ABSTRACT
Objectives
The 8-week Micronutrients for ADHD in Youth (MADDY) randomized controlled trial (N = 126, age 6–12) of broad-spectrum multinutrients for ADHD with emotional dysregulation found 3 times as many responders with multinutrients (54%) compared to placebo (18%) by Clinical Global Impression-Improvement (CGI-I). Our primary aim for this analysis tests the hypothesis that those with poor overall diet quality at baseline benefit more. The second aim is to explore whether specific components of diet quality moderate treatment response.
Methods
124 children (69 multinutrients, 55 placebo) had diet quality assessed using the Healthy Eating Index-2015 (HEI-2015). For each potential moderator, the outcome CGI-I at week 8 (RCT-end), was modeled two ways: (1) as a dichotomous variable: responder/non-responder, with responders defined by a rating of 1 or 2 ‘very much’ or ‘much improved,’ all else equals non-responder using logistic regression, and (2) as a dimensional improvement outcome from 1 = very much improved to 7 = very much worse, using linear regression.
Results
HEI-2015 total score did not moderate treatment response [odds ratio = 1.00 (95% CI: 0.90,1.10), p = 0.984] or improvement [β = −0.01 (95% CI: −0.06,0.04), p = 0.648]. However, total vegetable intake moderated level of improvement in exploratory analysis [β = −0.48 (95% CI: −0.82, −0.13), p = 0.007]: those with higher baseline vegetable intake showed greater benefit from multinutrients compared to placebo.
Conclusions
Multinutrients may benefit children with ADHD and irritability regardless of overall diet quality. The finding that higher baseline vegetable intake may improve response to multinutrients deserves further exploration, including dietary effect on gut microbiota and absorption of multinutrients and parental factors.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder affecting up to 10% of children in the United States (US) [Citation1]. ADHD includes symptoms of inattention and/or hyperactivity/impulsivity that interfere with functioning in two or more settings for at least 6 months [Citation2]. Up to half of children with ADHD also exhibit symptoms of emotional dysregulation such as anger, irritability, rage, and low tolerance for distress [Citation3]. The etiology of ADHD is multi-factorial, involving genetic, environmental, social, and neurobiological factors [Citation4]. Dietary composition is an increasingly studied environmental factor with emerging studies finding ADHD associated with a Western dietary pattern [Citation5, Citation6] and inversely associated with better adherence to healthy Mediterranean [Citation7] and Dutch [Citation8] dietary patterns. In a randomized controlled trial (RCT), ADHD symptoms in children were significantly improved by adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet [Citation9]. Additionally, lower frequency of consuming fruits and vegetables was associated with higher prevalence of ADHD diagnosis and increased level of inattention in a previous cross-sectional analysis of this sample of children with ADHD [Citation7, Citation10].
Two-thirds of children with ADHD in the US take medication for ADHD symptoms [Citation11]. Stimulants are the most common medication class and are effective at reducing symptoms in most children; however, in recent years, concerns over the potential adverse side effects, including appetite suppression and growth delay, sleep disturbances, cardiovascular concerns, and other potential long-term effects have emerged [Citation11]. In response to these concerns and calls for more research on non-pharmacological treatment options for ADHD [Citation12], several randomized controlled trials (RCTs) have been conducted using broad-spectrum multinutrients to treat ADHD in adults and ADHD and emotional dysregulation in children [Citation13–15]. When investigating new treatments, it is important to determine who may benefit most from the treatment. Moderator analysis specifies for whom and under what conditions the treatment works or works best [Citation16]. There has been speculation that nutrient supplementation may only work for those with poor diets [Citation17], although previous analyses suggested that healthy eating behavior did not moderate treatment response to broad-spectrum multinutrients for ADHD [Citation18, Citation19].
These moderator analyses are based on a fully blinded RCT of children with ADHD and emotional dysregulation that showed benefit for multinutrients over placebo in clinician-rated global improvement [Citation15]. Since diet is the main source of nutrient intake, and diet quality corresponds with nutritional outcomes, the primary aim of this study examines whether overall diet quality at baseline moderates treatment response. The hypothesis is that participants with lower diet quality scores will benefit more from treatment with multinutrients than those with higher diet quality. The secondary aim is an exploratory analysis to examine whether any specific dietary components moderate treatment response.
