Abstract
Seasonal phytoplankton blooms dominated by cyanobacteria in Upper Klamath Lake, Oregon, produce toxic microcystins at concentrations that may be detrimental to local wildlife. In 2009, water column, sediment trap, and surficial sediment samples were collected to relate the occurrences of microcystins in these samples to other environmental variables. Microcystin concentrations in sediment trap and surficial sediment samples were lower than in water column samples, and sediment trap samples contained higher concentrations than surficial sediment samples, with a peak in one sample (1107 μg/g) that exceeded the maximum concentration measured in any other water or sediment sample from this area. Concentrations of microcystins and cells of Microcystis aeruginosa increased following the decline of the first bloom dominated by non-microcystin-producing Aphanizomenon flos-aquae in response to an increase in nitrogen and phosphorus concentrations. Results of this study also show that nitrogen fixation by A. flos-aquae during spring and early summer provided nitrogen for growth of toxigenic M. aeruginosa and that phosphorus availability may have played a greater role overall in determining the pattern of microcystin occurrence in the lake, given the apparent influence of phosphorus on growth and decline of the A. flos-aquae bloom. This study is the first report of microcystins in sediments of Upper Klamath Lake, the largest lake (by surface area) in Oregon, and contributes to understanding the conditions under which elevated microcystin concentrations occur.
[Supplementary materials are available for this article. Go to the publisher's online edition of Lake and Reservoir Management for the following free supplemental resources: microscope images of major phytoplankton species, microcystin concentrations in surficial sediment samples from randomly selected sites, loadings of the first 2 principal components from PCA analysis of microcystin and water quality data, and the medians and ranges of measured limnological variables.]
High exogenous nutrient loading, warm weather, and calm wind promote the development of cyanobacterial blooms that produce toxic secondary metabolites, primarily microcystins, which have been associated with gastroenteritic and hepatic diseases and tumor promotion in humans, livestock, domestic animals, and wildlife (Sivonen and Jones Citation1999). In Upper Klamath Lake, Oregon, more than 90% of the phytoplankton community (by biovolume) is composed of non-microcystin-producing Aphanizomenon flos-aquae during periods of peak bloom density (Kann Citation1998, Kann and Smith Citation1999, Carmichael et al. Citation2000). The most common microcystin producer globally, Microcystis aeruginosa, is also present in the lake. On a water column mean basis, however, M. aeruginosa is a minor component of the total biomass, usually <1% (Kann Citation1998). Despite its low abundance, M. aeruginosa has been directly linked to the presence of microcystins in Upper Klamath Lake (Saker et al. Citation2007) and in Agency Lake (Jacoby and Kann Citation2007). Members of the genus Aphanizomenon produce cylindrospermopsins and other toxins in laboratory cultures (Preussel et al. Citation2006), but A. flos-aquae is not known to produce microcystins in Upper Klamath Lake, and since 2007, cylindrospermopsins have been found only at concentrations near or below the detection limit (S. Eldridge et al. Citation2012).
Changes in the occurrence of microcystins in water column samples from Upper Klamath Lake are associated with parameters, particularly nutrients, which affect water quality and are similar to, but noncoincident with, the growth dynamics of the A. flos-aquae-dominated bloom (S. Eldridge et al. Citation2012). In addition, previous work showed depletion in dissolved iron concentrations by the end of the late-season (Aug) bloom period, relative to early season conditions (Apr–May; Kuwabara et al. Citation2009). Iron is known to promote microcystin production in other systems (Sevilla et al. Citation2008, Alexova et al. Citation2011), so the seasonal depletion observed in 2006 may signify a possible role for iron availability in microcystin occurrence in Upper Klamath Lake.
Sedimentation is one of the most important loss processes for phytoplankton (Takamura and Yasuno Citation1988) and may be a major destination for microcystins and toxigenic cells (Chen et al. Citation2008, Wörmer et al. Citation2011). Because sediment microcystins likely originate and are transferred from the water column, the same environmental factors that influence water quality and A. flos-aquae bloom dynamics in Upper Klamath Lake may be related to the occurrence of microcystins in the water column and sediment samples. Understanding the spatial and temporal distribution of microcystins has major implications for monitoring the health of the federally endangered Lost River (Deltistes luxatus) and shortnose (Chasmistes brevirostris) suckers in Upper Klamath Lake. The decline in these fish populations has been attributed, in part, to water quality degradation associated with the growth of cyanobacteria (Williams Citation1988, Buettner and Scoppettone Citation1990, Perkins et al. Citation2000) and to microcystin exposure through ingestion (S. Eldridge et al. Citation2012). Because these fish are benthic feeders, they may be exposed to the toxin at or near lake sediments.
The goal of this study was to examine potential associations between sediment and water column (suspended sediment) microcystin concentrations and measurements of other environmental parameters, including iron. The results presented here may provide data necessary for resource managers to make decisions regarding restoration of the Upper Klamath River basin and, specifically, minimization of the risk of exposure to endangered fish and other wildlife.
Materials and methods
Study site
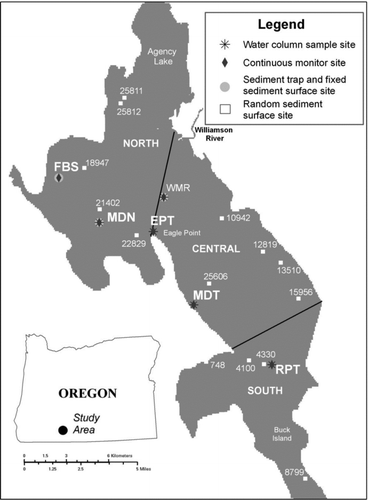
Upper Klamath Lake, including contiguous Agency Lake, is one of the largest natural freshwater lakes in the western United States and is the largest lake in Oregon. Although large by surface area (305 km2), the average depth is 2.6 m, and, with the exception of a deeper 15 m trench along the western shoreline between Eagle Point and Buck Island (), more than 90% of the lake is <4 m deep.
Figure 1 Locations of sediment sampling (sediment surface and sediment trap) sites, water column sample collection sites, and continuous monitoring sites, Upper Klamath Lake, Oregon, 2009. Only continuous monitoring sites where water and/or sediment samples were also collected are shown.
The lake's drainage basin is 9415 km2 and is composed of phosphorus-rich, volcanic soils. The Upper Klamath Lake system has been naturally eutrophic since at least the mid-1800s (the earliest known records; Phinney and Peek Citation1961). But, major changes in land use and hydrology of the lake over the past century by water diversions and agricultural changes in the upper watershed and by construction of a dam at the outlet in 1921 have increased nutrient loading both to the lake and to its tributaries. This has led to increased biomass and decreased species diversity of the phytoplankton blooms that occur in the lake during the summer and fall (Bradbury et al. Citation2004, Eilers et al. Citation2004). Both the timing and quantity of lake-flushing flows essential for nutrient export have been greatly altered, and the lake level has been reduced to below the pre-1921 levels. As a result of these and other changes, phosphorus loading in Upper Klamath Basin tributaries is elevated substantially above natural levels (∼65 μg/L; Kann and Walker Citation1999, ODEQ Citation2002), and the lake is now classified as hypereutrophic. The Upper Klamath Lake system has been the primary water source for agricultural activity within the Upper Klamath Basin since 1921 (BOR Citation2000). It is also the largest stopover in the Pacific Flyway for waterfowl and home to the largest wintering congregation of bald eagles in the lower 48 states (Carmichael et al. Citation2000).
