Abstract
Phenolics produced during xylooligosaccharide production might inhibit xylanases and enhance the antioxidant and antimicrobial activities of XOS. The effects of phenolic compounds on xylanases may depend on the type and concentration of the compound, the plant biomass used, and the enzyme used. Understanding the effects of phenolic compounds on xylanases and their impact on XOS is critical for developing viable bioconversion of lignocellulosic biomass to XOS. Understanding the complex relationship between phenolic compounds and xylanases can lead to the development of strategies that improve the efficiency and cost-effectiveness of XOS manufacturing processes and optimise enzyme performance.
1. Introduction
The production of xylooligosaccharide (XOS) from lignocellulosic biomass has received a lot of attention, and it helps to meet the ever-increasing demand for functional food supplements [Citation1–3]. The main components of these feedstocks are hemicellulose, cellulose, and lignin. Xylan, the most common hemicellulose constituent, has been linked to the production of XOS [Citation4]. Endo-(1,4)-ß-xylanases are used to hydrolyze xylan enzymatically to produce XOS. For economically viable xylan degradation in many cases, efficient and low-cost xylanases are required [Citation5]. Xylanases with a wider range of optimal conditions, consistent performance, minimal substrate loss, and product generation with minimal side effects are preferred in commercial xylan hydrolysis. However, very few xylanases are unaffected by one or more of the critical parameters that govern their catalytic activity and effectiveness. Improved efficiency and resistance to inhibitors meet environmental, financial, and industry demands [Citation5,Citation6].
Chemical hydrolysis, autohydrolysis, enzymatic hydrolysis, or a combination of these methods were used to create XOS. However, pretreatment is required in the enzymatic production of XOS to separate the cell wall constituents, primarily by degrading lignin bonds in lignocellulosic materials, allowing xylanases to effectively metabolize xylan and create XOS [Citation7]. During the pretreatment of lignocellulosic material, native lignin is frequently transformed, resulting in oligomers, monomeric phenolics, or other degradation modifications. As a result, non-soluble lignin may persist in processed lignocellulosic materials and co-exist with hemicellulose or cellulose, and it frequently inhibits enzymatic hydrolytic activity (cellulases or hemicellulases) and lowers product yields. Unfortunately, by-products of the physio-chemical pre-treatment of lignocellulosic biomass wastes continue to be an issue in sectors that integrate saccharification and fermentation.
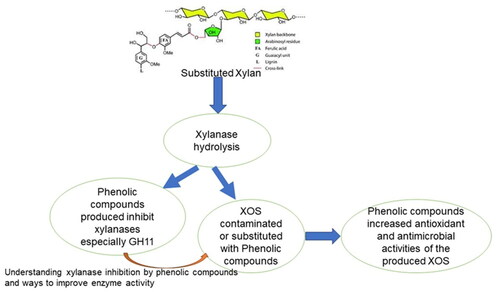
Furthermore, lignin and lignin-derived phenolic compounds continue to be a major source of concern in XOS production because they inhibit xylanases, especially at high phenolic concentrations relevant to high biomass-loading xylanase hydrolysis systems [Citation8–11]. As a result, efficient xylan degradation is difficult. The negative impact of lignin-derived phenolics on xylanases has long been considered and addressed in lignocellulosic material hydrolysis research and development activities, but most solutions are expensive.
Regardless of the lignocellulosic biomass substrate, various pre-treatment procedures generate lignin-derived phenolic compounds (alkaline, acidic, or steaming). The type and quantity of lignin-derived phenolic compounds are determined by the lignocellulosic residue species and pre-treatment processes, and phenolic compound concentrations can be modified by solid loading in pre-treatment and enzymatic digestion. The concentration of phenolic compounds increases as the solid input in the hydrolysis process increases. The inhibitory effects of lignin derivatives/phenolic compounds on enzymes differ depending on the source and chemical composition [Citation12,Citation13]. Furthermore, pre-treatment by-product size has been identified as the primary driving force behind the inhibition of Bacillus firmus K-1 glycoside hydrolases family 11 (GH11) xylanase (Xyn11A) by lignin and lignin derivatives [Citation14]. The inhibition effect of macromolecular lignin could be reduced, as with cellulases, by increasing the enzyme/substrate concentration, protecting proteins like bovine serum albumin, and transforming inhibitors into less inhibitory substances [Citation15]. With phenolics, this is not the case. The inhibitory properties of phenolics and lignin can be attributed primarily to their xylanase-binding behavior and properties (size, polarity, chemical reactivity, and concentration). There are only a few macromolecular lignin adsorption sites. However, because phenolic compounds have multiple binding sites, eliminating the inhibitory effects of monomeric phenolic compounds by increasing enzyme concentrations is difficult [Citation15].
