognitive measurements on day 2 can be found in Supplemental Table 2. Within this group, the interaction treatment*period was significant (P = 0.013) and, therefore, the treatment effect is reported separately for each period. In this group, NWT-03 consumption only had a significant effect on response time in the second experimental period (P = 0.001), but not in the first experimental period (P = 0.176). In the first experimental period the mean changes in response times were not significantly different between the control (−3.6 ± 31.9 ms) and NWT-03 intervention period (5.5 ± 30.9 ms). However, in the second experimental period, the decrease in response time (−8.3 ± 33.6 ms) was on average significantly different from the increase in the control period (15.2 ± 35.0 ms).
Table 2. Average response times (ms) of the anti-cue reaction time test before and after the control and intervention period (n = 37).
Psychomotor vigilance test
For the psychomotor vigilance test, the response times at the start of both periods were also not significantly different (P = 0.490). For this cognitive test, the final statistical model contained treatment and period, but not the interaction term, indicating that the treatment effect did not significantly depend on period. After the intervention period with NWT-03, the change in response times (11.8 ± 53.6 ms) was not significantly different from the change during the control period (8.0 ± 67.7 ms; P = 0.487; ). The results of the subjects that did not complete the cognitive measurements on day 2 can be found in Supplemental Table 3. In this group, NWT-03 consumption had a significant effect on response time (P = 0.001), which did not significantly depend on period. After the intervention with NWT-03, the change in response time (3.1 ± 43.2 ms) was significantly different from the change after the control period (16.9 ± 39.7 ms).
Table 3. Average response times (ms) of the psychomotor vigilance test before and after the control and intervention period (n = 37).
Serum BDNF concentrations
Serum BDNF concentrations at the start of both periods were not significantly different (P = 0.288). The final statistical model evaluating the effect of NWT-03 versus placebo only contained treatment. NWT-03 consumption did not have a significant effect on serum BDNF concentrations (P = 0.241; ). After the intervention with NWT-03, the change in serum BDNF concentrations did not significantly differ between the intervention (931 ± 6850 pg/ml) and the control period (−445 ± 7071 pg/ml). Also in the subgroup that completed all cognitive measurements at day 0, 2 and 27, the change in serum BDNF concentrations did not significantly differ (P = 0.776; ) between the intervention (2 ± 6529 pg/ml) and control period (426 ± 6763 pg/ml). No significant correlations were found between changes in serum BDNF concentrations and changes in response time in the anti-cue reaction time test (r = −0.147; P = 0.242) or psychomotor vigilance test (r = −0.002; P = 0.985) within the intervention period. The results of the subjects that did not complete the cognitive measurements on day 2 can be found in Supplemental Table 4. Also in this subgroup, the changes in serum BDNF concentrations were not significantly different between the control (−1569 ± 7407 pg/ml) and NWT-03 intervention period (2131 ± 7172 pg/ml; P = 0.0503).
Table 4. Brain-derived neurotrophic factor concentrations (pg/ml) before and after the control and intervention period of all subjects (n = 71) and the subjects that completed all cognitive measurements (n = 40).
Discussion
In this randomized, double-blind, placebo-controlled study in men and women with the metabolic syndrome, consumption of the protein hydrolysate NWT-03 significantly decreased the response time in the anti-cue reaction time test compared to the control group, but did not show a significant effect on the change in response time in the psychomotor vigilance test, or serum BDNF concentrations.