Sample design, collection, and analyses
Sediment samples
Surficial sediment samples were collected weekly at 3 fixed sites, MDN, WMR, and FBS (), between 15 June and 6 October 2009 using either a benthos gravity corer or a hand-coring device. Cylindrical sediment traps were also deployed weekly near the sediment surface at these sites 4 days before core extractions. Site selection was based on results of previous work describing the distribution and habitat use of juvenile suckers in the lake (Bottcher and Burdick Citation2010). Additional cores were extracted on 31 July, 14 August, and 18 September at 5 randomly chosen sites. Weekly sampling at the 3 fixed sites provided higher temporal resolution, whereas sampling from randomly selected sites allowed data collection from a larger area of the lake. At each site, a sediment core approximately 6.5 cm in diameter was extracted, and the top 1 cm was removed and homogenized. Replicate cores were collected from one site each week (alternating between sites during subsequent samplings) to measure variability in sample collection. Samples were frozen and shipped overnight for microcystin analysis to the US Geological Survey (USGS) Columbia Environmental Research Center (CERC, Columbia, MO) within 3 days.
Sediment traps consisting of triplicate columns, each a 35 × 7 cm cylinder with a height:diameter ratio of 5:1, were positioned vertically in the water column at 0.5 m above the sediment. Sediment traps were deployed weekly for 24 h beginning 18 June at all fixed sites and until 12 August at WMR, 26 August at FBS, and 16 September at MDN. The bottom 100 mL from one cylinder in the trap at each site was collected and shipped overnight to CERC for microcystin analysis. An additional cylinder in the FBS trap was sampled concurrently with and in the same manner as all other cylinders between 18 June and 12 August to measure the variation (standard deviation) in microcystin concentration between trap columns.
Between 1 July and 16 September, additional samples from sediment-trap columns were retained for microscopic observation of the phytoplankton community and were preserved in Lugol's solution. The lower 100 mL (consisting primarily of suspended sediment) and the 200 mL of overlying lake water were collected and analyzed separately. Cells were far more abundant in the overlying 200 mL, so this fraction was screened to qualitatively estimate changes in the relative abundances over time of the groups most commonly observed that year: A. flos-aquae, M. aeruginosa, Woronichinia naegeliana, and Gloeotrichia echinulata. Estimates of increasing or decreasing abundance were based on sample photographs and recorded observations made during sample collection and screening. Differences between sites were difficult to distinguish, so relative abundance estimates were based on combining observations made at all sites. Samples were screened in triplicate or greater.
Water column samples
Water column samples were collected weekly at 2 of the 3 fixed sediment sites, MDN and WMR, as part of a USGS long-term water quality monitoring program. At these sites and at 3 additional sites in the monitoring program, MDT, EPT, and RPT (), samples were collected for analyses of total microcystins, chlorophyll a, dissolved inorganic phosphorus (DIP; PO4 3−), dissolved inorganic nitrogen (DIN: NH3, and NO2 − + NO3 −), total phosphorus (TP), and total nitrogen (TN) following the procedures outlined in D. Eldridge et al. (Citation2012) and S. Eldridge et al. (Citation2012). Samples collected for microcystin analysis at sites MDN, WMR, and RPT on 10 August 2009 and thereafter were collected by depth integration. Additional water column samples (all constituents) were collected from site MDN for quality control (blank, replicate, and split samples). The collection procedures and results of quality control analyses are discussed in D. Eldridge et al. (Citation2012) and in S. Eldridge et al. (Citation2012). At sites MDN, WMR, and FBS, 1-L grab samples were also collected from approximately 0.5 m below the lake surface and analyzed for cellular iron analysis by flow-injection inductively coupled plasma mass spectrometry (ICP-MS) as described in Topping and Kuwabara (Citation2003) at the USGS in Menlo Park, California. Samples collected at all sites 1 July and 22–29 July and at sites MDN and WMR on 16 September contained insufficient phytoplankton biomass for trace metal analysis.
Continuous water quality monitors (YSI, Inc., Yellow Springs, OH) were deployed at all sites prior to sample collection to measure water temperature, dissolved oxygen concentration and percent saturation, pH, and specific conductance according to D. Eldridge et al. (Citation2012). Calibration, maintenance, and data handling were performed as outlined in Wagner et al. (Citation2006).
Microcystin analysis
Water column samples were fractionated (filtered) to measure microcystin concentrations in the different phytoplankton size classes separately. Samples were filtered with a 63 μm sieve to isolate large colonies (the large particulate, or cell-associated, fraction). The small particulate fraction, 1.5–63 μm, was collected by filtering the 63 μm fraction filtrate. The filtrate from this step was considered the dissolved fraction and retained for determination of dissolved microcystin (<1.5 μm) concentrations. All fractions were analyzed for microcystin concentrations using the congener-independent enzyme-linked immunosorbent assay (ELISA) as described in S. Eldridge et al. (Citation2012). Microcystin concentrations in the particulate and dissolved fractions of each sample were added together to give total microcystin concentrations (Graham and Jones Citation2007). The detection limit for the microcystin assay was 0.1 μg/L. Values <0.1 μg/L that were not censored (as <MQL, the method quantitation limit) were considered detections, although they appeared to be less than the detection limit because concentrations were calculated from the total extracted biomass and sample volumes. Sediment samples were dried for 96 h and extracted following a protocol modified from Chen et al. (Citation2006) in which sonication was replaced by use of the Dionex Automated Solvent Extractor (Dionex, Sunnyvale, CA). Following extraction, samples were analyzed for microcystin concentrations using ELISA.
Sedimentary flux of microcystins
The total sedimentary flux (μg/m2/h) of microcystins collected in sediment traps was measured at MDN and WMR by dividing the microcystin concentrations in settled trap material (micrograms of microcystins measured in dried sediment per meter square of surface area in each trap column) by the total time that each trap was in the water. To determine the fraction of trap material collected by resuspension, a modification of the equations proposed by Gasith (Citation1975; as presented by Bloesch Citation1994) was used:
where T is the mass of material in each trap that settled from the water column (suspended sediment), S is the total mass of entrapped material, and R is the mass of trap material that was resuspended from lake bottom sediments. In Gasith (Citation1975), f T, f S, and f R represented the organic fractions of resuspended material, but in the current study, these were replaced with the microcystin fractions found in water column, sediment trap, and surficial sediment samples, respectively.