Furthermore, no detoxification procedure completely removes all inhibitors from pre-treatment hydrolysates from the feedstock. This poses a problem for manufacturers who combine saccharification and fermentation. Furthermore, the detoxification process reduced xylan levels, resulting in a significant decrease in XOS production. As a result, some inhibitors must be considered digestion barriers as well as valuable compounds for other purposes, such as antioxidants (phenolic acid). As a result, developing extraction methods that preserve the inhibitors while producing XOS could be critical. This can be accomplished by optimizing xylanases, particularly those that are resistant to inhibition. To avoid the inhibitory effects of lignin, derivatives, and phenolic chemicals, computational simulation models and protein engineering techniques are used. In vitro, site-directed mutagenesis is used to validate predictions.
Despite this, phenolic compounds are far more inhibiting than sugars, furan derivatives, and organic acids because they can precipitate and permanently inhibit enzymes. Our understanding of phenolic inhibition, however, is insufficient to solve the problem. Understanding and reducing phenolic inhibitory effects in xylan hydrolysis is therefore critical for assessing the productivity of lignocellulosic biomass hydrolysis by xylanases into XOS. This review summarizes the inhibitory effects and detoxifying strategies of lignin-derived phenolic compounds derived from agricultural wastes on endo-acting −1,4 bacterial xylanases, identifies knowledge gaps, and evaluates the effect of phenolic content on XOS antioxidant activity.
2. Phenolic compounds and their inhibitory effects on xylanases
2.1. Phenolic compounds classification
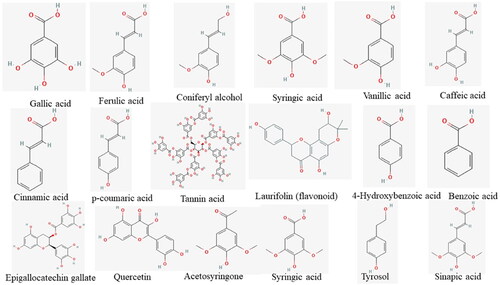
Much lignocellulosic biomass contains phenolic compounds with a common structural feature: an aromatic ring with at least one hydroxyl functional group, that is, a phenol [Citation16,Citation17]. Although most lignocellulosic biomass waste contains phenolic compounds, which are polymerized into larger molecules such as tannins and flavonoids, a basic classification divides the large group of phenolic compounds into simple and polyphenolic compounds based solely on the number of phenol subunits present [Citation16–18]. depicts the classification of phenolic compounds, with simple phenolics classified as phenolic acids and coumarins. Phenolic acids, which are abundant in lignocellulosic biomass, have been linked to a variety of biological effects [Citation17]. Hydroxybenzoic and hydroxycinnamic acids are two types of phenolic acids. The most prominent hydroxycinnamic acids are FA, p-coumaric acid, and caffeic acid, while GA is the most abundant benzoic acid [Citation17]. depicts a schematic representation of numerous phenolic compounds found in lignocellulosic biomass. These phenolic compounds are produced during the pretreatment of lignocellulosic biomass and inhibit xylanases during hydrolysis. Vanillin, 4-hydroxybenzoic acid, and catechol are the most common phenolics produced during lignocellulosic biomass waste pretreatments [Citation18–20]. Most phenolics are derived from lignin decomposition, with a few derived from extractives [Citation3,Citation10].
Figure 1. Overview of the classification of phenolic compounds [Citation16–18].
![Figure 1. Overview of the classification of phenolic compounds [Citation16–18].](/cms/asset/f12273c2-0254-4b9b-822d-c34d77f38a34/ganp_a_2328723_f0001_c.jpg)
2.2. Xylanase inhibition by phenolic compounds
Among the by-products of pre-treatment of agricultural wastes, phenolic compounds are the most inhibitory chemicals, causing precipitate and persistent blockage of enzyme processes [Citation21,Citation22]. In a study by Mathibe et al. [Citation12], kraft lignin, coumaric acid, FA, syringic acid, and guaiacol were among the most negligible inhibitive phenolic compounds on Thermocyces lanuginosus VAPS-24 (XynA) activity. In contrast, vanillin, GA, and vanillic acid were the most potent inhibitors at a concentration of 2 mg/ml [Citation12]. According to Michelin et al. [Citation23], phenolic compounds from pretreated SCB inhibited xylanase. At high doses, phenolic compounds (protocatechuic acid, vanillic acid, and acetovanillone) inhibited commercial xylanase (Igaarazyme-40S) [Citation24]. Another study revealed that phenolic compounds from SCB inhibited Cellulomonas flavigena PR-22 xylanases by 74% [Citation21]. Moreira et al. [Citation25] discovered that phenolic compounds significantly alter the activity of xylanase (XylT1) from Aspergillus terreus, with 4-hydroxybenzoic acid having the most significant effect and cinnamic acid, FA, p-coumaric acids, and vanillin deactivating the enzyme. To inhibit xylanase (Tx-Xyl) from Thermobacillus xylanilyticus, a noncompetitive multi-site inhibitory mechanism of phenolic compounds, including cinnamic acid, p-coumaric acid, caffeic acid, FA, and 3,4,5-trimethoxy-cinnamic acid, was proposed [Citation13]. Molecular docking revealed that enzyme-phenolic compound interactions were most likely to include residues on the surface of Tx-Xyl, indicating that the inhibitory effects of phenolic compounds on Tx-Xyl were most likely caused by enzyme conformational changes that caused steric inactivation [Citation13].
