Abstract
A global dataset was compiled to examine relations between the total nitrogen to total phosphorus ratio (TN:TP) and microcystin concentration in lakes and reservoirs. Microcystin concentration decreased as TN:TP ratios increased, suggesting that manipulation of the TN:TP ratio may reduce microcystin concentrations. This relationship was experimentally tested by adding ammonium nitrate to increase the TN:TP ratio in large-scale (70 m3), in situ mesocosms located in a eutrophic reservoir that routinely experiences toxic blooms of cyanobacteria. At a TN:TP ratio >75:1, chlorophytes dominated the phytoplankton community in the mesocosms, while cyanobacterial biovolume was significantly reduced and microcystin was not detected. In contrast, the unmanipulated reservoir was dominated by cyanobacteria, and microcystin was detected. Secchi depths were 1.1 to 1.8 times greater in the mesocosms relative to the reservoir. Cladoceran zooplankton had a larger body size (0.14 mm on average) in the mesocosms compared to conspecifics in the reservoir, which was likely related to the higher quality food. Combined, these empirical and experimental data indicate that although nutrient addition is counterintuitive to current cyanobacteria management practices, increasing the TN:TP ratio by adding nitrogen may be a potential short-term management strategy to reduce cyanobacteria and cyanotoxins when other alternatives (e.g., phosphorus reduction) are not possible. Additional experimental studies with careful controls are needed to define best management practices and identify any potential unintended consequences before nitrogen addition is implemented as a lake and reservoir management practice.
Globally, the increasing occurrence of cyanobacterial blooms is degrading water resources (Bennett et al. Citation2001, Paerl and Scott Citation2010, Paerl et al. Citation2011). Negative impacts of these blooms include, but are not limited to, unsightly surface scums, taste- and odor-causing compounds, and the production of a suite of potent toxins (cyanotoxins) that affect a wide range of aquatic and terrestrial organisms, including humans (Chorus and Bartram Citation1999). Of the cyanotoxins, microcystin is the most commonly occurring and most studied toxin worldwide (Chorus and Bartram Citation1999). One cause of increased cyanobacterial blooms is cultural eutrophication (Bennett et al. Citation2001, Carpenter Citation2008). Although most developed countries have substantially reduced point source inputs of nutrients to aquatic ecosystems via regulation, the reduction of nonpoint sources has been more challenging (Schindler and Vallentyne Citation2008). Given the current rates of nutrient loading, solutions combining short-term, in-lake management strategies and long-term watershed approaches are needed to maintain and recover water quality in surface waters (Carpenter Citation2008, Schindler and Vallentyne Citation2008, Hudnell Citation2013).
Table 1 Number of observations (n), mean, median, maximum (max), and minimum (min) value for total nitrogen (TN), total phosphorus (TP), and the TN:TP ratio with microcystin concentrations >1, >6, and >20 μg/L for the global dataset.
The total nitrogen to total phosphorus ratio (TN:TP) has been suggested to indicate cyanobacterial dominance in phytoplankton communities (Smith Citation1983). TN:TP ratios >30 (by weight) were proposed as a threshold to reduce blooms of cyanobacteria (Smith Citation1983, Pick and Lean Citation1987); however, this threshold is not universal (Stockner and Shortreed Citation1988, Canfield et al. Citation1989, Levine and Schindler Citation1999). Recent regional surveys in the Midwestern United States and Canada have indicated that cyanobacterial biovolume and microcystin concentration may be reduced at a much higher ratio (∼75; Graham et al. Citation2004, Orihel et al. Citation2012) than originally proposed. Overall, this research suggests that increasing the TN:TP ratio by removing P or adding N may offer a potential in-lake management strategy to control cyanobacteria and cyanotoxins. However, the reduction of cyanotoxins by N addition has not been tested experimentally at large scales. This study examined if (1) microcystin concentrations decreased as TN:TP ratios increased (Graham et al. Citation2004, Orihel et al. Citation2012) by compiling and analyzing a global dataset, and (2) if cyanobacterial biovolume and microcystin concentration was reduced by increasing the TN:TP ratio by adding N in large in-reservoir experimental mesocosms.
Materials and methods
Global dataset
To determine the relation between microcystin concentrations and the TN:TP ratio worldwide, a global dataset with 5101 data points from 2073 lakes and reservoirs was compiled. Researchers that have conducted regional studies were contacted to obtain TN, TP, and total microcystin data (Supplemental ); cyanobacterial abundance/biovolume data were not available for most studies. The 2007 US Environmental Protection Agency National Lake Assessment (USEPA Citation2007) database was also queried for relevant data. Microcystin data were split into 3 groups indicating relatively low, moderate, and high microcystin concentrations: <6, 6–20, and >20 μg/L, respectively. These break points were chosen because >6 μg/L is one of the lowest US state (i.e., Washington) guidance levels for lake and reservoir advisory/closure, and >20 μg/L was used by Graham et al. (Citation2009) and the World Health Organization (WHO; Chorus and Bartram Citation1999) to denote a high concentration. If microcystin was not detected (n = 3273), the analytical detection limit was substituted (Manly Citation2009).
Figure 1 Willow Creek Reservoir, Heppner, OR. The position of the mesocosm array is denoted by a white box in the reservoir and the US Army Corps of Engineers main site is denoted by a white circle. Image source: Willow Creek Reservoir, OR. 45°20′48″N; 119°32′32″W. Source: Google Earth; accessed 5 Feb 2011.