Data analysis
Seasonal trends and site comparisons were based on weekly median values of measured parameters. Water column stability was calculated as the median relative thermal resistance to mixing (RTRM) according to Jones and Welch (Citation1990) and Kann and Welch (Citation2005). To compare variables between sites and study years, nonparametric statistical analyses were performed using SigmaPlot, version 11 (Systat Software, Inc., San Jose, CA) because the data continued to deviate from normality and homoscedasticity after log transformation. The Mann-Whitney rank sum one-way analysis of variance (ANOVA) and the Tukey test were used for pairwise comparisons. Correlations between microcystin, chlorophyll a, and nutrient concentrations and physiochemical data were determined using the Spearman's rank-order correlation analysis. Correlations were considered significant at p < 0.05. Autocorrelation of measured parameters was evaluated with the methods described in Helsel and Hirsch (Citation2002).
To reveal the internal structure of the data in a way that best explained the variance in the data, a principal components analysis (PCA) was performed using the median values of 15 parameters from each site and following the procedures described in Parinet et al. (Citation2004). To compare differences in the data over time, surficial sediment and suspended sediment microcystin concentrations, water column pH, dissolved oxygen, water temperature, and concentrations of chlorophyll a, TP, TN, dissolved nutrients, and cellular iron in water samples (all sites combined on each sample date) were reduced to 5 principal components, which accounted for >97% of the variation in the data.
Results
Qualitative changes in phytoplankton community
Sediment trap samples were screened to estimate changes in the relative abundances of specific phytoplankton taxa. Relative changes were compared over time, not between the different taxa, although it was apparent that A. flos-aquae was the dominant species in all samples during bloom periods (micrographs of representative samples are shown in Supplement Fig. S1). Changes in the relative abundance of A. flos-aquae in sediment traps followed the pattern in water column chlorophyll a concentrations previously observed by Kann (Citation1998), Carmichael et al. (Citation2000), and Lindenberg et al. (Citation2009). Both A. flos-aquae colonies and chlorophyll a concentrations increased between late spring and early summer (before July), decreased during the mid-July bloom decline, and increased again as the bloom recovered in early August ( and ).
Figure 2 Relative abundances through the sampling season of cyanobacterial groups in phytoplankton observed in sediment trap samples between 1 July and 16 September, Upper Klamath Lake, Oregon, 2009.
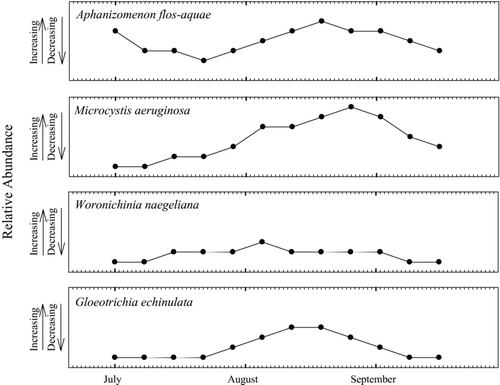
M. aeruginosa and W. naegeliana appeared in the water column in mid-July, and along with A. flos-aquae, M. aeruginosa persisted in the lake until late fall. M. aeruginosa first appeared in sediment traps on 15 July, when microcystin concentrations in water column and sediment trap samples were near or below the detection limit. The number of M. aeruginosa colonies steadily increased in sediment traps until early September, following the pattern observed for microcystin concentrations in the water column (, , and ) and in sediment trap material from site FBS and, to a limited extent, site MDN (). This increase in apparent M. aeruginosa cell density also coincided with increasing microcystin concentrations measured in sediment surface samples from sites FBS and WMR (). When present, M. aeruginosa was observed by field personnel as a thin film on the lake surface and as accumulations along the leeward shore during daily high wind events. G. echinulata, a known microcystin producer (Carey et al. Citation2007), first appeared in sediment trap samples on 29 July, so there may be some contribution from this species to the occurrence of microcystins in the lake.
Microcystin concentrations in sediment samples
Microcystins were detected in all surficial sediment and sediment trap samples collected in 2009; however, median concentrations in sediment samples were much lower than concentrations measured in the large particulate (cell-associated) fraction of water column samples (suspended sediments; expressed as μg/g dry weight of suspended solids) at sites MDN and WMR (; microcystin concentrations measured in water column samples from the deep sites, EPT and MDT, are reported in S. Eldridge et al. Citation2012). Sediment trap samples contained higher microcystin concentrations by weight than surficial sediment samples (Mann-Whitney, p < 0.001), with most concentrations ranging between 0.08 and 38.2 μg/g. One trap sample collected on 26 August 2009 from site FBS contained microcystins at a seasonal peak concentration of 1107 μg/g, which exceeded the maximum concentration measured in any other suspended material or sediment sample from this area. Exceptionally high biomass composed mostly of M. aeruginosa cells in the sediment trap collected on this date was also visually evident and noted by field and lab personnel. This high concentration was observed only once, but because of the toxicity of microcystins, it is particularly noteworthy.
Table 1 Medians (Med) and ranges of microcystin concentrations in water column and sediment samples collected at shallow (<10 m) sites in Upper Klamath Lake, Oregon, 2009. Values of total μg/L in water column samples are the sums of all fractions measured in the water samples. NA indicates that no samples were collected, and MQL represents the method quantitation limit.
Figure 3 Median concentrations of microcystins in particulates from water column and sediment trap samples and (a) chlorophyll a concentrations, (b) total phosphorus (TP) and total nitrogen (TN) concentrations, (c) dissolved inorganic phosphorus (DIP) and dissolved inorganic nitrogen (DIN; NH3 and NO2 − + NO3 −) concentrations, and (d) total and dissolved nutrient ratios, TN:TP and DIN:DIP.
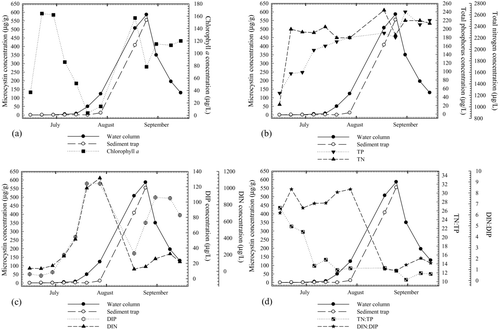
Figure 4 Microcystins as (a) total concentration (in dissolved + particulate fractions) in water samples measured volumetrically, (b) concentrations in particulates of water samples measured gravimetrically, (c) concentrations in surficial sediment samples from fixed sites (line graph) and from randomly selected sites (bar graph), and (d) concentrations in sediment trap samples. Deep sites are indicated by a solid line. Error bars indicate the standard deviations from mean values of replicate sediment surface samples (c) or duplicate columns sampled at site FBS (d).
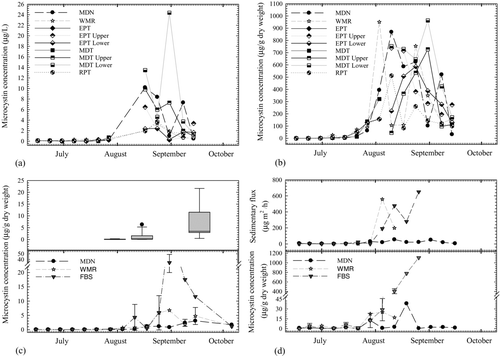
Median surficial sediment microcystin concentrations ranged from 0.004 to 34.4 μg/g and correlated positively with water sample concentrations ( and ). Microcystin concentrations measured from randomly selected surficial sediment samples (Supplement, Table S1) were not significantly different between regions (). Samples collected on 18 September were higher, ranging from 0.49 to 21.6 μg/g, than samples collected 31 July, which varied between 0.02 and 0.30 μg/g (; Supplement, Table S1).