The decrease in response time in the anti-cue reaction time test reflects an improved anti-cue performance. It relies on proactive cognitive control, rather than reactive cognitive control, and involves a switching mechanism that balances inhibition and excitation to promote speeded finger responses on the hand opposite to the site of the cue [Citation14]. Importantly, response inhibition is a key underlying mechanism because the response options on the hand congruent with side of the cue are suppressed [Citation17, Citation18]. Such cognitive control functions, that can be positioned within the executive function domain, have a wide clinical validity [Citation19]. It has been described that they decline in some neuropsychological pathologies (for example, Parkinson; [Citation20]) and with ageing [Citation18]. Interestingly, we found that 4 weeks consumption of the protein hydrolysate NWT-03 supports improvement of these cognitive control functions. However, this improvement, an effect size of 14 ms, is relatively small in comparison with effect sizes due to, for example, healthy ageing: mean anti-cue performance of 50–60 years old individuals is 90 ms slower than that of 18–25 years old individuals, and this increases in 70–80 years old individuals to 262 ms [Citation18]. In comparison proportionate to ageing, NWT-03 slows down cognitive decline with 5 years. In contrast to the improved score in the executive domain, the speed of responses in the psychomotor vigilance test, which represents the (sustained) attention domain, did not significantly change over time. The question is why executive function, as measured via the anti-cue reaction time test, did appear responsive to NWT-03 consumption, and attention, as measured via the psychomotor vigilance test, did not. The anti-cue reaction time test is a much more demanding, higher order cognition test [Citation21, Citation22] than the relatively easy psychomotor vigilance test, which requires a simple detection response [Citation23]. Cognitive tests within the executive function domain need more complex operations compared to tests that require simple transformations and reactions [Citation24]. Arguably, the involvement of different cognitive mechanisms in the two cognitive tests may be contributing to the present set of discrepant findings.
An intriguing question is how the effects of the protein hydrolysate NWT-03 on executive functioning as measured with the anti-cue reaction time test can be explained. First, it might that the effect of NWT-03 on cognition act via inhibition of DPP4. Pharmacological DPP4 inhibitors are known to improve insulin sensitivity in liver, muscle, and adipose tissue [Citation25]. At least in adipose tissue, these inhibitors act via interfering with the PI-3 K/AKT/GLUT4 insulin signaling pathway [Citation26], which can also be found in the central nervous system [Citation27, Citation28]. Even though DPP4 inhibitors cannot cross the blood–brain barrier under normal conditions, several studies indicate beneficial effects of these inhibitors on brain complications [Citation29] and improved cognitive outcomes upon DPP4 inhibition [Citation30]. As the brain is also sensitive to insulin [Citation31], which can cross the blood–brain barrier, it is suggested that peripheral insulin concentrations are the potential link with cognitive performance [Citation32], particularly in the memory domain [Citation33].
In addition to this indirect effect of NWT-03 via changes in peripheral insulin concentrations on cognition, there also might be a second, more direct pathway. As discussed earlier, a second explanation might be found in the assumption that the small protein fragments in NWT-03 can possibly cross the blood–brain barrier [Citation12], and directly affect local brain processes involved in cognitive performance.
As a third explanation, we speculate that it could also be possible that effects were mediated via changing BDNF concentrations. We have reported earlier that a number of other nutritional interventions indeed have the capacity to affect circulating BDNF concentrations [Citation34] and, subsequently, cognition [Citation35]. Particularly the link with inhibiting DPP4 becomes again interesting since DPP4 inhibition was found to increase BDNF concentrations in the brain, at least in mice [Citation36]. However, in our study the underlying mechanism of NWT-03 cannot be attributed to a change in serum BDNF concentrations since BDNF concentrations were not significantly changed and did not correlate with changes in cognitive performance. Of course, we cannot exclude the possibility that there was a change in BDNF concentrations in brain regions responsible for executive function.
