ABSTRACT
Introduction
Small fibre neuropathy (SFN) is an early manifestation of diabetic polyneuropathy. Although oxidative stress, inflammation and change of intestinal bacterial population are assumed to be their pathogenesis, the effects of dietary nutrition have not been evaluated. The relationship between dietary nutrition intake and pain sensation was evaluated in the Japanese population.
Methods
We conducted the Iwaki project, a population-based study recruiting 1,028 individuals, in 2018. The relationships between the pain threshold from intraepidermal electrical stimulation (PINT) and the amount of dietary nutrition evaluated by a brief-type self-administered diet history questionnaire were examined. The odds ratio was further explored after categorizing subjects based on low (< 63.7 μg/day), intermediate (63.7-159.2 μg/day), and high cryptoxanthin levels (> 159.2 μg/day).
Results
Univariate linear regression analyses showed significant correlations between PINT and cryptoxanthin intake even after adjustments for other nutritional intakes (ß = 0.107, p < 0.01). Multivariate logistic regression analysis revealed low and high cryptoxanthin intake as significant risk factors for abnormal PINT (≥ 0.20 mA). Multivariate linear regression analyses showed significant correlations between PINT and cryptoxanthin intake levels after adjustment for other clinically PINT-related factors (ß = 0.09, p < 0.01).
Conclusions
Adequate intake of cryptoxanthin is recommended to maintain the pain threshold in the Japanese population.
Introduction
Small fibre neuropathy (SFN) is a peripheral nerve disorder characterized by dysfunction exclusively in small myelinated and unmyelinated fibres, which can manifest as an initial symptom of diabetic polyneuropathy (DPN) [Citation1]. Small myelinated nerve fibres (Aδ fibres) maintain cold temperature and rapid pain sensitivity and small unmyelinated nerve fibres (C fibres) are sensitive to heat temperature and slow pain after thermal or mechanical stimulation of the skin [Citation1]. SFN manifests with a variety of symptoms, such as chronic and diffuse pain, paraesthesia and dysesthesia; however, the condition can also be asymptomatic, displaying no pain experience, reduced pain, or temperature sensation [Citation1].
Currently, SFN can be evaluated quantitatively by either invasive or noninvasive methods [Citation2]. We previously evaluated SFN in the general Japanese population, employing a new electrode for intraepidermal electrical stimulation (IES) [Citation3–7]. A significant increase in the pain threshold of intraepidermal nerve terminal from IES (PINT) was observed in patients with DPN compared with those without DPN. We further clarified that a normal-high HbA1c level, plasma lipopolysaccharide binding protein (LBP) level, tryptophan metabolism, urine 8-hydroxy 2’-deoxyguanosine level (an oxidative stress marker), and the intestinal Bacteroides genus proportion were correlated with PINT index. These findings may suggest that there are pathogenetic factors of SFN independent of abnormal glucose metabolism, which may synergistically aggravate SFN if diabetes is present. Nevertheless, it remains unclear what factors are involved in SFN in nondiabetic individuals.
Oxidative stress is thought to play a key role in the pathogenesis of DPN [Citation5, Citation8]. Antioxidant micronutrients such as vitamins and carotenoids exist in abundance in fruits and vegetables and contribute to the body’s defence [Citation9]. In DPN, vitamin E, an antioxidant vitamin, and lycopene, an antioxidant carotenoid, can suppress clinical and experimental neurological symptoms such as pain and hyperalgesia [Citation10]. β-cryptoxanthin, one of carotenoids, is known to have an antioxidant effect in vitro at most physiological concentrations [Citation11, Citation12]. Daily intake of cryptoxanthin supplement can ameliorate experimental neuropathic pain [Citation13]. These findings have attracted attention to the idea that nutritional therapy might be readily accepted as an additional recommended strategy for preventing SFN. In this study, we evaluated the correlation between the intake of micronutrients and the PINT index in a general Japanese population (Supplemental Figure 1).