Table 2 Spearman's rank-order correlations (r) between median microcystin concentrations in surficial sediment samples (MCsed), water column samples (MCwater; the sum of all fractions), and environmental variables measured by sample date (all sites combined). Correlations were considered significant at p < 0.05. Significant correlations are shown in bold. Correlations that were not statistically significant with either MCsed or MCwater are not shown.
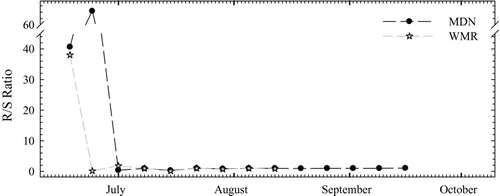
Changes in the ratio of total or gross sedimentation (including resuspended microcystins) to sedimentation of water column-derived particulate microcystins (R/S) were measured in samples collected from sites MDN and WMR. Sediment collected in traps before 1 July contained a considerable fraction (≥38%) of resuspended material from bottom sediments. Because microcystin concentrations were low in all samples and below detection in most water column samples during this time, the contribution of resuspended microcystins to concentrations measured in trap samples was negligible (). The contribution of resuspended microcystins to the concentrations in the traps throughout the rest of the season was also insignificant because the traps collected after 1 July contained around 1% or less of resuspended material.
Relation to environmental variables
In 2009, median lakewide chlorophyll a concentrations were elevated early in the sample season, around 1 July, and again between late August and early September, indicating 2 major bloom episodes at sampled sites (). Following the decline of the first major bloom, total microcystin concentrations in water column samples began to increase. Microcystin concentrations then increased more rapidly as the second bloom began to develop in early August. A late-season increase in microcystin concentration was also observed in sediment samples (), but these concentrations remained near the detection limit until mid-August. Microcystins began accumulating in sediment traps deployed at sites WMR and FBS the week of 3 August as median chlorophyll a concentrations neared the second peak of the season. During this time, the sedimentary flux of microcystins reached a maximum of 558 μg/m2/h at site WMR, when the peak microcystin concentration in suspended material from this site was measured (). In FBS trap samples, a maximum sedimentary flux of 650 μg/m2/h was measured during the week of 24 August, when the last sediment trap sample was collected from that site.
Figure 5 Changes in R/S, the ratio of sedimentation containing resuspended particles to sedimentation of settling material from the water column 0.5 m from the sediment surface.
Concentrations of TN and TP increased, although not at the same rate, while TN:TP ratios decreased between May and October 2009 ( and ). In addition, DIN and DIP concentrations increased through July and declined sharply along with DIN:DIP ratios after 1 August (). Water column microcystin concentrations also began increasing during this period of elevated DIN and DIP and continued to increase after DIN and DIP concentrations peaked in late July. Lower DIN and DIP concentrations (relative to the late-July maxima) were observed prior to the mid-August peak in water and sediment microcystins. Therefore, it seemed that concentrations of dissolved nutrients (particularly DIN) remained high enough until September to support growth of non-diazotrophic, microcystin-producing strains.
Figure 6 Intracellular iron concentrations in phytoplankton from filtered water column samples. The missing values on 1 July, between 15 July–5 August, and at sites MDN and WMR on 16 September represent samples with insufficient biomass for trace metal analysis or high amounts of inorganic material.
Based on Spearman's rank-order analysis, surficial sediment and water column microcystin concentrations were negatively correlated with total and dissolved nutrient ratios, TN:TP and DIN:DIP, and positively correlated with TP and DIP (). DIP correlated significantly with sediment microcystins only, and no significant correlations were found between surficial sediment or water column microcystins and DIN (NH3 or NO2 + NO3 −). Among the physiochemical parameters measured in 2009, pH, dissolved oxygen concentrations, water column stability (measured as RTRM), and site depths correlated positively with microcystin concentrations; pH and dissolved oxygen were significantly correlated with sediment microcystins only, and RTRM correlated significantly with total water column microcystin concentrations only.
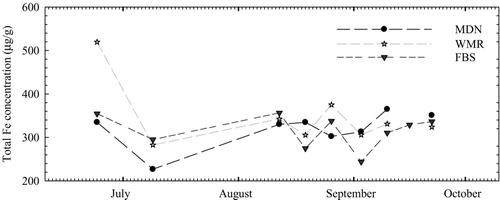
Intracellular concentrations (from phytoplankton biomass) of total iron were also measured in water column samples to evaluate the effects of this trace metal on microcystin occurrence. Median concentrations of cellular iron were similar between sites and were generally lower between July and September (), when microcystin levels were higher in the water column, than in the initial samples collected in June, when microcystin concentrations were near or below detection. Cellular iron concentrations were between 244 and 375 μg/g from 19 August to 16 September, when microcystin concentrations increased in water column, surficial sediment, and sediment trap samples. M. aeruginosa was identified in all samples collected for trace metal analysis after 1 August but not in samples collected earlier. In addition, cellular iron concentrations were not correlated with water column or sediment microcystin concentrations by Spearman's rank-order analysis.
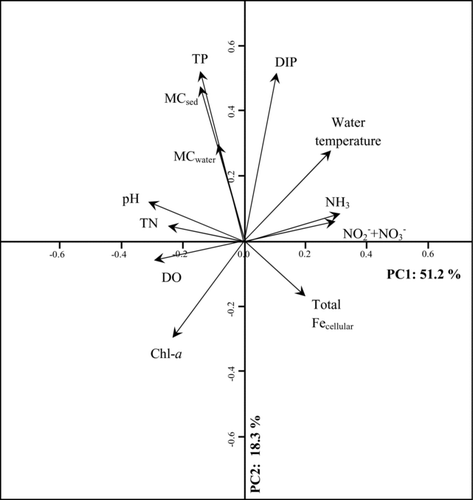
Figure 7 PCA ordination plot (loadings) of variables for microcystins in surficial sediment (MCsediment) and water column (MCwater) samples (median concentrations of all sites on each sample date) and other environmental parameters between 15 June and 16 September 2009. TP = total phosphorus, DIP = dissolved inorganic phosphorus, Chl-a = chlorophyll a, DO = dissolved oxygen, TN = total nitrogen.