Methods
Trial design and participants
Data for this analysis were from the Micronutrients for ADHD in Youth (MADDY) Study, an 8-week randomized double-blind placebo-controlled trial conducted from 2018 to 2020 that utilized a 36-ingredient vitamin/mineral supplement to treat ADHD and emotional dysregulation in a pediatric population. Additional details on study design and primary outcomes are published elsewhere [Citation15, Citation20]. Participants were 6–12 years old and met the DSM-5 criteria for ADHD as assessed by a clinical cut-off of 6 or more inattention and/or hyperactivity/impulsivity symptoms on the parent-reported Child and Adolescent Symptom Inventory-5 (CASI-5), and at least one impairing symptom of irritability or anger from the CASI-5 oppositional defiant disorder (ODD) or disruptive mood dysregulation disorder (DMDD) subscales. Participants were psychotropic-medication-free, being either medication-naïve or having discontinued medications for at least 2 weeks prior to starting the study. They were recruited from two sites in the United States (Columbus, OH and Portland, OR) and one in Canada (Lethbridge, Alberta). Recruitment was through referrals from pediatricians and mental health providers, social media, and flyers. Written informed consent from parents and assent from children were completed at the baseline visit prior to any study procedures. The study was approved on 10 August 2017 by Institutional Review Boards at The Ohio State University and Oregon Health & Science University and the Conjoint Health Research Ethics Board at University of Calgary for the University of Lethbridge.
Sample size
For the primary outcome in the MADDY RCT, a sample size of 123 was needed to detect differences in the CASI-5 composite score between groups using a 3:2 randomization ratio of active to placebo; 135 participants were recruited to allow for attrition [Citation15]. This study used data from 124 children (69 multinutrients/ 55 placebo) who were included in the intention-to-treat analysis of the MADDY RCT and who completed a food frequency questionnaire at baseline.
Primary intervention
The active intervention ‘multinutrients’ consisted of capsules containing a blend of vitamins, minerals, amino acids, and antioxidants. A dose of 9–12 capsules per day provided doses above the Recommended Dietary Allowance (RDA), but below the Upper Tolerable Intake Level (UL). The placebo capsule, which contained cellulose filler and 0.1 mg of riboflavin per capsule (total dose 0.9–1.2 mg/day, which is above RDA for this age group) added to mimic urine color when supplemented with B-vitamins, looked identical to the multinutrient capsule. Hardy Nutritionals (Raymond, AB, Canada; www.hardynutritionals.com) provided the active intervention formula, Daily Essential Nutrients, and the placebo capsules without cost, but had no role in the study design, data collection and analysis, or interpretation of the results.
Measures
Sociodemographic and anthropometric measures
Demographic information was collected at baseline using a Demographics and History questionnaire. Questions included gender, ethnicity, race, parent/guardians’ occupation, parent/guardians’ level of education, and family income. Body mass index (BMI) was calculated based on height, measured using a stadiometer with adjustable headpiece, and weight, measured using a calibrated digital scale.
Diet quality measures
Within 3 days of the baseline visit, a parent or caregiver completed the VioScreen digital food frequency questionnaire (FFQ) to report their child’s typical dietary intake. VioScreenTM (https://www.viocare.com/vioscreen.html; Viocare Inc, Kingston, NJ) is a validated, graphics-based dietary analysis software that uses 1500 food images with foods and portion sizes to provide the equivalent of 90 days of nutrition tracking via a parent-report questionnaire that takes 20 min to complete [Citation21]. It uses food and nutrient information from the Nutrition Coordinating Center Food and Nutrient Database (University of Minnesota Division of Epidemiology and Community Health in Minneapolis) to calculate a diet quality score using the Healthy Eating Index-2015 (HEI-2015). The HEI is a density-based measure (e.g. amounts per 1000 kcal), which has been validated for individuals ages 2 and up [Citation22]. It assesses the quality of a diet based on the Dietary Guidelines for Americans (DGA) recommendations. The DGA was developed to help Americans consume diets that promote health and prevent disease [Citation23]. The HEI-2015 total score, ranging from 0 to 100, is the sum of 13 component scores, each of which ranges from a minimum of 0 to a maximum score of either 5 or 10 depending on the component [Citation24]. A higher total score denotes better adherence to the DGA. Nine of the components are designated ‘adequacy components’ (total fruit, whole fruit, vegetables, greens and beans, whole grains, dairy, protein foods, seafood and plant proteins, and fatty acids), which represent food groups or dietary elements with recommendations for increased intake and are scored such that higher intake corresponds with higher scores. The remaining four components are ‘moderation components’ (refined grains, sodium, saturated fat, and added sugars) representing food groups and dietary elements with recommendations for limiting consumption and are reverse scored, with lower intake scored higher [Citation24].