Materials and methods
Subjects and demographics
The Iwaki health promotion project is a health promotion study of Japanese individuals over 10 years of age [Citation14]. This health evaluation in 2018 was conducted with voluntary participants living in Iwaki, located in northern Japan, a suburban area of Hirosaki city in Aomori Prefecture, comprising mainly farmland. For this study, 1,056 volunteers were originally included, of whom the following were excluded: 11 with incomplete clinical data and 17 with no PINT measurement. Thus, the total number of examined individuals (n) was 1,028 (sex: men, n = 427; women, n = 601; mean age: 52.9 ± 15.5 years) (). The participants’ demographic and detailed clinical profiles are shown in Supplemental Table 1. After the initial determination of fasting blood glucose (FBG) and HbA1c (the National Glycohemoglobin Standardization Program value), the enrolled individuals (n = 1,028) were divided into 3 groups, namely, normoglycaemic subjects, impaired fasting glucose subjects (IFG) and diabetic subjects, according to the Japan Diabetes Society criteria as follows [Citation15]:
Normoglycaemic subjects (n = 906): FBG < 110 mg/dl, HbA1c < 6%
IFG subjects (n = 41): FBG = 110–125 mg/dl, HbA1c = 6–6.5%
Diabetic subjects (n = 81): FBG ≥ 126 mg/dl or HbA1c levels ≥ 6.5%
Figure 1. Subject selection. A total of 1,028 participants (427 men and 601 women) out of 1,056 volunteers from the Iwaki Study 2018 were eligible for this study. The participants were further classified into 906 normoglycaemic subjects, 41 impaired fasting glucose (IFG) subjects, and 81 diabetic subjects. Independently, subjects were further divided into 336 low cryptoxanthin subjects (Cry-L), 343 intermediate cryptoxanthin subjects (Cry-I) and 349 high cryptoxanthin subjects (Cry-H) based on the amount of cryptoxanthin intake in the diet.
Clinical profiles
Blood samples were collected in the mornings under fasting conditions from peripheral veins while in a supine position. The following clinical measurements were recorded: height, body weight, body mass index (BMI), and waist circumference. Measurements of glucose metabolism included FBG, HbA1c, glycoalbumin, and C-peptide. For the determination of lipid metabolism, serum levels of triglyceride (Tg), total cholesterol (Tc), high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) were measured. For the determination of renal function, serum levels of blood urea nitrogen (BUN), creatinine and uric acid were measured. For the determination of inflammation markers, serum high-sensitivity C-reactive-protein (Hs-CRP) was measured by the nephelometry method. Subjective neuropathic foot symptoms (pricking, burning, and aching pain) were determined from the questionnaires. None of the participants were diagnosed with type 1 diabetes or inherited diseases that affected the HbA1c measured values.
Nutritional intake assessment
Nutritional consumption data for the study participants were obtained using the brief-type self-administered diet history questionnaire (BDHQ) [Citation16]. The BDHQ is a 4-page fixed-portion questionnaire that asks about the consumption frequency of selected foods, but not about portion size, to estimate the dietary intake of 58 food and beverage items, which are commonly consumed in Japan, during the preceding month. Based on the standard tables of food composition in Japan revised in 2010, crude estimates for dietary intake of total energy and 37 selected nutrients were calculated [Citation17]. Apart from the categorization according to glucose metabolism, the subjects were further divided based on cryptoxanthin intake levels evaluated by the BDHQ as follows:
Low cryptoxanthin subjects (Cry-L, n = 336): Cryptoxanthin < 63.7 μg/day.
Intermediate cryptoxanthin subjects (Cry-I, n = 343): Cryptoxanthin = 63.7–159.2 μg/day.
High cryptoxanthin subjects (Cry-H, n = 349): Cryptoxanthin > 159.2 μg/day.
PINT measurement
For nociceptive stimulation, the IES method was adopted using a disposable concentric bipolar needle electrode (NM-983W; Nihon Kohden Corp., Tokyo, Japan) connected to a specific stimulator for cutaneous Aδ and C fibres, as previously described (PNS-7000; Nihon Kohden) [Citation3, Citation6]. The stimulator consisted of an outer ring anode (1.3 mm diameter) and the cathode of an inner needle that protruded 0.025 mm from the level of the outer ring. The IES electrode was placed onto the skin of the instep (over the extensor digitorum brevis) to deliver weak continuous electrical stimulations. This stimulation can evoke a local pricking sensation. In instances where the keratinized layer of the skin was too thick and likely to interrupt the electronic stimulation, the electrode was moved elsewhere on the same foot to locate an area seemingly without a thick layer. The participants were instructed to push the button as quickly as possible only when they felt a sensation. Stimulation intensity was decreased by 0.05 mA stepwise from 0.2 mA until the participants reported a pricking sensation. The current intensity is directly proportional to the intensity of stimulation. PINT was defined as the minimum intensity at which the participants felt a pricking sensation in more than two trials. Therefore, PINT can basically evaluate the degree of hypoalgesia towards electrical pain stimulation.