The inhibitory effects of phenolic compounds on xylanases differ based on the plant’s chemical composition, source, and pre-treatment procedure [Citation9,Citation12,Citation13,Citation17,Citation26] (). Acidic pretreatment, for example, yields fewer inhibitors (furans, phenolics, and aliphatic carboxylic acids), whereas alkali pre-treatment yields more inhibitory compounds (acetic acids, dicarboxylic acids, phenolics, and furan aldehydes) [Citation10,Citation20]. Even though the agricultural residue species and pre-treatment processes determine the type and quantity of lignin-derived phenolic compounds, phenolic compound concentrations can be modified by solid loading in pre-treatment and enzymatic digestion [Citation12,Citation13]. Increasing the solid input in the hydrolysis process increases the concentration of the phenolic compounds. Furthermore, because of variances in enzyme binding behavior and pre-treatment by-product features, such as polarity, size, concentration, and chemical reactivity, the inhibitory impact of pre-treatment by-products such as lignin and phenolic compounds varies [Citation14,Citation27]. Furthermore, lignin and lignin-derived phenolic compounds remain a severe concern in XOS production because they inhibit xylanases, particularly at high phenolic compound concentrations relevant to a high biomass-loading xylanase hydrolysis system [Citation8–11]. This makes efficient xylan degradation problematic. The negative influence of lignin-derived phenolics on xylanases has long been thought of and addressed in lignocellulosic material hydrolysis research and development activities.
Table 1. Inhibitory effects of phenolic compounds on xylanases.
In another study by Hsieh et al. [Citation28], guaiacol, syringic acid, eugenol, and p-coumaric acid did not affect pulpzyme HC activity. Catechol and vanillin, on the other hand, inhibited the activity of pulpzyme HC [Citation28]. Pulpzyme HC was less resistant to catechol in alkaline than in neutral conditions, whereas vanillin inhibited pulpzyme HC in both neutral and alkaline pH [Citation28]. Catechol inhibited xylanase activity more than vanillin, according to statistical analysis [Citation28]. Furthermore, the concentrations for these two differ significantly, with vanillin concentrations less than 2.5 mM having no apparent impact on xylanase activity, but catechol concentrations as low as 0.5 mM significantly reducing xylanase activity [Citation28]. Morrison et al. [Citation8], also found that the greatest inhibition of XynA was observed in the presence of gallic acid (47%), followed by r-coumaric acid (38%). Several studies have also found that gallic acid was one of the most potent inhibitors of different xylanases. For example, Ullah et al. [Citation29] found that xylanase PcX1 tolerated vanillin, ferulic acid, coumaric acid, syringaldehyde, and hydroxybenzoic acid, with activity slightly inhibited (17%) by gallic and tannic acid. Gallic acid inhibited xylanases with greater than 50% inhibition in studies conducted by Mathibe et al. [Citation12], Hamann et al. [Citation15], and Gufe et al. [Citation30].
At low concentrations, phenolic compounds have been found to activate or increase the activities of some xylanases. For example, ferulic acid was found to activate two xylanases (Xyl-1/GH10 and Xyl-2/GH11) from Aspergillus tamarii [Citation31]. Oliveira et al. [Citation32] discovered that ferulic acid increased enzyme activity by 75% and catalytic velocity, efficiency, and catalytic rate constant by 2.5-fold while not affecting xylanase affinity for xylan. In some studies, such as those conducted by Silva et al. [Citation27] and Monclaro et al. [Citation31], some of the tested enzymes were found to be ferulic acid resistant. As a result, further research into the enzymes that can be inhibited or activated by these phenolic compounds is required to use the enzymes appropriately and efficiently. A compressive search for xylanases that are resistant to phenolic compounds found in lignocellulosic biomass is critical for XOS production.
2.3. The inhibition of lignin-derived phenolic compounds
Understanding and eliminating the inhibitory effects of phenolic compounds on xylan hydrolysis is critical for evaluating XOS production from agricultural wastes using xylanase enzymes. The inhibitory effect of macromolecular lignin on cellulases could be reduced by increasing the enzyme/substrate concentration and converting inhibitors into less inhibitory compounds with lignin-degrading enzymes such as laccases [Citation15]. This is not true of phenolic compounds. Our understanding of phenolic compound inhibition, however, is insufficient to remedy the situation. Although washing removes the phenolic compounds formed during lignocellulosic biomass waste pre-treatment, a significant amount of the substrate is lost. The inhibitory properties of phenolic compounds and lignin stem primarily from their xylanase-binding properties (size, polarity, chemical reactivity, and concentration). There are only a few macromolecular lignin adsorption sites. However, since phenolic compounds have multiple binding sites, increasing enzyme concentrations to eliminate the inhibitory effects of a monomeric phenolic compound is difficult [Citation15].