Behavioral measures
An a priori primary outcome measure used in the MADDY RCT was the blinded clinician-rated Clinical Global Impression (CGI) scale. CGI is a validated clinical measure with 2 subscales; one assessing symptom severity (CGI-S, rated from 1 = normal to 7 = among the most extremely ill) and the other symptom improvement from baseline (CGI-I, rated from 1 = very much improved to 7 = very much worse) [Citation25]. Trained and blinded study staff rated the week 8 CGI-I based on all available data, including interviews with parent and child at each visit, several behavioral questionnaires, in-clinic observations, and CGI-S ratings from baseline and week 8. To standardize ratings among sites, all CGIs were reviewed weekly at cross-site telephone calls with blinded senior study staff, including doctoral clinicians. The primary outcome measure was dichotomous with ‘treatment responders’ classified by a rating of 1 or 2 (‘very much improved’ or ‘much improved’) on the CGI-I at week 8.
Statistical methods
Descriptive statistics were used to analyze demographic variables and diet quality scores for all participants in this analysis. For the continuous variables, normally distributed data were reported as mean and standard deviation (SD) and non-normal data reported as median and interquartile range (IQR). Categorical variables were reported as frequency (percent). Two-sample t-tests for continuous variables and the Pearson chi squared or Fisher exact test (if expected counts <5) for categorical variables were used to compare the baseline variables between the multinutrient and placebo groups.
The moderator variable was participants’ HEI-2015 total score for the primary analysis, and the HEI-2015 component scores for the secondary exploratory analysis. For each potential moderator, two models were specified: (1) logistic regression using CGI-I at week 8 as a dichotomous outcome (referred to hereafter as ‘treatment response’) to estimate the odds ratio and 95% confidence interval (CI) and (2) ordinary least squares linear regression with CGI-I at week 8 as a dimensional outcome (referred to hereafter as ‘level of improvement’) to estimate the unstandardized beta coefficients (β) and 95% CI. If week 8 CGI-I data were missing, last observation carried forward from week 4 was used to inform CGI-I rating. A 3-way interaction of site-by-treatment group-by-diet quality score was first tested to determine whether there were significant site differences. If the 3-way interaction was not significant, the model included the potential moderator in an interaction term with treatment group, plus the individual terms for the potential moderator, treatment group, and site entered as a covariate. Sociodemographic characteristics and BMI were checked for potential confounding of HEI scores using Pearson correlation coefficients for continuous variables and one-way ANOVA for categorical variables for parametric data, or Spearman correlation coefficients for continuous variables, Mann–Whitney test for categorical variables with 2 categories, and Kruskal–Wallis test for categorical variables with 3 + categories for non-parametric data. Any variables identified as related to diet quality were then entered as covariates. For linear regression models, assumptions of linear regression were verified for any significant moderators using graphical and statistical methods including Shapiro-Wilks test for normality of residuals, Breusch–Pagan test for homoskedasticity of residuals, and variance inflation factors to assess independent variables for multicollinearity.
Since two models were used to test the primary hypothesis, Benjamini-Hochberg false discovery rate procedure was applied to adjust for multiple testing. The secondary exploratory analysis was hypothesis-generating and therefore these analyses did not correct for multiple testing. Statistical significance was defined as a two-sided p-value <0.05. Any significant interactions were probed to determine direction of moderation. All analyses were conducted using Stata version 17 software (College Station, TX: StataCorp LLC).