Statistical methods
The values of the clinical measurements are expressed as the means ± SD or medians (interquartile ranges, 25%–75%). The statistical significance of the differences in values between two groups (parametric) and case‒control associations among groups (nonparametric) was assessed using analysis of variance (ANOVA) with a post hoc test, followed by Bonferroni’s correction and χ2 tests, respectively. Correlations between PINT index and nutritional measurements were assessed by linear regression analyses. The risk of high cryptoxanthin intake with increased PINT index was calculated by multiple logistic regression analysis with an adjustment for factors identified to be associated with PINT index by univariate regression analysis and potentially cofounding variables for SFN from a previous study [Citation3]. For the calculation of odds ratios, SFN was designated as 0.20 mA and higher. A value of p < 0.05 was regarded as statistically significant. All analyses were performed using Jmp version 10.0.4 and StatView version 5.0.1 (SAS Institute Inc., Cary, NC. USA).
Results
Participant clinical profile and demographic correlations
The clinical profiles of the participants are displayed in Supplemental Table 1. The mean age was 53.0 ± 15.3 years for men and 53.2 ± 15.7 years for women. BMI, waist circumference, FBG and C-peptide were elevated in men than in women (BMI: 24.0 ± 3.3 vs. 22.3 ± 3.6, waist circumference: 83.8 ± 9.2 vs. 73.6 ± 9.1, p < 0.0001; FBG: 97.5 ± 17.0 vs. 92.8 ± 13.1 mg/dL, p < 0.0001; and C-peptide: 1.5 ± 0.7 vs. 1.4 ± 0.6 ng/mL, p < 0.0001). Tg was higher and HDL-c levels were lower in men than in women (Tg: 120.5 ± 89.1 vs. 80.8 ± 42.1 mg/dL, p < 0.0001; HDL-c: 59.6 ± 16.6 vs. 70.1 ± 16.9 mg/dL, p < 0.0001). Serum BUN and creatinine were higher in men than in women (BUN: 14.7 ± 3.9 vs. 13.7 ± 4.1 mg/dL, p < 0.0001; creatinine: 0.8 ± 0.5 vs. 0.6 ± 0.3 mg/dL, p < 0.0001). Hs-CRP was higher in men than in women (0.08 ± 0.11 vs. 0.06 ± 0.10 mg/dL, p < 0.01). The prevalences of IFG, diabetes, hypertension and dyslipidaemia were comparable, while those of alcohol consumption and smoking habits were significantly higher in men than in women. The PINT index was significantly higher in men than in women (0.13 ± 0.10 vs. 0.10 ± 0.10 mA, p < 0.01).
Estimated nutrient components of examined subjects evaluated by the BDHQ
The estimated energy requirement, net energy requirement and intake of nutrition weight, water, protein, total lipid and carbohydrate were significantly higher in men than in women (p < 0.0001) (Supplemental Table 2). Cryptoxanthin intake was comparable between men and women. The intake of carotenoids and vitamin C was significantly higher in women than in men (p < 0.0001 for α-carotene and β-carotene; p < 0.05 for vitamin C). The intake of tocopherols, saturated fatty acids, n-3/n-6 fatty acids, cholesterol and alcohol was significantly higher in men than in women (p < 0.0001 for β-tocopherol, γ-tocopherol, δ-tocopherol, n-6 fatty acids and alcohol; p < 0.001 for cholesterol; and p < 0.01 for saturated fatty acids and n-3 fatty acids).
Correlation between the nutrient factors and the PINT index
A univariate regression analysis revealed a close correlation between the PINT index and ingested nutritional factors such as nutrition weight, water, vegetable protein, cryptoxanthin, vitamin D, daidzein, genistein, and equivalent amount of salt ().
Table 1. Correlation between nutrient factors and PINT index.
The correlation between the PINT index and cryptoxanthin remained significant after adjustment for multiple factors positively correlated with PINT in univariate analysis (nutrition weight, water, vegetable protein, vitamin D, daidzein, genistein, and equivalent amount of salt) (ß = 0.1065; p = 0.0018). These correlations remained significant after adjustment for sex and age (ß = 0.1090; p = 0.013).