Only a few xylanases have been reported to be resistant to phenolic compounds, including xylanase XylT2 from Aspergillus terreus [Citation33] and xylanase X22 from Emericela nidulans [Citation27], which were shown to be resistant to coumaric acid, FA, cinnamic acid, hydroxybenzoic acid, tannic acid, and vanillin. Nonetheless, due to their propensity to effectively adsorb phenolic compounds, polymeric materials such as polyethene glycol and polyvinylpyrrolidone can avoid enzyme inhibition [Citation22]. To decrease enzymatic inhibition caused by these phenolic compounds, biomass-derived phenolic compounds in the crude extract should be reduced or eliminated before enzyme hydrolysis. These findings can be used to encourage the development of suitable biomass pre-treatment procedures that are less harmful to enzymatic hydrolysis [Citation12]. Unfortunately, no detoxifying method eliminates all inhibitors from agricultural waste hydrolysates.
Additionally, the detoxification procedure lowered xylan levels, resulting in a significant decrease in XOS generation. Although relatively few xylanases are resilient to all phenolic compounds, discovering such enzymes can be time-consuming and expensive. Enzyme augmentation by protein engineering techniques can boost enzyme resilience to phenolic compounds. Protein engineering is less costly and requires less time to produce resistant enzymes. Computational predictions of the inhibitory mechanism and how to solve it using in silico site-directed mutagenesis are used in protein engineering techniques, and the predictions are confirmed by in vitro inhibitory testing.
2.3.1. Enzymatic engineering as a solution for phenolic compound inhibition
Several microorganisms generate xylanase enzymes, including fungi (mainly filamentous fungi such as ascomycetes) and bacteria [Citation34–36]. The filamentous fungus secretes many more comprehensive xylanolytic enzymatic complexes than yeast and bacteria [Citation34]. Industrial xylanase preparations have been manufactured massively, mainly from Aspergillus, Trichoderma, and Penicillium fungi [Citation34,Citation36,Citation37]. However, low yield, poor stability, decreased activity, by-product formation, a complex purification procedure, and various other issues make the enzyme production sector difficult [Citation38,Citation39].
Novel advances in protein engineering, particularly the merging of interdisciplinary technologies such as post-translational enzymatic modification, structural-aided protein modification, and computational modelling strategies, have opened a new vista for more efficient enzyme production [Citation38,Citation39]. Random mutagenesis, site-directed mutagenesis, gene shuffling, and fusion proteins have all been used in protein engineering. Several studies have looked into the possibility of enzymatically engineering xylanases to improve their catalytic efficiency, pH tolerance, and thermostability. For example, Li et al. [Citation40] successfully engineered xylanase by the N-terminal region replacement and site-directed mutagenesis in the cord of xylanase from Streptomyces rochei L10904 to improve its catalytic properties, and they discovered that the engineered xylanases had higher specific activities, greater substrate affinity, and better hydrolysis characteristics than the native xylanase. Wang et al. [Citation41] subjected the Bacillus subtilis xylanase sequence to site saturation and iterative mutagenesis and discovered that the engineered xylanases had higher catalytic activity, and efficiency, and also proved to be more thermostable than the native xylanase. You et al. [Citation42] used a structure-based semi-rational design strategy to improve the low-temperature catalytic performance of xylanase from Bispora species. When compared to native xylanase, the engineered xylanase had higher specific activity (2.9-fold), catalytic efficiency (2.8-fold), and thermostability [Citation42]. Damis et al. (2019) discovered that engineered xylanases created using a combination of site-saturation, error-prone PCR, and site-directed mutagenesis had higher catalytic efficiency, yield, thermostability, and acid stability than native xylanase. Several other attempts at protein engineering have been made, for example, to improve primary sequences, structural folding, substrate specificities, temperature or pH stability, activity, and catalytic mechanisms [Citation2,Citation41,Citation43–46]. As a result, these findings suggest that engineering xylanases to improve their activity against phenolic compounds holds promise, but more research is needed to specifically target this application. There have been few attempts to improve xylanase resistance to phenolic compounds. Gufe et al. [Citation30] engineered xylanase from Bacillus firmus K-1, which was more resistant to phenolic compounds such as gallic acid, ferulic acid, and coniferyl alcohol than the native xylanase. It’s important to note that the success of engineering xylanases with enhanced resistance to phenolic compounds depends on a variety of factors, including the specific type of xylanase and phenolic compounds of interest, as well as the intended application.