Results
Study population characteristics
Sociodemographic characteristics, diet quality, and symptom scores of the study population are shown in . The study included 124 children (median age = 10.2 years, IQR = 8.5–11.2), 73% of whom were male. Approximately 79% of the sample identified as White. Over half of the sample (57%) had family income greater than $80,000/year. At baseline, the mean and standard deviation (SD) of the clinician-rated CGI-S score for the study population was 4.5 (0.7) and at week 8 was 4.0 (0.8). The average total HEI-2015 score for the study sample was 63.6 [SD = 8.7, range 40.8–81.2 (maximum score of 100 indicates perfect alignment with dietary guidance (DGA)]. Demographic variables and diet quality scores were equivalent between the multinutrient and placebo groups (). HEI-2015 total score was correlated with participant age (r = −0.31, p < 0.001), but not to other sociodemographic factors or BMI (Supplemental Table 1). Age was therefore included as an additional covariate in the moderator analysis of HEI-2015 total score.
Table 1. Characteristics of the study population.
Diet quality scores as moderators
As shown in , in the primary analysis, HEI-2015 total score did not significantly moderate treatment response [odds ratio = 1.00 (95% CI: 0.90,1.10), p = 0.984] nor level of improvement on the CGI-I [β = −0.01 (95% CI: −0.06,0.04), p = 0.648].
Table 2. Results of primary and exploratory analysis of overall diet quality and dietary component scores as moderators of treatment response.
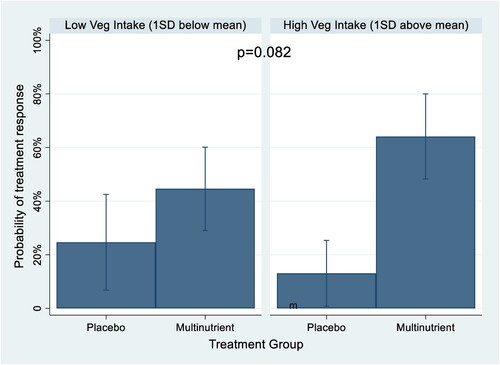
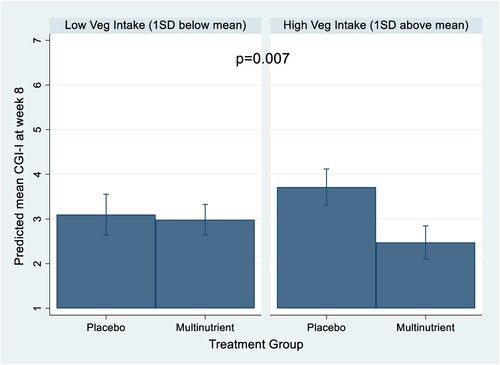
In the exploratory analysis, the HEI component scores did not moderate dichotomous treatment response/non-response, although total vegetable intake trended towards significance [odds ratio = 1.98 (95% CI: 0.92, 4.28), p = 0.082] (). illustrates this trend at various levels of vegetable intake. At high vegetable intake, those in the multinutrient group were predicted to be nearly 5 times more likely to be treatment responders than those in the placebo group (probability of treatment response of 0.64 vs. 0.13, respectively); while at low level of vegetable intake, those in the multinutrient group were about 1.8 times more likely to be treatment responders than those in the placebo group (probability of treatment response of 0.45 vs. 0.25, respectively). Further, the HEI component score for total vegetable intake significantly moderated level of improvement [β = −0.48 (95% CI: −0.82, −0.13), p = 0.007] (). illustrates the predicted difference between multinutrient and placebo improvement at various levels of vegetable intake: those with high vegetable intake had significantly more behavioral improvement (lower CGI-I score) on the multinutrients than the placebo. Because HEI total vegetable score was identified as a significant moderator, it was also evaluated for potential relationships with sociodemographic variables and BMI; none were significantly related and were thus not included as covariates in this moderation analyses. Additionally, given the previously published findings from this sample that HEI total vegetable score was negatively associated with baseline severity of inattention [Citation10], a post-hoc adjustment to the moderator analysis was performed with baseline inattention symptom score entered as a covariate. The results did not change appreciably, indicating the moderating effect of vegetable intake remained significant after controlling for inattention score.