Correlation between the ingested nutrients and the PINT index in normoglycaemic subjects
To exclude the influence of abnormal glucose metabolism on the PINT index, we examined the correlation between ingested nutrients and the PINT index except for IFG and type 2 diabetes. A univariate regression analysis revealed a similar correlation between the PINT index and nutrient factors observed in all subjects, including cryptoxanthin (). The correlation between the PINT index and cryptoxanthin remained significant after adjustment for multiple factors (ß = 0.1081; p = 0.0022). These correlations remained significant after adjustment for sex and age (ß = 0.1279; p = 0.004).
Table 2. Correlation of nutrient factors with PINT index excluding abnormal glycemic subjects.
The risk ratio of cryptoxanthin levels to an increased PINT index
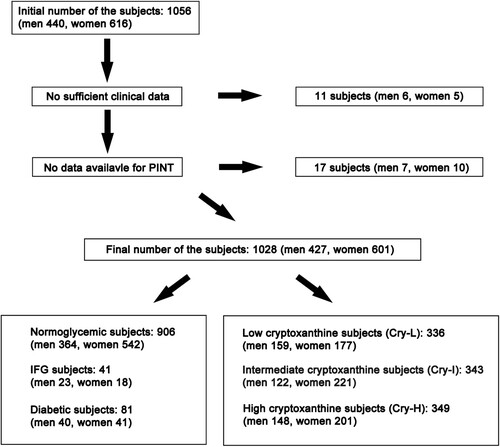
The correlation between the cryptoxanthin intake level and the PINT index was assessed further with stratification of the subjects into tertiles based on the cryptoxanthin level [low < 63.7 μg/day (Cry-L), intermediate = 63.7–159.2 μg/day (Cry-I), and high > 159.2 μg/day (Cry-H)] (). The risks of these tertiles were calculated with respect to an increased PINT index. Cry-H was a significant risk factor for increased PINT [odds ratio (OR) 1.80, 95% confidence interval (CI) 1.20–2.72] if SFN was designated as 0.20 mA and more (A). Furthermore, the risk was significant after adjustment for multiple factors (nutrition weight, water, vegetable protein, vitamin D, daidzein, genistein, and equivalent amount of salt) in the Cry-L and Cry-H groups compared to the Cry-I group (OR 1.58, 95% CI 1.03–2.44 and OR 1.62, 95% CI 1.10–2.50, respectively) (B). The risks of these cryptoxanthin tertiles excluding abnormal glycaemic subjects were also calculated. Similarly, Cry-H, excluding abnormal glucose subjects, remained a significant risk factor for increased PINT (OR 1.95, 95% CI 1.24–3.11) (C). The risk was also significant after adjustment for multiple factors in the Cry-L and Cry – H groups compared to the Cry-I group (OR 1.63, 95% CI 1.02–2.63 and OR 1.81, 95% CI 1.13–2.94, respectively) (D).
Figure 2. Logistic analysis of the clinical measures placing subjects at risk of increased PINT index. The odds ratios with 95% confidence intervals (CIs) for increased PINT (≧ 0.2 mA) are shown (A) and were adjusted for multiple factors (nutrition weight, water weight, vegetable protein, vitamin D, daidzein, genistein, and equivalent amount of salt) (B). The odds ratios with 95% CIs for increased PINT (≧ 0.2 mA) excluding IFG and diabetic subjects are shown (C) and were adjusted for multiple factors (nutrition weight, water weight, vegetable protein, vitamin D, daidzein, genistein, and equivalent amount of salt) (D). Cry, cryptoxanthin: Cry-L, Cry low: Cry-I, Cry intermediate: Cry-H, Cry high: Ref, reference: PINT, pain threshold of intraepidermal nerve terminal from intraepidermal electrical stimulation: IFG, impaired fasting glucose.
Correlation between the PINT index and cryptoxanthin adjusted for the measurements associated with a high pain threshold
Univariate regression analysis was performed to explore the reported clinical factors that correlated with the PINT index, including cryptoxanthin. Univariate regression analysis revealed a significant correlation between the PINT index and clinical measures such as sex, age, BMI, waist circumference, FBG, HbA1c, sBP, Tg, Hs-CRP, history of IFG, diabetes, hypertension, dyslipidaemia, and cryptoxanthin (). The correlation between the PINT index and age (β = 0.1006, p < 0.01), history of hypertension (β = 0.0905, p < 0.05) and cryptoxanthin (β = 0.0921, p < 0.01) remained significant after adjustment for multiple factors (sex, age, BMI, waist circumference, FBG, HbA1c, sBP, Tg, Hs-CRP, history of hypertension or dyslipidaemia, and cryptoxanthin).