2.4. Effect of phenolic compounds on antioxidant and antimicrobial properties of xylooligosaccharides
It has been reported that phenolic compounds in polysaccharides such as xylan have high antioxidant activity because they produce a resonance-stabilized phenoxy radical, stop free radical chain reactions (the electron donation and hydrogen atom transfer to free radicals), and are associated with modifying some cellular signalling processes such as the inhibition of lipid peroxidation, all of which contribute to the reduction of peroxidation, cellular damage, or death [Citation47–51]. Since XOS has a xylose backbone, which can have a side chain that is mostly substituted with arabinose and can contain ester-linked phenolic compounds such as ferulic, coumaric, and caffeic acids, influences their physicochemical properties of XOS [Citation47]. It was indicated that the feruloyl oligosaccharides could efficiently protect normal rat erythrocytes against hemolysis induced by free radicals under in vitro conditions, indicating the anti-oxidative potential of feruloyl oligosaccharides derived from wheat bran insoluble dietary fiber [Citation52].
Additionally, the antioxidant activities of free and bound ferulic acids were documented from native and malted finger millet, and recently, the strong antioxidant activity of water-soluble arabinoxylans isolated from rice and ragi was demonstrated [Citation52]. Furthermore, the presence of phenolic compounds, sugars with uronyl/acetyl groups, and the degree/nature of polymerization impart strong antioxidant activity of the polysaccharides [Citation47–49,Citation51–54]. According to Xiao et al. [Citation55], bamboo XOS had superoxide radical scavenging index values of 0.95, which was comparable to commercial antioxidant BHT (0.79). When compared to BHT and bamboo-produced XOS, commercial antioxidant BHA (0.2) demonstrated the greatest scavenging ability [Citation55]. Furthermore, the bamboo XOS had a comparable hydroxyl radical scavenging index value of 0.65 to that of BHT (0.57), while BHA (0.17) demonstrated significantly better hydroxyl radical scavenging activity than BHT and XOS [Citation55]. When compared to the commercial antioxidant BHT, XOS produced by autohydrolysis demonstrated acceptable scavenging activity in superoxide radicals and hydroxyl radicals [Citation55]. The antioxidant activity of bamboo XOS was attributed to the presence of sugars with uronic/acetyl groups, phenolic acids, degree of polymerization, linkage type, substituted groups and position and ester-linked phenolic acids. Reddy et al. [Citation54] extracted XOS and phenolic acids from several lignocellulosic biomass such as sugarcane bagasse, bamboo, rice straw, and wheat bran using crude enzyme of Bacillus subtilis and found that phenolic compounds and XOS varied depending on plant biomass and their antioxidant activity correlated well with hydroxycinnamic acids content. Rashad et al. [Citation56] found that XOS mixtures produced using Bacillus amyloliquifaciens NRRL B-14393 xylanase from mango and orange peels showed very high antioxidant activities (96% and 76.84%, respectively) and total phenolic acid contents (156.32 and 133.74 mg GAE/litre of extract, respectively) compared to water hyacinth plant and sugarcane bagasse XOS mixtures. Si et al. [Citation47] discovered that the total antioxidant activity of hydrolysate was 141.8 mg ascorbic acid equivalents per g crude xylan, and the highest 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity reached 92.7%, and there was a correlation between phenolic acid concentrations and antioxidant activity of the produced XOS. Phenolic compounds also have increased the antimicrobial activities of XOS against Klebsiella pneumonia and Pseudomonas aeruginosa [Citation47]. In another study, XOS derived from garlic straw demonstrated strong antimicrobial activities against K. pneumoniae, Enterococcus faecalis, M. luteus, and P. aeruginosa but not against E.coli, Bacillus thuringiensis, Staphylococcus aureus and Listeria monocytogenes [Citation57]. The antioxidant and antibacterial actions of this XOS were linked to the XOS's average molecular weight (). Furthermore, XOS produced from sugarcane bagasse exhibited significant antimicrobial properties against Bacillus subtilis [Citation58]. The antibacterial efficacy of XOS produced from birchwood xylan against Helicobacter pylori, S. aureus, Bacillus cereus, and Micrococcus flavus was significant, but not against P. aeruginosa and Proteus mirabilis. The antibacterial and antioxidant activities of the XOS were linked to its 4-O-methyl-D-glucuronic acid substitutions [Citation59].
Table 2. Effects of phenolic compounds on the antioxidant and antimicrobial activity of XOS.
Considering the dual role of phenolic compounds (inhibition of xylanases and enhancing antioxidant/antimicrobial activities of XOS) allows for a holistic approach to process optimization and downstream applications. It enables the development of sustainable and efficient bioprocesses that not only mitigate the inhibitory effects of phenolics but also harness their value as valuable resources. This approach contributes to the utilization of phenolics in a more sustainable and economically viable manner while promoting the transition towards a circular bioeconomy. Therefore, by optimizing the processes and downstream applications, phenolics can be efficiently utilized along with other biomass components, such as sugars, lignin, and proteins, to create a diverse range of products, including biofuels, chemicals, materials, XOS, and energy. Xylanases that are resistant to phenolic compounds could be a promising source of enzymes for the production of XOS with high phenolic contents, which will enhance XOS’s antioxidant/antimicrobial activities.