Discussion
In this study, we explored moderating effects of overall diet quality at baseline on treatment response to multinutrient supplementation for ADHD and emotional dysregulation. As recently demonstrated, multinutrient supplementation had three times as many treatment responders as placebo according to the clinician-rated CGI-I scale [Citation15]. In the primary analysis presented here, we examined the moderating effects of diet quality on treatment response and level of improvement based on CGI-I rating at week 8. Participants’ age was negatively correlated with HEI-2015 total score in this sample, suggesting that younger participants had better diet quality than older participants. This is consistent with findings from a nationally representative sample of American children [Citation26]. Counter to our hypothesis, overall diet quality at baseline, as measured by the HEI-2015 total score, did not moderate odds of treatment response or level of improvement based on the CGI-I. This study aligns with previous findings that healthy eating behavior was not a moderator of outcomes in two previous RCTs of multinutrient supplementation to treat ADHD in adults and children [Citation18, Citation19]. Both previous studies relied on a brief dietary intake questionnaire modified from Baker et al. (2003) to identify healthy eating behaviors including questions on consumption of fruit and vegetables, breakfast, consumption of fast foods, and eating when full, producing a single total score with higher numbers indicative of healthier eating [Citation18, Citation27]. The modified tool, however, did not allow for the calculation of a validated index of diet quality. The use of a dietary assessment tool in this analysis, along with a validated diet quality index, allows us to confirm the moderating effects of diet quality in multinutrient interventions in ADHD and emotional dysregulation, adding to the extant literature. The lack of moderation by overall diet quality suggests that there may be individual differences in the level of nutrients needed, above and beyond adequate intakes from dietary sources, which may be linked to genetic diatheses [Citation28], a hypothesis that could be investigated in future studies.
As an exploratory analysis, we examined the moderating effects of individual diet quality components. In this analysis, the HEI component of total vegetable intake moderated the level of improvement. The direction of moderation indicated that those with higher vegetable intake showed significantly greater improvement (lower CGI-I) in the multinutrient group compared to placebo. This finding also counters the original hypothesis that those with an unhealthy diet would benefit more from the multinutrient supplement. It is not clear how higher vegetable intake is linked to greater improvement. Possible explanations include evidence showing that vegetable intake affects the composition of gut microbiota, which could potentially affect absorption and/or utilization of vitamin and mineral components [Citation29]. Additionally, the gut microbiota of children with ADHD differs from healthy controls [Citation30]; however, it is unclear at this time how various disparate factors affecting the microbiota may interact in the case of ADHD and influence changes in symptom severity and treatment response. A second possibility is that vegetable intake may represent other factors that enhanced treatment response; for instance, research has shown child fruit and vegetable intake is related to parental rules about serving fruit/vegetable everyday [Citation31]. Speculatively, children with higher vegetable intake may have parents who utilize more structure in their parenting style, evidenced by getting their child to eat more vegetables, which also allowed for better adherence to the treatment. Such parental structuring could even speculatively include limiting of screen time, provision of adequate sleep, promotion of physical exercise, and other lifestyle advantages so that multinutrients were the only thing lacking for optimal function.
Clinical significance
This study indicates that multinutrients may benefit children with ADHD and irritability regardless of overall diet quality. While a diet higher in fruit and vegetable intake than the typical American diet (e.g. the Mediterranean diet) may improve symptoms of ADHD and emotional dysregulation, multinutrient supplementation may provide an additional, and possibly complementary treatment option, for individuals interested in nutritional treatments for ADHD.
Strengths and limitations
The use of a validated, reliable dietary index in children is a strength of this study. This is the first study to look at an a priori-defined diet index as a moderator of response to a multinutrient intervention for ADHD. This adds to existing research by providing a detailed description of dietary patterns to understand which components may affect response to the treatment. Additionally, this analysis used the placebo-control condition provided by the design of the RCT to attribute effects directly to the multinutrient intervention.
One limitation is the increased chance of Type I error in the exploratory analysis since we did not correct for multiple testing (although p = 0.007 is compelling). In this case, the risk from a Type 1 error is low since the findings will be used to inform future studies to test the generated hypotheses. While FFQ’s are a validated tool to track dietary intake, a second limitation is the vulnerability of self-reported dietary intake methods to random and systematic errors [Citation32]. The use of the VioscreenTM tool addresses some limitations of traditional FFQs by offering photographs to capture serving size and limiting respondent burden through use of branching logic to minimize survey questions based on respondents’ previous answers [Citation32].