Table 3. Correlation between PINT index and clinical factors associated with high pain threshold.
Comparison of the PINT indices and pathogenic factors of DPN among the stratified groups of graded cryptoxanthin intake
Three groups based on graded serum cryptoxanthin levels were examined to explore their relationship with the PINT indices and known pathogenic factors that contribute to the onset and development of DPN. The cryptoxanthin level gradually increased in the order of Cry-L, Cry-I and Cry-H (p < 0.01, Cry-L vs. Cry-I, and p < 0.01, Cry-H vs. Cry-I) (A). The cryptoxanthin level was significantly increased in the 60s, 70s, and 80s compared to other generation, while the level was similar among the 60s, 70s, and 80s (Supplemental Figure 2A).
Figure 3. Pathological factors for DPN in each group of graded participants according to cryptoxanthin intake. Cryptoxanthin intake gradually increased in the order of Cry-L, Cry-I and Cry-H (A). The PINT index was significantly lower in the Cry-I group than in the Cry-L group. The PINT index of the Cry-H group was higher than that of the Cry-I group (B). The average age in the Cry-L group was comparable to that in the Cry-I group (C). The average age in the Cry-H group was the highest among all groups. FBG was significantly increased in Cry-I and Cry-H groups compared to Cry-I group, while FBG was comparable between the Cry-I and Cry-H groups (D). HbA1c in the Cry-H group was the highest among all groups (E). sBP was similar among all groups (F). Bars represent the mean ± SD. PINT, pain threshold of intraepidermal nerve terminal from intraepidermal electrical stimulation: Cry, cryptoxanthin: Cry-L, Cry low: Cry-I, Cry intermediate: Cry-H, Cry high: FBG, fasting blood glucose: sBP, systolic blood pressure. *p < 0.01 vs. Cry-L, †p < 0.01 vs. Cry-I, ‡p < 0.05 vs. Cry-L.
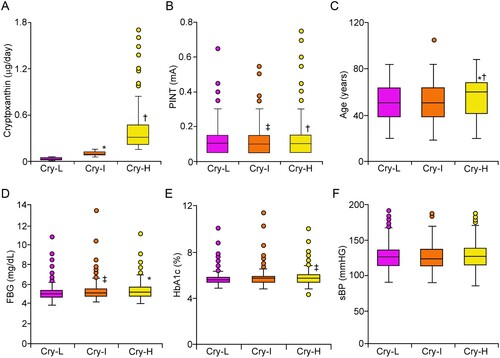
The PINT index of Cry-L group was significantly higher than those of Cry-I group (p < 0.05) (B). Similarly, the PINT index of the Cry-H group was significantly higher than that of the Cry-I group (p < 0.01). The PINT index was comparable between Cry-L and Cry-H groups. The PINT index was significantly increased in the 80s compared to the generations (Supplemental Figure 2B). Age was comparable between the Cry-L and Cry-I groups (C). The age of the Cry-H group was the highest among all groups (p < 0.01 vs. Cry-L and Cry-I). The FBG levels of Cry-I and Cry-H groups were significantly higher than those of Cry-M group (p < 0.05 and p < 0.01, respectively), while the values were comparable between Cry-I and Cry-H groups (D). HbA1c levels were comparable between Cry-L and Cry-I groups, while those of Cry-H group were significantly higher than those of Cry-L group (p < 0.05) (E). No apparent change in systolic blood pressure was observed among any of the groups (F).
Discussion
We previously clarified that a significant increase in the PINT index was correlated with normal-high HbA1c, oxidative stress, and plasma LBP levels and a reduced proportion of the Bacteroides genus in the gut [Citation3–6]. Of note, oxidative stress is known to be a mechanism of DPN, including SFN. Indeed, the β-cryptoxanthin intake was significantly associated with a reduced risk of type 2 diabetes mellitus, in which oxidative stress is profoundly involved in its development [Citation18]. Furtehrmore, daily intake of 10 mg/L cryptoxanthin supplement can ameliorate experimental neuropathic pain [Citation13]. Nevertheless, our results showed that a high intake of cryptoxanthin was significantly correlated with a risk of a high PINT index in uni – and multivariate analyses. Intriguingly, nonvitamin A-forming carotenoids such as lycopene protect against DNA damage in almost all circumstances, but vitamin A-forming carotenoids such as cryptoxanthin can enhance DNA damage, especially when they are present at high concentrations [Citation12]. High concentration of carotenoid can elicit the generation of reactive oxidative species and DNA injury [Citation19]. These suggest that carotenoids may be cytotoxic depending on certain conditions, such as high concentrations and genetic vulnerability. Thus, an excessive intake of cryptoxanthin may cause tissue damage even in the peripheral nervous system, while a low intake of cryptoxanthin decreases antioxidant defence in the peripheral nervous system. Validation study would be required in future.