Principal components analysis (PCA)
Principal components analysis (PCA) identified 5 composite variables (principal components) that accounted for 97% of the total variability in the larger dataset. Most of the variability among the tested parameters was accounted for by the first 2 principal components, PC1 and PC2, which represented 51 and 18% of the variability in the original data, respectively. PC1 was primarily defined by characteristics of A. flos-aquae-dominated biomass and activity, including chlorophyll a concentrations, DIN concentrations, dissolved oxygen concentrations, and pH (; Supplement, Table S2). Loadings of PC1 and PC2 (correlations between the original parameters and the principal components) showed 2 primary “modes” of variation in the original data. Positive correlations were found between PC1 and DIN (NH3, NO2 − + NO3 −), water temperature, and cellular iron. PC1 was negatively correlated with pH, TN, dissolved oxygen, and chlorophyll a concentrations. PC2 was mainly influenced by surficial sediment microcystin and phosphorus (total and dissolved) concentrations, based on the loading scores obtained for these variables. Similar results were obtained when sediment trap microcystin concentrations were included or when the seasonal median values at each site were compared. The variability in water column microcystin concentrations was not captured by either principal component because these loading scores were relatively low on both PC1 and PC2.
Figure 8 Scores for each sample date between 15 June and 16 September 2009 on the first 2 principal components. Group 1 represents sample dates during the decline of the first bloom of the season (weeks of 6 Jul–3 Aug); group II includes dates of slow decline in the second bloom (weeks of 23–30 Aug); and group III consists of sample dates when water column samples contained elevated microcystin concentrations (week of 10 Aug) and maximum lakewide chlorophyll a concentrations (weeks of 29 Jun and 17 Aug). The period of rapid growth in the Aphanizomenon flos-aquae-dominated bloom (weeks of 15–22 June) early in the season is shown in group IV.
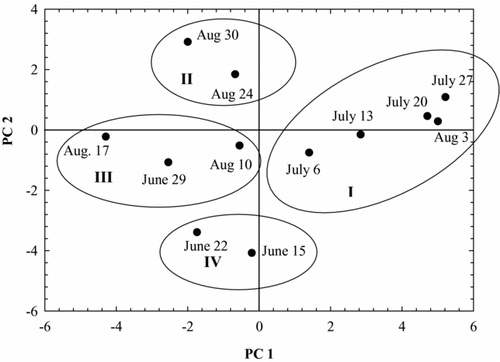
The PCA score plot () shows that sample dates seem to have clustered into 4 periods between 15 June and 16 September 2009, based on the relative median concentrations of the tested parameters. Cluster I received positive scores on PC1 primarily due to the increase in DIN between 6 July and 3 August. Chlorophyll a concentrations were decreasing during this time, but the negative correlation between PC1 and chlorophyll a may have been due to the relatively wide range (across PC1, low to high) in values on sample dates within this cluster. Sample dates in cluster II, 24 and 30 August, scored positively on PC2 because maximum median microcystin concentrations were detected in sediment-trap samples collected on those dates. Low DIN and high DIP concentrations were also detected in water column samples during that time. Clusters III and IV included sample dates with negative scores on both PC1 and PC2 as a result of increasing or peak bloom development (as indicated by higher chlorophyll a, concentrations, pH, and dissolved oxygen concentrations). Cluster III scores were higher (closer to 0) on PC2 than cluster IV scores due to high microcystin concentrations in sediment and water samples as the first bloom of the season peaked.
Discussion
This study is the first to document the occurrence of microcystins in sediments of Upper Klamath Lake and supports a previous analysis of microcystins in concurrently collected water column samples (S. Eldridge et al. Citation2012). In the current work, microcystins were detected in all sediment samples at concentrations lower than in the suspended material collected from the water column. Because the sediment traps were positioned to capture negatively buoyant, actively settling colonies, these concentration differences suggested that microcystins were produced or concentrated in positively buoyant cells and accumulated in the benthic sediments to a limited extent during major bloom episodes. In addition, the lower sediment microcystin concentrations measured in surficial sediments (relative to suspended material) suggested rapid biodegradation at or near the sediment surface. Degradation at the sediment surface has been observed in other shallow lakes where, for example, in Lake Taihu, the half-lives of microcystin RR and LR were recently reported to be about 1 day in sediment and about 6 days in the water column (Chen et al. Citation2008).
Although high dissolved microcystin concentrations in the water column may promote direct adsorption onto bottom sediments, microcystin adsorption may be limited in shallow systems (Grützmacher et al. Citation2010). In Upper Klamath Lake, a strong negative correlation was observed between microcystin concentrations and site depth in surficial sediment samples from randomly selected sites (). Also, higher sedimentation rates were observed during the second bloom, while microcystin concentrations were elevated in suspended material. These patterns indicated that microcystins were transferred to the lake sediments primarily through settling or sedimentation of intact cells during bloom episodes.
Sedimentation has been shown through other studies to be a major destination for microcystins and toxigenic cells (Takamura and Yasuno Citation1988, Wörmer et al. Citation2011). In addition, acoustic backscatter and vertical velocities from acoustic Doppler current profilers previously deployed in Upper Klamath Lake indicated that, during stratification, colonies floated to the surface and settle to the lake bottom (Wood and Gartner Citation2010). Comparisons of benthic chlorophyll a and phaeophytin concentrations measured in 2009 further reflect the settling and degradation of phytoplankton biomass during the bloom cycle (Kuwabara et al. Citation2010). Live, sedimented toxigenic cells maintained their toxin content in other studies, although concentrations may differ from neutrally or positively buoyant cells (Wörmer et al. Citation2011). Therefore, cell-associated microcystins (within the large particulate fraction) from the water column of Upper Klamath Lake were likely transferred to the sediment, which increased the potential for benthic or benthic-feeding organisms to be exposed.
Although not measured in this study, it is possible that cells of microcystin-containing (and other) cyanobacteria migrated from the sediments to the water column. Several studies showed the importance of nutrient and microcystin translocation via migration from the sediments in other lakes (e.g., Johnston and Jacoby Citation2003). If this also occurs in Upper Klamath Lake, then the sediments would not only be a major destination of microcystins and microcystin-containing cells, but also a source of toxic cells to the water column, particularly under certain temperature and light conditions. Additional sampling and analysis during the winter and early spring may help to better understand this exchange.
Maximum surficial sediment concentrations measured in this study far exceeded those of other studies where similar sampling and analytical techniques were used. For example, Mohamed et al. (Citation2007) measured concentrations <0.092 μg/g in the Nile River and irrigation canal sediments in Egypt, and Tsuji et al. (Citation2001) reported a maximum concentration in Japanese lake sediments of 2.33 μg/g. In other studies, microcystin concentrations in lake or reservoir sediments were below 0.5 μg/g (e.g., Babica et al. Citation2006). Differences in the results between these and the current study may be due to their different extraction methods. Concentrations measured from adsorbed microcystins in sediment samples may vary considerably by the solvent system used, and the microcystin variants may exhibit different extraction efficiencies based on their relative hydrophobicities (Babica et al. Citation2006). Chen et al. (Citation2006) reported lower recoveries using extraction in the acetic acid in trifluoroacetic acid (TFA)-methanol solvent system (used in Babica et al. Citation2006 and Mohamed et al. Citation2007) than when the EDTA/Na4P2O7 TFA system, performed in the current study, was used. Results obtained using the former method may, therefore, be underestimated.