Implications for future research
These findings suggest several avenues for future research. Other baseline characteristics such as sociodemographic variables, baseline symptom severity, ADHD subtype, comorbidities, blood nutrient levels, markers of inflammation, antioxidant levels, microbiome should be evaluated as potential moderators to determine for which sub-populations treatment with multinutrients is effective. Vegetable intake appears to be an important aspect of diet quality in relation to ADHD and should be further explored by investigating the relationship between participants’ dietary patterns, microbiome, genetics, and response to the multinutrient treatment. Saliva, blood, and stool samples from MADDY participants will enable future research on the microbiome, diet quality, genetics, and other biomarkers that may be related to treatment response. Finally, future interventions could be tested combining multinutrient supplementation with increased vegetable intake to potentially optimize treatment effectiveness.
Conclusions
Overall diet quality as measured by HEI-2015 total score was not a moderator of treatment outcomes in the MADDY RCT. These findings are clinically important because they suggest that multinutrient supplementation may benefit children with ADHD and irritability independent of overall diet quality. The finding that higher vegetable intake may increase chance of behavioral improvement with multinutrients is interesting and merits further exploration of potential mechanisms. Hypotheses include microbiome changes from higher intake of vegetables that may improve nutrient absorption or utilization and parental factors that may influence treatment adherence or other relevant lifestyle factors.
Supplemental Material
Download MS Word (18.9 KB)Acknowledgements
The authors wish to acknowledge our study participants, the study staff, and student researchers, as well as our funders.
Disclosure statement
Dr. Arnold has received research funding from Axial, Forest, Lilly, Noven, Otsuka, Shire, Supernus, Roche, Yamo, and YoungLiving (as well as NIH and Autism Speaks); has consulted with Pfizer, Tris Pharma, and Waypoint; and been on advisory boards for Arbor, Ironshore, Otsuka, Pfizer, Roche, Seaside Therapeutics, Shire. The other authors declare that the research was conducted without any conflict of interest.
Additional information
Funding
Notes on contributors
Lisa M. Robinette
Lisa Robinette, MS is a PhD student in the Interdisciplinary PhD in Nutrition Program, OSU
Irene E. Hatsu
Irene E. Hatsu, PhD is an Associate Professor of Human Nutrition at the Department of Human Sciences, OSU.
Jeanette M. Johnstone
Jeanette Johnstone, PhD is an Assistant Professor in Child and Adolescent Psychiatry, OHSU
Alisha M. Bruton
Alisha Bruton, ND, MS is a research scientist in the Department of Psychiatry, OHSU.
Brenda M.Y. Leung
Brenda Leung, PhD is an Associate Professor of Public Health in the Faculty of Health Sciences, University of Lethbridge, and holds the Emmy Droog Chair in Complementary and Alternative Healthcare
L. Eugene Arnold
L. Eugene Arnold, MD is a Professor Emeritus of Psychiatry & Behavioral Health, OSU.
References
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among US children and adolescents, 2016. J Clin Child & Adoles Psychol. 2018;47(2):199–212.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington (DC); 2013.
- Faraone SV, Rostain AL, Blader J, Busch B, Childress AC, Connor DF, Newcorn JH. Practitioner review: emotional dysregulation in attention-deficit/hyperactivity disorder–implications for clinical recognition and intervention. J Child Psychol Psychiatry. 2019;60(2):133–50.
- Lange KW, Hauser J, Makulska-Gertruda E, Nakamura Y, Reissmann A, Sakaue Y, et al. The role of nutritional supplements in the treatment of ADHD: what the evidence says. Curr Psychiatry Rep. 2017;19(2):8.
- Howard AL, Robinson M, Smith GJ, Ambrosini GL, Piek JP, Oddy WH. ADHD is associated with a “western” dietary pattern in adolescents. J Atten Disord. 2011;15(5):403–11.
- Abbasi K, Beigrezai S, Ghiasvand R, Pourmasoumi M, Mahaki B. Dietary patterns and attention deficit hyperactivity disorder among Iranian children: a case-control study. J Am Coll Nutr. 2019;38(1):76–83.
- Ríos-Hernández A, Alda JA, Farran-Codina A, Ferreira-García E, Izquierdo-Pulido M. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. 2017;139(2).
- Mian A, Jansen PW, Nguyen AN, Bowling A, Renders CM, Voortman T. Children's attention-deficit/hyperactivity disorder symptoms predict lower diet quality but not vice versa: results from bidirectional analyses in a population-based cohort. J Nutr. 2019;149(4):642–8.