Besides various animal studies, there are a number of human studies that showed positive effects of other protein hydrolysates on cognition. For example, an acute low-dose supplementation with a tryptophan-rich egg protein hydrolysate in male and female students improved attention in the psychomotor vigilance test [Citation37]. Another study with the same hydrolysate for 19 days in healthy women decreased reaction times in both simple and complex reaction time tests, indicating an improved sustained attention [Citation38]. Finally, 2 g tryptophan-rich egg protein hydrolysate also prevented fatigue in older women [Citation39]. This tryptophan-rich egg protein hydrolysate increased the ratio of L-tryptophan to other large neutral amino acids (TRP:LNAA), thereby promoting the passage of tryptophan over the blood–brain barrier and enhance serotonin synthesis in the brain. This neurotransmitter plays a role in cognitive functions, such as attention and information processing [Citation40]. Furthermore, hydrolysates different than egg protein such as silk fibroin hydrolysate has also been described to improve cognitive functions including attention [Citation41]. Mechanisms of action that have been postulated to explain the effects of this silk fibroin hydrolysate on cognition were an increased blood circulation and glucose uptake in the brain, protection from reactive oxygen species, and prevention of protein aggregation. In future studies, it would be interesting to explore these mechanisms regarding NWT-03 as well.
In contrast to the subjects that completed all measurements, the subjects that did not perform an extra cognitive measurement at day 2, so in between the test days at the start and the end of the 4 weeks intervention period, NWT-03 consumption significantly decreased the response time only in the second experimental period of the anti-cue reaction time test, and in the psychomotor vigilance test. The anti-cue reaction time test is a form of motor training, which can lead to structural changes in the brain, at least in mice [Citation42]. It could be that the extra cognitive measurement on day 2 enhanced the effect of NWT-03 or vice versa. Another possible explanation for the treatment effect in the second, but not in the first experimental period in subjects that did not perform an extra cognitive measurement, is that NWT-03 has a prolonged effect. This could result in a carry-over effect despite a wash-out period of minimum two weeks. Regarding the psychomotor vigilance test, the extra test for the subjects that completed all measurements could have diminished the beneficial effects of NWT-03 on psychomotor vigilance test performance due to lack of engagement. Repeated testing contributes to reduced task engagement [Citation43], which is positively correlated with performance [Citation44]. However, this seems unlikely as the findings of the anti-cue reaction time test do not follow the same pattern.
In conclusion, the egg protein hydrolysate NWT-03 has shown its potential to improve cognitive function in men and women with the metabolic syndrome, specifically within the executive function domain. Besides the beneficial metabolic effects and improvements in the peripheral vascular function that have been shown earlier, the current study suggests an additional improvement of central risk factors. Further research is needed to examine whether this effect of NWT-03 on the brain has a direct or indirect origin.
Acknowledgements
We thank Sanne van der Made for critically reading the study protocol and the manuscript. We thank Resy Smeets, Dorien Reijnders, and Bibi Waterval for their practical work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
Notes on contributors
Elske Gravesteijn
Elske Gravesteijn received her PhD from the department of Nutrition and Movement Sciences, NUTRIM School of Nutrition and Translational Research in Metabolism of Maastricht University. Her research interest is the effects of diet on metabolic health, vascular function and cognition.
Jos J. Adam
Jos J. Adam is an Associate Professor of Movement Sciences at Maastricht University. He works at the department of Nutrition and Movement Sciences and is involved in research projects within the Faculty of Health, Medicine and Life Sciences (FHML) and the Faculty of Psychology and Neurosciences (FPN). His expertise lies within the field of cognition and action in a life span perspective. In terms of teaching, he contributes to the Biomedical Sciences program at Maastricht University.
Ronald P. Mensink
Ronald P. Mensink is Professor of Molecular Nutrition at Maastricht University with emphasis on lipid metabolism. His research interest is the relationships between nutritive and nonnutritive diet components with chronic metabolic stress, in particular cardiovascular disease.
Bjorn Winkens
Bjorn Winkens is an Associate Professor of Statistics at Maastricht University. He works at the department of Methodology and Statistics and is involved in many research projects within Care and Public Health Research Institute (CAPHRI) and other research schools. His expertise lies within the field of (linear) mixed model analysis. In addition, he is involved as a teacher and/or course coordinator in several education programs within the Faculty of Health, Medicine and Life Sciences (FHML).