The seasonal variability in environmental parameters observed in 2009 was similar to conditions in Upper Klamath Lake previously reported (Kann Citation1998, Hoilman et al. Citation2008, Lindenberg et al. Citation2009, D. Eldridge et al. Citation2012; Supplement, Table S3) and reflects the environmental influences on growth and senescence of the phytoplankton bloom, which may also have implications for the ecological relation between A. flos-aquae (the dominant bloom former) and M. aeruginosa (the primary microcystin producer). In most years, an early A. flos-aquae bloom is followed by a rapid decline between the second half of July and the first half of August. After 1–3 weeks, a second A. flos-aquae bloom follows between mid- to late August and early September. Supersaturated dissolved oxygen concentrations and pH values near 9.5 coincide with the chlorophyll a maximum, while minimum chlorophyll a concentrations typically occur with undersaturated dissolved oxygen concentrations, decreased pH, and peak dissolved nutrient concentrations. As was observed in 2009, dissolved nutrient concentrations are usually low through the first bloom, peak with the rapid decline of this bloom, and decrease but remain elevated during the rapid growth and stable phase of the second bloom.
The trends in chlorophyll a and microcystin concentrations observed in water column samples collected between June and October 2009 indicated a relation between microcystin occurrence in Upper Klamath Lake and the mid-season decline and subsequent increase in the A. flos-aquae-dominated bloom. Microcystin concentrations increased at all sites in the water column and sediment following the decline of the first major bloom and remained elevated during growth of the second bloom as a result of the increase in available nutrients. Before the first bloom, microcystin concentrations were likely minimal because nutrient concentrations were consumed during rapid A. flos-aquae bloom growth. As indicated by the PCA score plot (based on the first 2 principle components; ), sample dates between 15 June and 30 August clustered into 4 distinct periods. In comparison to the growth and decline of the A. flos-aquae-dominated bloom, these periods were qualitatively characterized as (I) declining or minimum chlorophyll a concentrations and increasing nutrient (primarily DIN) concentrations after the first major bloom, (II) slow decline of the second major bloom, (III) high microcystin concentrations in water and sediment during peak concentrations of chlorophyll a, and (IV) the rapid increase in chlorophyll a concentrations early in the sample season. A similar pattern was observed in 2007 but not in 2008, when the bloom peaked much later and did not fully recover lakewide (S. Eldridge et al. Citation2012).
Dissolved nitrogen (NH3) seemed to be a growth-limiting nutrient in Upper Klamath Lake, given the dominance of diazotrophic A. flos-aquae; heterocysts were present on A. flos-aquae filaments during the entire bloom season. M. aeruginosa does not fix atmospheric nitrogen (N2), unlike A. flos-aquae, and is therefore dependent on the dissolved form. Rapid increases in both TN and chlorophyll a in late June and late August indicated that development of the major A. flos-aquae blooms was facilitated by atmospheric nitrogen sequestration. In Upper Klamath Lake, nitrogen addition to the water column may also occur through benthic fluxes (Kuwabara et al. Citation2007) and riverine inputs, but these have been reported to be much smaller sources of nitrogen to the lake than nitrogen fixation (Kann and Walker Citation1999). Therefore, as shown previously in a marine environment (Agawin et al. Citation2007), growth of M. aeruginosa was likely facilitated by nitrogen addition through N2 fixation by A. flos-aquae early in the season, when concentrations of A. flos-aquae were highest and microcystin levels were lowest.
Results of this study, along with S. Eldridge et al. (Citation2012), suggest that phosphorus availability and the ecological relation between M. aeruginosa and A. flos-aquae may have been the most influential overall factors on microcystin occurrence in the lake. Correlation does not indicate cause, but the significant correlations between microcystin concentrations and TP and/or DIP concentrations in sediment or water column samples, the lack of correlation between microcystin concentrations and TN or DIN concentrations in these samples, and the seasonal trends in nutrient concentrations and ratios (discussed in S. Eldridge et al. Citation2012) suggest that high phosphorus levels during mid- to late summer may have played a greater role than nitrogen availability in the increased microcystin concentrations. Similar correlations between water column microcystin and nutrient concentrations were observed in 2007 and 2008 (S. Eldridge et al. Citation2012). In addition, PCA showed that sediment microcystin concentrations were more related to total and dissolved phosphorus concentrations than to the other measured parameters.
Previous data collected from Upper Klamath Lake indicate potentially phosphorus-limiting conditions characterized by chlorophyll a:TP ratios >1 (White Citation1989) and TN:TP ratios >17 (Forsberg and Ryding Citation1980) early in the sampling season (Jun–Jul; Lindenberg et al. Citation2009). In 2009, increases in chlorophyll a:TP ratios over time (S. Eldridge et al. Citation2012) indicated that phytoplankton biomass was less phosphorus limited after the first major bloom decline than during early bloom development, particularly at sites MDT and MDN. A higher rate of organic matter mineralization occurs in most years in the deepest part of the lake (along the western shoreline, near site MDT) from decay of the greater biomass typical of this area. This elevates dissolved nutrient (DIN and DIP) concentrations and returns DIP to the water column, where it stimulates development of a second major bloom. Furthermore, the steady increase in TP observed in 2009 (and in most other years) during the sampling season, which is common in shallow lakes, suggested that internal recycling from lake sediments was not episodic. Given the likely dependence of M. aeruginosa on A. flos-aquae to supply new nitrogen to the system, it seemed that toxigenic M. aeruginosa growth and/or microcystin occurrence was stimulated directly by the release of DIN during the bloom decline. Microcystin occurrence may have been dependent overall, however, on the presence of phosphorus to regulate growth and decline of A. flos-aquae because the A. flos-aquae bloom was controlled largely by changes in phosphorus availability.
Other environmental factors were found in this study to be potentially associated with microcystin occurrence in Upper Klamath Lake, but Spearman's rank-order analysis showed generally mixed results, and no other measured factors seemed to be more important for increasing microcystin concentrations than phosphorus. Suspended sediment microcystin concentrations (in water column samples) correlated positively with water column stability (RTRM). This indicated that, as with other bloom formers in the lake (primarily A. flos-aquae), the accumulation of microcystins (or toxigenic cells) in the water column was enhanced or promoted when the water column was stable. Along with chlorophyll a concentrations, higher pH and dissolved oxygen concentrations are typically associated with the metabolic activity of the major bloom; therefore, the positive correlations between pH and dissolved oxygen with sediment microcystin concentrations may have reflected the corresponding increases in sediment microcystin and chlorophyll a concentrations during the second, late-summer bloom.
Results of cellular trace metal analysis failed to show any influence of cellular iron concentrations on microcystin occurrence. Iron concentrations were lower when microcystin levels were elevated in the water column, and M. aeruginosa was not identified in the samples collected for trace metal analysis before 1 August when microcystin concentrations were highest at site WMR. This is supported by the lack of correlation between cellular iron concentrations and water column or sediment microcystin concentrations.
Understanding the ecological relation between M. aeruginosa and A. flos-aquae has implications for the management of Upper Klamath Lake. If these species compete directly for nutrients or other resources, the occurrence of microcystins may increase if nutrient management (reduction of phosphorus inputs, the focus of most proposed plans) successfully limits the A. flos-aquae bloom. If the relation between these species is facilitative, as the results of this study indicate, however, then an overall decrease of A. flos-aquae in the lake also may eliminate (toxigenic) M. aeruginosa because this species already occurs in low abundance here.