3. Future research needs
There is still much to learn about the complex interactions between phenolic compounds, enzymes, microbes, and biochemical processes. It should be noted that the efficacy of these strategies varies depending on the specific phenolic compounds and biological systems involved. To successfully overcome the inhibitory effects of phenolic compounds, a multidisciplinary approach combining molecular biology, biotechnology, chemistry, and process engineering may be required. Several strategies and research directions can be used to overcome the inhibitory effects of phenolic compounds. Such potential strategies can include the following:
Further studies on the genetic modification strategies to improve xylanase tolerance to phenolic compounds. Some amino acid residues on xylanases that interact with phenolics have been identified, allowing scientists to modify enzyme sequences to disrupt these interaction sites. This can include introducing genes that degrade phenolic compounds or increasing the expression of endogenous genes involved in detoxification pathways.
Further studies into enzymes or microorganisms that can help break down phenolic compounds into less inhibitory or non-inhibitory forms.
More research into chemical modifications to phenolic compounds to lessen their inhibitory effects. This can include structural changes to change their chemical properties and reduce inhibitory effects while retaining desired functionalities.
Further studies on adsorbents or techniques for immobilisation to remove or sequester phenolic compounds from the system. Adsorbents such as activated carbon, resins, and other materials can bind phenolic compounds selectively, reducing their inhibitory effects on xylanase.
Further research on ways to reduce the inhibitory effects of phenolic compounds by optimising process conditions and bioreactor design. This can include adjusting factors like temperature, pH, oxygen levels, or substrate concentration to create a less inhibitory environment for target xylanase or processes.
Investigate the possibility of combining phenolic compounds with other compounds or treatments that can reduce their inhibitory effects. Certain antioxidants or chemical additives, for example, may counteract the inhibitory effects of phenolic compounds.
4. Conclusion
There are numerous ways to improve the process if xylan hydrolysis inhibitors are discovered. By first optimizing the pretreatment and hydrolysis parameters, inhibitor formation can be reduced. Second, hydrolysis can be predicted using hydrolysate analysis, and third, specific detoxifying methods can be developed to effectively remove inhibitors from highly inhibitory substituted xylan just before it degrades. By better understanding inhibitor inhibition mechanisms, interactions, and the impact of hydrolysis factors such as pH, the conditions for producing XOS can be improved. When designing the ideal hydrolysis process, the rates of bioconversion and the adaptive response of xylanases to inhibitors must also be taken into account. The production of xylooligosaccharides with high phenolic content is critical and has great potential in the development of functional foods or feeds. The phenolic compounds’ resistivity and production of XOS with high phenolic compounds by some xylanases demonstrated the biotechnological potential in the hydrolysis of agro-industrial residue lignocellulosic biomass. The bi-functionality of these xylanases allowed for the simultaneous extraction of XOS and antioxidant compounds from plant biomass. Further research is needed to find more suitable xylanases with high XOS yields that contain strong antioxidant and antimicrobial activities.
CRediT author contributions
CG: Conceptualization, writing, revision.
All authors: writing, revision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- J.A. Linares-Pasten, A. Aronsson, and E.N. Karlsson, Curr. Protein Pept. Sci .19, 48 (2018).
- S. Zhang, K. Zhang, X. Chen, X. Chu, F. Sun, and Z. Dong, Biochem. Biophys. Res. Commun. 395, 200 (2010).
- X. Chen, R. Zhai, Y. Li, X. Yuan, Z.H. Liu, and M. Jin, Biotechnol. Biofuels. 13, 44 (2020).
- A.A. Aachary, and S.G. Prapulla, Comp. Rev. Food. Sci. Food. Safe. 10, 2 (2011).
- U. Javed, A. Ansari, A. Aman, and S.A.U. Qader, Biocatal. Agric. Biotechnol. 21, 101341 (2019).
- T.Y. Chen, J.L. Wen, B. Wang, H.M. Wang, C.F. Liu, and R.C. Sun, Bioresour. Technol. 244, 717 (2017).
- L. Kumar, V. Arantes, R. Chandra, and J. Saddler, Bioresour. Technol. 103, 201 (2012).
- D. Morrison, J.S. van Dyk, and B.I. Pletschke, BioResources 6, 3132 (2011).
- A. Oliva-Taravilla, E. Tomás-Pejó, M. Demuez, C. González-Fernández, and M. Ballesteros, J. Biotechnol. 218, 94 (2016).
- L.J. Jönsson, and C. Martín, Bioresour. Technol. 199, 103 (2016).
- P.T. Adeboye, M. Bettiga, and L. Olsson, AMB. Express. 4, 46 (2014).
- B.N. Mathibe, S. Malgas, L. Radosavljevic, V. Kumar, P. Shukla, and B.I. Pletschke, 3 Biotech. 10, 349 (2020).
- I. Boukari, M. O’Donohue, C. Rémond, and B. Chabbert, J. Mol. Catal. B: Enzym. 72, 130 (2011).
- A. Muhammad, P. Khunrae, and T. Sutthibutpong, J. Mol. Model. 26, 124 (2020).