The major food sources of cryptoxanthin in most diets are citrus fruit, including orange and fruit juices [Citation11]. Cryptoxanthin consumption varies widely by country, with unusually high consumption in Spain and Japan [Citation20], because mandarin oranges, which contain high amounts of β-cryptoxanthin at 100 µg/100 g orange, are consumed daily in Japan. Because carotenoids do not meet the criteria for being considered vitamins, there is no daily recommended intake for them. Considering that the median cryptoxanthin intake was 97.5 µg/day in our study and that the blood absorption of β-cryptoxanthin is greater than that of other common carotenoids, ideally, the ingestion of less than two mandarin oranges a day, but not concentrated supplement ingestion, may be considered a good custom for preventing an increase in the PINT index [Citation21]. However, there are not a small number of people who eat two or more mandarin oranges a day in their daily lives in Japan. Therefore, our results are required to be further verification through clinical studies.
On the other hand, the amount of β-cryptoxanthin intake seasonally and regionally can fluctuate because plasma β-cryptoxanthin concentrations correlate with the total intake of fruits and vegetables, as is typical for carotenoids [Citation22]. In particular, mandarin oranges are harvested in winter, and its consumption is increased in Japan. Therefore, the plasma β-cryptoxanthin concentration can reach a maximum in winter compared to other seasons [Citation23]. The Iwaki health promotion project takes place annually in June, so the amount of cryptoxanthin intake obtained in this study may be the lowest of the year. In the future, it will be necessary to evaluate the correlation between cryptoxanthin intake in each season and the PINT index.
In rodent models and human subjects with DPN, vitamin E and lycopene ameliorate neuropathy symptoms such as neuropathic pain, hyperalgesia and inflammatory reactions. To date, there have been no clinical or experimental trials of cryptoxanthin for DPN. The concentrations of β-cryptoxanthin in most mammalian tissues are generally low compared with those of other dietary antioxidants, such as vitamin E [Citation24], while β-cryptoxanthin appears to be much better absorbed than other carotenoids. Therefore, considering our results, clinical trials for DPN with moderate doses of cryptoxanthin as an adjunctive treatment along with diabetes treatment are expected in the future.
When dividing subjects based on the amount of cryptoxanthin intake, the fasting blood glucose levels, HbA1c levels and systolic blood pressure were comparable between the Cry-H and Cry-I groups. However, the average age was significantly higher in the Cry-H group than in the remaining groups. These results suggest that the ageing factor may be synergistically or additively involved in the increase in the PINT index of Cry-H subjects, unlike that of Cry-L subjects. Previous reports have shown that β-cryptoxanthin levels remain unchanged with ageing in Caucasians [Citation25]. The reason for our result is unclear, but elderly individuals in Iwaki Prefecture may consume more daily fruits and vegetables because Iwaki Prefecture is a major apple-growing region in Japan and agriculture is the prefecture's core industry.
Small nerve fibre function is considered less susceptible to the effects of aging than large myelinated nerve function. The results of the present study showed a marked increase in PINT index in the 80s, but only a mild increase in the 50s to 70s. On the other hand, cryptoxanthin intake was significantly increased in the 60s to 80s compared to other age groups, while the levels were similar. These results may suggest that excessive intake of cryptoxanthin may contribute to the age-related deterioration of PINT index, while insufficient intake of cryptoxanthin may not contribute. The high PINT index, especially in the 80s, could be ascribed to subclinical orthopaedic or central nervous system diseases that are involved in pain perception.
Kobayashi et al examined the relative validity of food group intakes estimated by the BDHQ in Japanese populations, using a 16 day semi-weighed dietary records as reference [Citation16]. Compared with dietary records, BDHQ could estimate intakes well for about half of the food groups with correlation coefficient values were >0.40. This suggests BDHQ has reasonable ranking ability for many food groups. However, several kinds of foods such as potatoes and seaweeds were not estimated well in the BDHQ. Because potatoes and seaweeds contained little or no cryptoxanthin, it was not considered to have a significant effect on the present results.