Jogchum Plat
Jogchum Plat is Professor Physiology of Nutrition at Maastricht University. He works at the department of Nutrition and Movement Sciences and is involved in many research projects within NUTRIM School of Nutrition and Translational Research in Metabolism. His expertise lies within the field of exploring the relationship between nutrition and health. In addition, he is teaching within the biomedical domain of education programs as part of the Faculty of Health, Medicine and Life Sciences (FHML).
References
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7.
- Assuncao N, Sudo FK, Drummond C, de Felice FG, Mattos P. Metabolic syndrome and cognitive decline in the elderly: a systematic review. PLoS One. 2018;13(3):e0194990.
- Rouch I, Trombert B, Kossowsky MP, Laurent B, Celle S, Ntougou Assoumou G, et al. Metabolic syndrome is associated with poor memory and executive performance in elderly community residents: the PROOF study. Am J Geriatr Psychiatry. 2014;22(11):1096–104.
- Giugliano D, Ceriello A, Esposito K. Are there specific treatments for the metabolic syndrome? Am J Clin Nutr. 2008;87:8–11.
- Al-Qawasmeh RH, Tayyem RF. Dietary and lifestyle risk factors and metabolic syndrome: literature review. Curr Res Nutr Food Sci J. 2018;6(3):594–608.
- Plat J, Severins N, Morrison S, Mensink RP. Effects of NWT-03, an egg-protein hydrolysate, on blood pressure in normotensive, high-normotensive and mild-hypertensive men and women: a dose-finding study. Br J Nutr. 2017;117(7):942–50.
- Wang Y, Landheer S, van Gilst WH, van Amerongen A, Hammes HP, Henning RH, et al. Attenuation of renovascular damage in Zucker diabetic fatty rat by NWT-03, an egg protein hydrolysate with ACE- and DPP4-inhibitory activity. PLoS One. 2012;7(10):e46781.
- Gerstein HC. Cardiovascular and metabolic benefits of ACE inhibition. Moving beyond blood pressure reduction. Diabetes Care. 2000;23(7):882–3.
- Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol. 2020;11:178.
- Plat J, Severins N, Mensink RP. Improvement of pulse wave velocity and metabolic cardiovascular risk parameters through egg protein hydrolysate intake: a randomized trial in overweight or obese subjects with IGT or T2DM. J Funct Foods. 2019;52:418–23.
- Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plast. 2018;4(1):65–79.
- Banks WA, Kastin AJ. Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci. 1996;59(23):1923–43.
- International Diabetes Federation. IDF consensus worldwide definition of the metabolic syndrome. July 2020 [cited 2021 Dec 8]. Available from: https://idf.org/our-activities/advocacy-awareness/resources-and-tools/60:idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html.
- Adam JJ, Jennings S, Bovend'Eerdt TJ, Hurks PP, Van Gerven PW. Switch hands! Mapping temporal dynamics of proactive manual control with anticues. Acta Psychol. 2015;161:137–44.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):449–502.
- Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118.
- Emmerling F, Duecker F, de Graaf TA, Schuhmann T, Adam JJ, Sack AT. Foresight beats hindsight: the neural correlates underlying motor preparation in the pro- anti-cue paradigm. Brain Behav. 2017;7(5):e00663.
- Van Gerven PW, Hurks PP, Bovend'Eerdt TJ, Adam JJ. Switch hands! Mapping proactive and reactive cognitive control across the life span. Dev Psychol. 2016;52(6):960–71.
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–68.
- Adam JJ, van Houdt H, Scholtissen B, Visser-Vandewalle V, Winogrodzka A, Duits A. Executive control in Parkinson's disease: effects of dopaminergic medication and deep brain stimulation on anti-cue keypress performance. Neurosci Lett. 2011;500(2):113–7.
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci. 2009;4(1):1–9.
- Nestler EJ, Hyman SE, Holtzman DM, Malenka RC. Higher cognitive function and behavioral control. molecular neuropharmacology: a foundation for clinical neuroscience, 3e. New York (NY): McGraw-Hill Education; 2015.