- Khoshbakht Y, Moghtaderi F, Bidaki R, Hosseinzadeh M, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on attention-deficit hyperactivity disorder (ADHD) symptoms: a randomized controlled clinical trial. Eur J Nutr. 2021;60(7):3647–58.
- Robinette LM, Hatsu IE, Johnstone JM, Tost G, Bruton AM, Leung BMY, et al. Fruit and vegetable intake is inversely associated with severity of inattention in a pediatric population with ADHD symptoms: the MADDY study. Nutr Neurosci. 2022: 1–10.
- Brown KA, Samuel S, Patel DR. Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: a review for practitioners. Transl Pediatr. 2018;7(1):36–47.
- Charach A. Editorial: time for a new conversation on stimulant use. J Am Acad Child Adolesc Psychiatry. 2020;59(8):929–30.
- Rucklidge JJ, Frampton CM, Gorman B, Boggis A. Vitamin-mineral treatment of attention-deficit hyperactivity disorder in adults: double-blind randomised placebo-controlled trial. Br J Psychiatry. 2014;204:306–15.
- Rucklidge JJ, Eggleston MJF, Johnstone JM, Darling K, Frampton CM. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: a fully blinded, randomized, placebo-controlled trial. J Child Psychol Psychiatry. 2018;59(3):232–46.
- Johnstone JM, Hatsu I, Tost G, Srikanth P, Eiterman LP, Bruton AM, et al. Micronutrients for attention-deficit/hyperactivity disorder in youths: A placebo-controlled randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2022;61(5):647–61.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82.
- Hurt EA, Arnold LE. An integrated dietary/nutritional approach to ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23(4):955–64.
- Rucklidge JJ, Johnstone J, Gorman B, Boggis A, Frampton CM. Moderators of treatment response in adults with ADHD treated with a vitamin-mineral supplement. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:163–71.
- Rucklidge JJ, Eggleston MJF, Darling KA, Stevens AJ, Kennedy MA, Frampton CM. Can we predict treatment response in children with ADHD to a vitamin-mineral supplement? An investigation into pre-treatment nutrient serum levels, MTHFR status, clinical correlates and demographic variables. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:181–92.
- Johnstone JM, Leung B, Gracious B, Perez L, Tost G, Savoy A, et al. Rationale and design of an international randomized placebo-controlled trial of a 36-ingredient micronutrient supplement for children with ADHD and irritable mood: the micronutrients for ADHD in youth (MADDY) study. Contem Clin Trials Commun. 2019;16:100478.
- Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, Paskett ED. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. 2014;114(4):613–21.
- Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, et al. Evaluation of the healthy eating index-2015. J Acad Nutr Diet. 2018;118(9):1622–33.
- Department of Health and Human Services. Executive summary. Dietary guidelines for Americans, 2015-2020. Washington (DC): USDA; 2015.
- Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602.
- Guy W. ECDEU assessment manual for psychopharmacology; 1976.
- Thomson JL, Tussing-Humphreys LM, Goodman MH, Landry AS. Diet quality in a nationally representative sample of American children by sociodemographic characteristics. Am J Clin Nutr. 2019;109(1):127–38.
- Baker CW, Little TD, Brownell KD. Predicting adolescent eating and activity behaviors: the role of social norms and personal agency. Health Psychol. 2003;22(2):189–98.
- Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002;75(4):616–58.
- Cui J, Lian Y, Zhao C, Du H, Han Y, Gao W, et al. Dietary fibers from fruits and vegetables and their health benefits via modulation of Gut microbiota. Compr Rev Food Sci Food Saf. 2019;18(5):1514–32.
- Wang N, Gao X, Zhang Z, Yang L. Composition of the Gut microbiota in attention deficit hyperactivity disorder: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:838941.
- Jones LR, Steer CD, Rogers IS, Emmett PM. Influences on child fruit and vegetable intake: sociodemographic, parental and child factors in a longitudinal cohort study. Public Health Nutr. 2010;13(7):1122–30.
- McClung HL, Ptomey LT, Shook RP, Aggarwal A, Gorczyca AM, Sazonov ES, et al. Dietary intake and physical activity assessment: current tools, techniques, and technologies for use in adult populations. Am J Prev Med. 2018;55(4):e93–e104.