There are several limitations in this study. First, we need to confirm our results in other cohorts because this study is based on a single cohort in a health promotion study, which consists of healthy volunteers and has insufficient numbers of overt-diabetic or prediabetic subjects. Second, as mentioned above, the amount of β-cryptoxanthin intake and blood levels can fluctuate seasonally, so it is necessary to examine the effects in different seasons. Because the Iwaki project usually takes place annually in June, cryptoxanthin levels are likely to be low. It is necessary to examine the questionnaire in winter, when mandarin oranges and fruits are abundantly ingested, and to evaluate the correlation between cryptoxanthin intake and the PINT index. Finally, invasive examinations were not permitted, and thus, evaluating nerve conduction velocities, the molecular and pathological changes of the skin, or the sural nerve was not possible. We need to confirm the findings obtained here by direct evaluation of skin and peripheral nerve tissues or nerve functions in the future.
In conclusion, our current study showed that a moderate intake of cryptoxanthin may prevent an increase in the PINT index. This effect is independent of abnormal glycaemic states. Rather than an excessive intake of cryptoxanthin from supplements, adequate intake of cryptoxanthin in an amount that is ingested from approximately 1–2 mandarin oranges/day would be useful to maintain a proper PINT index.
Supplementary Material
Download PDF (509.7 KB)Acknowledgements
The authors would like to acknowledge the participants as a part of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data used during the study are available from the corresponding author (HM) by request.
Additional information
Funding
Notes on contributors
Masaki Ryuzaki
Masaki Ryuzaki, MD, an assistant of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Hiroki Mizukami
Hiroki Mizukami, MD, PhD, a professor and head of Pathology and Molecular Medicine focuses on diabetic polyneuropathy, diabetic β-cell pathology and cancer pathology with diabetes.
Yuki Takeuchi
Yuki Takeuchi, MD, PhD, an assistant professor of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Sho Osonoi
Sho Osonoi, MD, PhD, an assistant professor of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Takanori Sasaki
Takanaroi Sasaki, BS, MT, an assistant professor of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Zhenchao Wang
Zhenchao Wang, MD, a PhD student of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Hanae Kushibiki
Hanae Kushibiki, MD, an assistant professor of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Takahiro Yamada
Takahiro Yamada, MD, a PhD student of Gastrointestinal Surgery has an interest in the mechanism of diabetic polyneuropathy and pancreas ductal carcinoma.
Keisuke Yamazaki
Keisuke Yamazaki, MD, a PhD student of Gastrointestinal Surgery has an interest in the mechanism of diabetic polyneuropathy and pancreas ductal carcinoma.
Saori Ogasawara
Saori Ogasawara, BS, MT, a technician of Pathology has interest in the mechanism of diabetic polyneuropathy.
Takefusa Tarusawa
Takefusa Tarusawa, MD, a PhD student of Pathology and Molecular Medicine has an interest in the mechanism of diabetic polyneuropathy.
Tatsuya Mikami
Tatsuya Mikami, MD, PhD, a professor of Innovation Center for Health Promotion specializes in public health.
Kenichi Hakamada
Kenichi Hakamada, MD, PhD, a professor, Gastrointestinal Surgery focuses on cancer treatment.
Shigeyuki Nakaji
Shigeyuki Nakaji, MD, PhD, a professor of Social Medicine specializes in public health.
References
- Hoitsma E, Reulen JP, de Baets M, Drent M, Spaans F, Faber CG. Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci. 2004;227(1):119–30. doi: 10.1016/j.jns.2004.08.012.
- Rosenberg ME, Tervo TMT, Immonen IJ, Muller LJ, Gronhagen-riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;130(6):865–2921. doi:10.1016/s0002-9394(00)00839-4.
- Itabashi C, Mizukami H, Osonoi S, Takahashi K, Kudo K, Wada K, et al. Normal High HbA1c a Risk factor for abnormal pain threshold in the Japanese population. Front Endocrinol (Lausanne). 2019;11:651, doi:10.3389/fendo.2020.00130.
- Kudoh K, Mizukami H, Itabashi C, Fuke N, Osonoi S, Takeuchi Y, et al. Lipopolysaccharide-binding protein is a distinctive biomarker of abnormal pain threshold in the general Japanese population. BMJ Open Diabetes Res Care. 2020;8(1):e001739, doi:10.1136/bmjdrc-2020-001739.