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. 2001;35:146–60.
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30.
- Rameshrad M, Razavi BM, Ferns GAA, Hosseinzadeh H. Pharmacology of dipeptidyl peptidase-4 inhibitors and its use in the management of metabolic syndrome: a comprehensive review on drug repositioning. Daru. 2019;27(1):341–60.
- Liu Z, Xu L, Xing M, Xu X, Wei J, Wang J, et al. Trelagliptin succinate: DPP-4 inhibitor to improve insulin resistance in adipocytes. Biomed Pharmacother. 2020;125:109952.
- Gabbouj S, Ryhanen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, et al. Altered insulin signaling in Alzheimer's disease brain – special emphasis on PI3K-Akt pathway. Front Neurosci. 2019;13:629.
- Zaulkffali AS, Md Razip NN, Syed Alwi SS, Abd Jalil A, Abd Mutalib MS, Gopalsamy B, et al. Vitamins D and E stimulate the PI3K-AKT signalling pathway in insulin-resistant SK-N-SH neuronal cells. Nutrients. 2019;11(10):2525.
- Darsalia V, Johansen OE, Lietzau G, Nystrom T, Klein T, Patrone C. Dipeptidyl peptidase-4 inhibitors for the potential treatment of brain disorders; a mini-review with special focus on linagliptin and stroke. Front Neurol. 2019;10:493.
- Dumbrill JL, Moulton CD. Effects of incretin-based therapies on neurocognitive function in humans: a systematic review of the literature. Prim Care Diabetes. 2018;12(1):51–8.
- Milstein JL, Ferris HA. The brain as an insulin-sensitive metabolic organ. Mol Metab. 2021;52:101234.
- Strachan MW. Insulin and cognitive function in humans: experimental data and therapeutic considerations. Biochem Soc Trans. 2005;33(5):1037–40.
- Cetinkalp S, Simsir IY, Ertek S. Insulin resistance in brain and possible therapeutic approaches. Curr Vasc Pharmacol. 2014;12(4):553–64.
- Gravesteijn E, Mensink RP, Plat J. Effects of nutritional interventions on BDNF concentrations in humans: a systematic review. Nutr Neurosci. 2021;25(7):1425–36.
- Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–93.
- Yang D, Nakajo Y, Iihara K, Kataoka H, Yanamoto H. Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res. 2013;1517:104–13.
- Markus CR, Verschoor E, Firk C, Kloek J, Gerhardt CC. Effect of tryptophan-rich egg protein hydrolysate on brain tryptophan availability, stress and performance. Clin Nutr. 2010;29(5):610–6.
- Mohajeri MH, Wittwer J, Vargas K, Hogan E, Holmes A, Rogers PJ, et al. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br J Nutr. 2015;113(2):350–65.
- Gibson EL, Vargas K, Hogan E, Holmes A, Rogers PJ, Wittwer J, et al. Effects of acute treatment with a tryptophan-rich protein hydrolysate on plasma amino acids, mood and emotional functioning in older women. Psychopharmacology. 2014;231(24):4595–610.
- Riedel WJ, Sobczak S, Schmitt AJ. Tryptophan modulation and cognition. Adv Exp Med Biol. 2003;527:207–13.
- Kang YK, Lee BY, Bucci LR, Stohs SJ. Effect of a fibroin enzymatic hydrolysate on memory improvement: a placebo-controlled, double-blind study. Nutrients. 2018;10(2):233.
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462(7275):915–9.
- Lumsden J, Skinner A, Coyle D, Lawrence N, Munafo M. Attrition from web-based cognitive testing: a repeated measures comparison of gamification techniques. J Med Internet Res. 2017;19(11):e395.
- Matthews G, Warm JS, Smith AP. Task engagement and attentional resources. Hum Factors. 2017;59(1):44–61.