Supplementary_File.docx
Download MS Word (339.9 KB)Acknowledgments
We thank the reviewers who provided comments on this manuscript. The personnel of the USGS Klamath Falls Field Station are acknowledged for the use of field equipment and laboratory space. Mary Lindenberg, Kristofor Kannarr, Sara Caldwell Eldridge, Dan Blake Eldridge, Kristin Harbin, Cynthia King, and Matthew Wilson from the USGS in Klamath Falls performed the fieldwork. We also thank Amy Brooks, Micelis Doyle, and Matthew Johnston from the USGS Oregon Water Science Center for data processing and supportive work and Kevin Feltz, Lynne Johnson, and Jesse Arms who did the microcystin analysis at the USGS Columbia Environmental Research Center.
This work was funded by the Bureau of Reclamation (Reclamation), US Department of Interior, as part of Reclamation's mission to manage, develop, and protect water and related resources in an environmentally and economically sound manner in the interest of the American public. Funding was provided through Interagency Agreement 05AA204049. The views in this report are the authors’ and do not necessarily represent the views of Reclamation. Additional funding was provided by the USGS. Use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
This article not subject to US copyright law.
References
- Agawin , N SR , Rabouille , S , Veldhuis , M JW , Servatius , L , Hol , S , van Overzee , H MJ and Huisman , J . 2007 . Competition and facilitation between unicellular nitrogen-fixing cyanobacteria and non-nitrogen-fixing phytoplankton species . Limnol Oceanogr. , 52 : 2233 – 2248 .
- Alexova , R , Fujii , M , Birch , D , Cheng , J , Waite , D , Ferrari , B C and Neilan , B A . 2011 . Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation . Environ Microbiol. , 13 : 1064 – 1077 .
- Babica , P , Kohoutek , J , Blaha , L , Adamovsky , O and Marsalek , B . 2006 . Evaluation of extraction approaches linked to ELISA and HPLC for analyses of microcystin-LR, -RR, and - YR in freshwater sediments with different organic material contents . Anal Bioanal Chem. , 385 : 1545 – 1551 .
- Bloesch , J. 1994 . A review of methods used to measure sediment resuspension . Hydrobiologia. , 284 : 13 – 18 .
- Bottcher , J L and Burdick , S M . 2010 . Temporal and spatial distribution of endangered juvenile Lost River and shortnose suckers in relation to environmental variables in Upper Klamath Lake, Oregon: 2009 annual data summary . US Geological Survey Open-File Report 2010–1261; [cited 23 July 2012] Available from: http://pubs.usgs.gov/of/2010/1261/
- Bradbury , J P , Colman , S M and Reynolds , R L . 2004 . The history of recent limnological changes and human impact on Upper Klamath Lake, Oregon . J Paleolimnol. , 31 : 151 – 165 .
- Buettner , M and Scoppettone , G G . 1990 . Life history and status of catostomids in Upper Klamath Lake, Oregon—Completion report , 119 Reno (NV) : US Fish and Wildlife Service, National Fisheries Research Center, Reno Field Station .
- [BOR] Bureau of Reclamation . 2000 . Klamath Project–Historical operation Klamath Falls (OR) [cited 23 July 2012] Available from: http://www.usbr.gov/mp/kbao/docs/Historic%20Operation.pdf
- Carey , C C , Haney , J F and Cottingham , K L . 2007 . First report of microcystin-LR in the cyanobacterium Gloeotrichia echinulata . Environ Toxicol. , 22 : 337 – 339 .
- Carmichael , W W , Drapeau , C and Anderson , D M . 2000 . Harvesting of Aphanizomenon flos-aquae Ralfs ex Born & Flah. Var. flos-aquae (Cyanobacteria) from Klamath Lake for human dietary use . J Appl Phycol. , 12 : 585 – 595 .
- Chen , W , Li , L , Gan , N and Song , L . 2006 . Optimization of an effective extraction procedure for the analysis of microcystin in soils and lake sediments . Environ Pollut. , 143 : 241 – 246 .
- Chen , W , Song , L , Peng , L , Wan , N , Zhang , X and Gan , N . 2008 . Reduction in microcystin concentrations in large and shallow lakes: Water and sediment-interface contributions . Water Res. , 42 : 763 – 773 .
- Eilers , J , Kann , J , Cornett , J , Moser , K , St. Amand , A and Gubala , C . 2004 . Recent paleolimnology of Upper Klamath Lake, Oregon . Hydrobiologie. , 520 : 7 – 18 .
- Eldridge , D B , Eldridge , S LC , Schenk , L N , Tanner , D Q and Wood , T M . 2012 . Water-quality data from Upper Klamath and Agency Lakes, Oregon, 2009–10 . US Geological Survey Open-File Report 2012–1142; [cited 2 November 2012] Available from: http://pubs.usgs.gov/of/2012/1142/
- Eldridge , S LC , Wood , T M and Echols , K R . 2012 . Spatial and temporal dynamics of cyanotoxins and their relation to other water quality variables in Upper Klamath Lake, Oregon, 2007–09 . US Geological Survey Scientific Investigations Report 2012–5069; [cited 23 July 2012] Available from: http://pubs.usgs.gov/sir/2012/5069/
- Forsberg , C and Ryding , S-O . 1980 . Eutrophication parameters and trophic state indices in 30 Swedish waste-receiving lakes . Arch Hydrobiol. , 89 : 189 – 207 .
- Gasith , A. 1975 . Tripton sedimentation in eutrophic lakes – simple correction for the resuspended matter . Verh Int Verein Limnol. , 19 : 116 – 122 .
- Graham , J L and Jones , J R . 2007 . Microcystin distribution in physical size class separations of natural plankton communities . Lake Reserv Manage. , 23 : 161 – 168 .
- Grützmacher , G , Wessel , G , Klitzke , S and Chorus , I . 2010 . Microcystin elimination during sediment contact . Environ Sci Technol. , 44 : 657 – 662 .
- Helsel , D R and Hirsch , R M . 2002 . Statistical methods in water resources–hydrologic analysis and interpretation. US Geological Survey Techniques of Water-Resources Investigations, Book 4, Chapter A3; [cited November 2] . Available from: http://pubs.usgs.gov/twri/twri4a3/
- Hoilman , G R , Lindenberg , M K and Wood , T M . 2008 . Water quality conditions in Upper Klamath and Agency Lakes, Oregon, 2005 . US Geological Survey Scientific Investigations Report 2008–5026; [cited 23 July 2012] Available from: http://pubs.usgs.gov/sir/2008/5026/
- Jacoby , J M and Kann , J . 2007 . The occurrence and response to toxic cyanobacteria in the Pacific Northwest, North America . Lake Reserv Manage. , 23 : 123 – 143 .
- Johnston , B R and Jacoby , J M . 2003 . Cyanobacterial toxicity and migration in a mesotrophic lake in Western Washington, USA . Hydrobiologia. , 495 : 79 – 91 .