- Osonoi S, Mizukami H, Itabashi C, Wada K, Kudoh K, Igawa A, et al. Increased oxidative stress underlies abnormal pain threshold in a normoglycemic Japanese population. Int J Mol Sci. 2020;21(21):8306, doi:10.3390/ijms21218306.
- Takeuchi Y, Mizukami H, Kudoh K, Osonoi S, Sasaki T, Kushibiki H, et al. The diversity and abundance of gut microbiota are associated with the pain sensation threshold in the Japanese population. Neurobiol Dis. 2022;173:105839, doi:10.1016/j.nbd.2022.105839.
- Kushibiki H, Mizukami H, Osonoi S, Takeuchi Y, Sasaki T, Ogasawara S, et al. Tryptophan metabolism and small fibre neuropathy: a correlation study. Brain Commun. 2024;6(2):fcae103, doi:10.1093/braincomms/fcae103.
- Mizukami H, Osonoi S. Pathogenesis and molecular treatment strategies of diabetic neuropathy collateral glucose-utilizing pathways in diabetic polyneuropathy. Int J Mol Sci. 2022;22(1):94, doi:10.3390/ijms22010094.
- Gutteridge JM. Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact. 1994;91(2-3):133–40. doi:10.1016/0009-2797(94)90033-7.
- Rajanandh MG, Kosey S, Prathiksha G. Assessment of antioxidant supplementation on the neuropathic pain score and quality of life in diabetic neuropathy patients - a randomized controlled study. Pharmacol Rep. 2014;66(1):44–48. doi:10.1016/j.pharep.2013.08.003.
- Maiani G, Castón MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53:S194–S218. doi:10.1002/mnfr.200800053.
- Azqueta A, Collins AR. Carotenoids and DNA damage. Mutat Res. 2012;733(1-2):4–13. doi: 10.1016/j.mrfmmm.2012.03.005.
- Park G, Horie T, Iezaki T, Okamoto M, Fukasawa K, Kanayama T, et al. Daily oral intake of β-cryptoxanthin ameliorates neuropathic pain. Biosci Biotechnol Biochem. 2017;81(5):1014–7. doi: 10.1080/09168451.2017.1280661.
- Nakaji S, Ihara K, Sawada K, Parodi S, Umeda T, Takahashi I, et al. Social innovation for life expectancy extension utilizing a platform-centered system used in the Iwaki health promotion project: a protocol paper. SAGE Open Med. 2021;9:205031212110026, doi:10.1177/20503121211002606.
- Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1(5):212–28. doi:10.1111/j.2040-1124.2010.00074.x.
- Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14(7):1200–11. doi:10.1017/S1368980011000504.
- Watanabe T. Food composition tables of Japan and the nutrient table/database. J. Nutr. Sci. Vitaminol (Tokyo). 2015;61:S25–S27. doi:10.3177/jnsv.61.S25.
- Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27(2):362–6. doi:10.2337/diacare.27.2.362.
- Palozza P, Calviello G, Serini S, Maggiano N, Lanza P, Ranelletti FO, Bartoli GM. β-carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic Biol Med. 2001;30(9):1000–7. doi:10.1016/s0891-5849(01)00488-9.
- Matsuda-Inoguchi N, Date C, Sakurai K, Kuwazoe M, Watanabe T, Toji C, et al. Reduction in estimated vitamin A intake induced by new food composition tables in Japan, where vitamin A is taken mostly from plant foods. Internat J Food Sci Nutr. 2006;57:279–91. doi:10.1080/09637480600789958.
- Burri BJ, La Frano MR, Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr Rev. 2016;74(2):69–82. doi:10.1093/nutrit/nuv064.
- Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA, Bogle ML, Tucker KL. Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr. 2008;11(10):989–97. doi:10.1017/S1368980007001310.
- Sugiura M, Masaya K, Matsumoto H, Nagao A, Yano M. Serum concentration of BETA-cryptoxanthin in Japan reflects the frequency of Satsuma mandarin (Citrus unshiu Marc.) consumption. J health science. 2002;48(4):350–3. doi:10.1248/jhs.48.350.
- United States Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington DC: National Academy Press; 2000.
- Carroll YL, Corridan BM, Morrissey PA. Carotenoids in young and elderly healthy humans: dietary intakes, biochemical status and diet-plasma relationships. Eur J Clin Nutr. 1999;53(8):644–53. doi:10.1038/sj.ejcn.1600827.