- P.R.V. Hamann, T.C. Gomes, L. de M.B.Silva, and E.F. Noronha, Process. Biochem. 92, 1 (2020).
- L. Panzella, F. Moccia, R. Nasti, S. Marzorati, L. Verotta, and A. Napolitano, Front. Nutr. 7, 60 (2020).
- L.D. Shirahigue, and S.R. Ceccato-Antonini, Cienc. Rural. 50, e20190857 (2020).
- V.D. Mitchell, C.M. Taylor, and S. Bauer, Bioenerg. Res. 7, 654 (2014).
- E. Palmqvist, and B. Hahn-Hägerdal, Bioresour. Technol. 74, 25 (2000).
- J.S. Van Dyk, and B.I. Pletschke, Biotechnol. Adv. 30, 1458 (2012).
- E. González-Bautista, J.C. Santana-Morales, F.J. Ríos-Fránquez, H.M. Poggi-Varaldo, A.C. Ramos-Valdivia, E. Cristiani-Urbina, and T. Ponce-Noyola, Fuel. 196, 32 (2017).
- R.I.S. Ladeira-Ázar, T. Morgan, G.P. Maitan-Alfenas, and V.M. Guimarães, Appl. Biochem. Biotechnol. 188, 29 (2019).
- M. Michelin, E. Ximenes, M.D.L.T. de Moraes, and M.R. Ladisch, Bioresour. Technol. 199, 275 (2016).
- F. Kaya, J.A. Heitmann, and T.W. Joyce, J. Biotechnol. 80, 241 (2000).
- L.R. de Souza Moreira, M. De Carvalho Campos, P.H.V.M. De Siqueira, L.P. Silva, C.A.O. Ricart, P.A. Martins, R.M.L. Queiroz, and E.X. Ferreira Filho, Fungal. Genet. Biol. 60, 46 (2013).
- A.T.W.M. Hendriks, and G. Zeeman, Bioresour. Technol. 100, 10 (2009).
- C. d O. G. Silva, E.N. Aquino, C.A.O. Ricart, G.E.O. Midorikawa, R.N.G. Miller, and E.X.F. Filho, FEMS. Microbiol. Lett. 362, 094 (2015).
- P. Hsieh, C. Liu, C. Ko, B. Yang, and P. Lin, BioResources 16, 8111 (2021).
- S.F. Ullah, A.A. Souza, P.R.V. Hamann, A.R.P. Ticona, G.M. Oliveira, J.A.R.G. Barbosa, S.M. Freitas, and E.F. Noronha, Int. J. Biol. Macromol. 127, 385 (2019).
- C. Gufe, T. Sutthibutpong, A. Muhammad, A. Ngenyoung, T. Rattanarojpong, and P. Khunrae, Biocatal. Agric. Biotechnol. 36, 102147 (2021).
- A.V. Monclaro, G.L. Recalde, F.G. da Silva, S.M. de Freitas, and E.X. Ferreira Filho, Enzyme. Microb. Technol. 120, 16 (2019).
- I.C.M. Oliveira, A.V. Garay, A.A. Souza, N.F. Valadares, J.A.R.G. Barbosa, F.P. Faria, and S.M. Freitas, Sci. Rep. 12, 11409 (2022).
- L.R. Moreira, and E.X. Filho, Appl. Microbiol. Biotechnol. 100, 5205 (2016).
- M. Sizova, J. Izquierdo, N. Panikov, and L. Lynd, Appl. Environ. Microbiol. 77, 2282 (2011).
- J. van den Brink, and R.P. de Vries, Appl. Microbiol. Biotechnol. 91, 1477 (2011).
- M. Michelin, T.M. Silva, V.M. Benassi, S.C. Peixoto-Nogueira, L.A.B. Moraes, J.M. Leão, J.A. Jorge, H.F. Terenzi, and T. Maria de Lourdes, Carbohydr. Res. 345, 2348 (2010).
- M. Lafond, A. Tauzin, V. Desseaux, E. Bonnin, E.H. Ajandouz, and T. Giardina, Microb. Cell. Fact. 10, 1 (2011).
- A. Sharma, G. Gupta, T. Ahmad, S. Mansoor, and B. Kaur, Food. Rev. Int. 37, 121 (2021).
- T. Dinmukhamed, Z. Huang, Y. Liu, X. Lv, J. Li, G. Du, and L. Liu, Syst. Microbiol. Biomanuf. 1, 15 (2021).
- Q. Li, B. Sun, H. Jia, J. Hou, R. Yang, K. Xiong, Y. Xu, and X. Li, Int J. Biol. Macromol. 101, 366 (2017).
- X. Wang, R. Ma, X. Xie, W. Liu, T. Tu, F. Zheng, S. You, J. Ge, H. Xie, B. Yao, and H. Luo, Sci. Rep. 7, 15287 (2017).