- Jones , C A and Welch , E B . 1990 . Internal phosphorus loading related to mixing and dilution in a dendritic shallow prairie lake . Res J Water Pollut C. , 62 ( 7 ) : 847 – 852 .
- Kann , J. 1998 . Ecology and water quality dynamics of a shallow hypereutrophic lake dominated by cyanobacteria (Aphanizomenon flos-aquae) , Chapel Hill (NC) : University of North Carolina . [dissertation]
- Kann , J and Smith , V H . 1999 . Estimating the probability of exceeding elevated pH values critical to fish populations in a hypereutrophic lake . Can J Fish Aquat Sci. , 56 : 2262 – 2270 .
- Kann , J and Walker , W W . 1999 . Nutrient and hydrologic loading to Upper Klamath Lake, Oregon, 1991–1998 , Klamath Falls (OR) : Klamath Tribes Natural Resources Department-U.S. Bureau of Reclamation Cooperative Studies .
- Kann , J and Welch , E B . 2005 . Wind control on water quality in shallow, hypereutrophic Upper Klamath Lake, Oregon . Lake Reserv Manage. , 21 ( 2 ) : 149 – 158 .
- Kuwabara , J S , Lynch , D D , Topping , B R , Murphy , F , Carter , J L , Simon , N S , Parchaso , F , Wood , T M , Lindenberg , M K , Wiese , K and Avanzino , R J . 2007 . Quantifying the benthic source of dissolved nutrients to the water column of Upper Klamath Lake, Oregon . US Geological Survey Open-File Report 2007–1276; [cited 23 July 2012] Available from: http://pubs.usgs.gov/of/2007/1276/
- Kuwabara , J S , Topping , B R , Lynch , D D , Carter , J L and Essaid , H I . 2009 . Benthic nutrient sources to hypereutrophic Upper Klamath Lake, Oregon, USA . Environ Toxicol Chem. , 28 ( 3 ) : 516 – 524 .
- Kuwabara , J S , Topping , B R , Carter , J L , Parchaso , F , Cameron , J M , Asbill , J R , Fend , S V , Duff , J H and Engelstad , A C . 2010 . The transition of benthic nutrient sources after planned levee breaches adjacent to Upper Klamath and Agency Lakes, Oregon . US Geological Survey Open File Report 2010–1062; [cited 23 July 2012] Available from: http://pubs.usgs.gov/of/2010/1062/
- Lindenberg , M K , Hoilman , G R and Wood , T M . 2009 . Water quality conditions in Upper Klamath and Agency Lakes, Oregon, 2006. US Geological Survey Scientific investigations report 2008–5201 . [cited 23 July 2012] Available from: http://pubs.usgs.gov/sir/2008/5201/
- Mohamed , Z A , El-Sharouny , H M and Ali , W S . 2007 . Microcystin concentrations in the Nile River sediments and removal of microcystin-LR by sediments during batch experiments . Arch Environ Contam Toxicol. , 52 : 489 – 495 .
- [ODEQ] Oregon Department of Environmental Quality . 2002 . Upper Klamath Lake drainage total maximum daily load (TMDL) and water quality management plan (WQMP) . State of Oregon Department of Environmental Quality, Portland, Oregon; [cited 4 February 2013] Available from: http://www.deq.state.or.us/wq/tmdls/docs/klamathbasin/ukldrainage/tmdlwqmp.pdf
- Parinet , B , Lhote , A and Legube , B . 2004 . Principal component analysis: an appropriate tool for water quality evaluation and management–application to a tropical lake system . Ecol Model. , 178 : 295 – 311 .
- Perkins , D L , Kann , J and Scoppettone , G G . 2000 . The role of poor water quality and fish kills in the decline of endangered Lost River and shortnose suckers in Upper Klamath Lake , 39 US Geological Survey, Biological Resources Division . Prepared for Bureau of Reclamation, Klamath Falls Project Office, Klamath Falls, Oregon. Contract 4-AA-29–12160
- Phinney , H K and Peek , C A . 1961 . Klamath Lake, an instance of natural enrichment. Transactions of the Seminar on Algae and Metropolitan Wastes, April 27–29, 1960 , 22 – 27 . US Public Health Service .
- Preussel , K , Stüken , A , Wiedner , C , Chrous , I and Fastner , J . 2006 . First report on cylindrospermopsin- producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes . Toxicon. , 47 : 156 – 162 .
- Saker , M L , Welker , M and Vasconcelos , V M . 2007 . Multiplex PCR for the detection of toxigenic cyanobacteria in dietary supplements produced for human consumption . Appl Microbiol Biot. , 73 : 1136 – 1142 .
- Sevilla , E , Martin-Luna , B , Vela , L , Bes , M T , Fillat , M F and Peleato , M L . 2008 . Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806 . Environ Microbiol. , 10 : 2476 – 2483 .
- Sivonen , K and Jones , G . 1999 . Cyanobacterial toxins , Edited by: Chorus , I and Bartram , J . 41 – 111 . London (UK) : E & FN Spon . Toxic cyanobacteria in water: A guide to their public health consequences, monitoring, and management
- Takamura , N and Yasuno , M . 1988 . Sedimentation of phytoplankton populations dominated by Microcystis in a shallow lake . J Plankton Res. , 10 : 283 – 299 .
- Topping , B R and Kuwabara , J S . 2003 . Dissolved nickel and benthic flux in South San Francisco Bay: A potential for natural sources to dominate . Bull Environ Contam Toxicol. , 71 : 46 – 51 .
- Tsuji , K , Masui , H , Uemura , H , Mori , Y and Harada , K I . 2001 . Analysis of MCYSTs in sediments using MMPB method . Toxicon. , 39 : 687 – 692 .
- Wagner , R J , Boulger , R W Jr , Oblinger , C J and Smith , B A . 2006 . Guidelines and standard procedures for continuous water-quality monitors: Station operation, record computation, and data reporting . US Geological Survey Techniques and Methods 1-D3; [cited 23 July 2012] Available from: http://pubs.usgs.gov/tm/2006/tm1D3/
- White , E. 1989 . Utility of relationships between lake phosphorus and chlorophyll a as predictive tools in eutrophication control studies . New Zeal J Mar Fresh. , 23 ( 1 ) : 35 – 41 .
- Williams , JE. 1988 . Endangered and threatened wildlife and plants—Determination of endangered status for the shortnose sucker and the Lost River sucker . Federal Register. , 50 : 27130 – 27134 .
- Wood , T M and Gartner , J W . 2010 . Use of acoustic backscatter and vertical velocity to estimate concentration and dynamics of suspended solids in Upper Klamath Lake, south-central Oregon: Implications for Aphanizomenon flos-aquae . U.S. Geological Survey Scientific Investigations Report 2010–5203; [cited 23 July 2012] Available from http://pubs.usgs.gov/sir/2010/5203/
- Wörmer , L , Cirés , S and Quesada , A . 2011 . Importance of natural sedimentation in the fate of microcystins . Chemosphere. , 82 ( 8 ) : 1141 – 1146 .