- S. You, Z. Zha, J. Li, W. Zhang, Z. Bai, Y. Hu, X. Wang, Y. Chen, Z. Chen, J. Wang, and H. Luo, Biotechnol. Biofuels. 14, 195 (2021).
- H. Boonyaputthikul, A. Muhammad, S. Roekring, T. Rattanarojpong, P. Khunrae, and T. Sutthibutpong, Arch. Biochem. Biophys. 672, 108068 (2019).
- T. Sutthibutpong, T. Rattanarojpong, and P. Khunrae, J. Biomol. Struct. Dyn. 36, 3978 (2018).
- A.S. Prajapati, V.A. Pawar, K.J. Panchal, A.P. Sudhir, B.R. Dave, D.H. Patel, and R.B. Subramanian, BMC. Biotechnol. 18, 9 (2018).
- N. Han, H. Miao, J. Ding, J. Li, Y. Mu, J. Zhou, and Z. Huang, Biotechnol. Biofuels. 10, 133 (2017).
- D. Si, T. Shang, X. Liu, Z. Zheng, Q. Hu, C. Hu, and R. Zhang, Biotechnol. Rep. (Amst) 27, e00511 (2020).
- A. Kawee-Ai, A. Srisuwun, N. Tantiwa, W. Nontaman, P. Boonchuay, A. Kuntiya, T. Chaiyaso, and P. Seesuriyachan, Ultrason. Sonochem. 31, 184 (2016).
- P. Boonchuay, R. Wongpoomchai, S. Jaturasitha, S. Mahatheeranont, M. Watanabe, and T. Chaiyaso, Food. Biosci. 40, 100895 (2021).
- A. Fuso, W. Dejonghe, L. Cauwenberghs, G. Rosso, F. Rosso, I. Manera, and A. Caligiani, J. Funct. Foods. 101, 105417 (2023).
- G. Victoria Gautério, C. Amorim, S.C. Silvério, B.B. Cardoso, L.F. Ballesteros, J.I. Alves, M. Alcina Pereira, S.P. Silva, E. Coelho, M.A. Coimbra, S. Juliano Kalil, and L.R. Rodrigues, Food. Chem. 391, 133231 (2022).
- L. Rondini, M.N. Peyrat-Maillard, A. Marsset-Baglieri, G. Fromentin, P. Durand, D. Tomé, M. Prost, and C. Berset, J. Agric. Food. Chem. 52, 4338 (2004).
- B.R. Veenashri, and G. Muralikrishna, Food. Chem. 126, 1475 (2011).
- S.S. Reddy, and C. Krishnan, Food. Biotechnol. 27, 357 (2013).
- X. Xiao, C.Z. Wang, J. Bian, and R.C. Sun, RSC. Adv. 5, 106219 (2015).
- M.M. Rashad, A.E. Mahmoud, M.U. Nooman, H.A. Mahmoud, A.E.D.M. El-Torky, and A.T. Keshta, J. App. Pharm. Sci. 6, 030 (2016).
- F. Kallel, D. Driss, S.E. Chaabouni, and R. Ghorbel, Appl. Biochem. Biotechnol. 175, 950 (2015).
- T.S. Milessi, F.A.S. Corradini, J.V.M. Marçal, T.O. Baldez, W. Kopp, R.C. Giordano, and R.L.C. Giordano, Ind. Crops. Prod. 172, 114056 (2021).
- P. Christakopoulos, P. Katapodis, E. Kalogeris, D. Kekos, B.J. Macris, H. Stamatis, and H. Skaltsa, Int. J. Biol. Macromol. 31, 171 (2003).
- F. Liu, W.F. Xu, H. Mu, Z.R. Lv, J. Peng, C. Guo, H.M. Zhou, Z.M. Ye, and X.H. Li, Biosci. Biotechnol. Biochem. 84, 1788 (2020).
- A. Raj, S. Kumar, S.K. Singh, and M. Kumar, Sci. World J. 2013, 1 (2013).
- J. Bian, F. Peng, X.P. Peng, P. Peng, F. Xu, and R.C. Sun, Bioresour. Technol. 127, 236 (2013).
- C. Huang, X. Wang, C. Liang, X. Jiang, G. Yang, J. Xu, and Q. Yong, Biotechnol. Biofuels. 12, 189 (2019).
- D. Gowdhaman, and V. Ponnusami, Int. J. Biol. Macromol. 79, 595 (2015).
- N. Miguez, D. Fernandez-Polo, P. Santos-Moriano, B. Rodríguez-Colinas, A. Poveda, J. Jimenez-Barbero, A.O. Ballesteros, and F.J. Plou, Biorefin. 1 (2022).
- M. Gupta, R. Bangotra, S. Sharma, S. Vaid, N. Kapoor, H.C. Dutt, and B.K. Bajaj, Ind. Crops. Prod. 178, 114591 (2022).
- C. Valls, F.I.J. Pastor, T. Vidal, M.B. Roncero, P. Díaz, J. Martínez, and S.V. Valenzuela, Carbohydr. Polym. 194, 43 (2018).