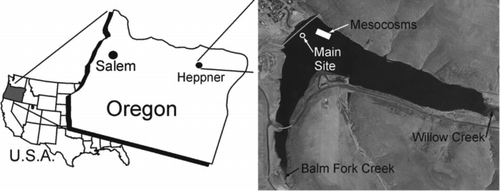
Study site
Willow Creek Reservoir (WCR) is a small (surface area = 0.52 km2; volume = 237.3 × 104 m3; zmax = 27 m), eutrophic (mean TP concentration = 30 μg/L, summer chlorophyll a concentration = 10–15 μg/L) impoundment located in the high desert of eastern Oregon (). Willow Creek is the main (∼90% of total inflow not including precipitation) perennial inflow from the south, while the smaller and seasonally intermittent Balm Fork Creek enters from the southwest (; USACE Citation2007). The primary purpose of the reservoir is flood control for the town of Heppner, while other uses include recreation and irrigation for agriculture. Since the completion of the dam in 1983, WCR has had annual cyanobacterial blooms during summer (>100,000 cells/mL; USACE Citation2007). Microcystin testing began in 2006, and concentrations have exceeded the Oregon Department of Health Services guideline for primary contact recreation (8 μg/L) each summer, resulting in advisories to the public to avoid contact with the water (USACE Citation2007, ODHS Citation2008). These advisories have caused economic and recreational losses at WCR (Adams Citation2012).
Mesocosm experiments
The mesocosm design (3 m diameter; 10 m deep; total volume = 70.37 m3) was a modification of that used by Sarnelle (Citation1993, Citation1997, Citation2007; described in detail in Supplemental Information 1a). Three replicate mesocosms were suspended in bays of a floating modular dock (EZ Doc, Huntersville, NC). The floating dock was anchored at a depth of 19–22 m in the northwest corner of the reservoir (). Mesocosms were open to the atmosphere at the surface and to hypolimnetic water (and sediments) at the bottom. The thermocline (WCR stratifies between 5–8 m) and subsequent anoxia in the hypolimnion were used to isolate the top part of the water column in each mesocosm for experimental manipulation.
Mesocosm experiments were conducted in 2009 and 2010; only 3 mesocosms were used each year, and during 2009, mesocosms were not manipulated. Water quality conditions in the mesocosms and the reservoir were compared to determine if and how well mesocosms reflected the reservoir environment. In 2010, N (as NH4NO3 because of its near neutral pH) was added to all 3 mesocosms; water quality conditions in the mesocosms were compared to those in the reservoir to determine the effects of N addition. The lack of control (untreated) mesocosms is a recognized limitation of the experimental design; however, the 2009 data suggested that the mesocosms were generally indicative of reservoir conditions, and any large differences in subsequent studies could be attributed to treatment effects (2009 methods, results, and discussion in Supplemental Information1b, 1c, and 1d, respectively). Additionally, Sarnelle (Citation2007) has shown that whole-lake phenomena were closely replicated by large-scale mesocosms, indicating that in the absence of whole-lake experiments, large-scale mesocosms provide data reflective of whole-lake conditions. Because the mesocosms used were approximately 7 times the size of those used by Sarnelle (Citation1997, Citation2007), and given the similarities (mean TN difference = 30 μg/L, mean TP difference = 7 μg/L) between the mesocosms and the reservoir in 2009 (Supplemental Information 1c and 1d), we concluded that the mesocosms generally reflected water quality conditions in WCR. Thus, responses to the manipulations observed in the mesocosms may be indicative of manipulations at a whole-lake scale.
Mesocosm set up and sampling in 2010
Mesocosms were installed on 30 June 2010 and removed on 8 October 2010; the reservoir was thermally stratified throughout the entire experimental period. Sampling commenced immediately after installation and continued at approximately weekly intervals until 1 September 2010, after which sampling was reduced to once every 2 weeks. To achieve a TN:TP ratio >75, 500 g of NH4NO3 dissolved in 1 L of deionized water was added to the surface of each mesocosm on 7 July 2010. The amount of N added was calculated from reservoir nutrient concentrations on 30 June (TN = 255 μg/L; TP = 27 μg/L) and the volume of the epilimnion in each mesocosm. To maintain the TN:TP ratio ≥75 throughout the experiment, additional N was added to the mesocosms on 17 August 2010. Total N concentrations were allowed to decrease after 17 August 2010 to examine the TN:TP ratio below which cyanobacteria and microcystin concentration increased.
Sampling methods in 2010 were similar to sampling methods in 2009 (Supplemental Information 1b). On each sampling occasion, all measurements and samples were collected from the reservoir and each mesocosm. Reservoir samples were collected on the south-facing side of the mesocosm floating dock. Profiles of temperature, pH, dissolved oxygen (DO), and conductivity were collected at 1 m intervals from the water surface to a depth of 11 m. Secchi depth was measured using a standard 0.2 m disk. Triplicate water samples for the analysis of TN, TP, and microcystin were collected from a depth of 0.5 m using a 2 L Van Dorn sampler. Samples were collected at a discrete depth because nutrient concentrations in epilimnetic waters were assumed to be homogeneously mixed. Triplicate integrated samples for the analysis of phytoplankton were collected by lowering a 2 L weighted sampler through the photic zone, determined as 2 times the Secchi depth (Wetzel Citation2001), on each sampling occasion. Integrated samples were collected to avoid potential bias due to motile species. A 100 mL subsample was withdrawn and preserved with Lugol's iodine (Eaton et al. Citation2005) for identification and analysis of phytoplankton. Zooplankton were collected with an 80 μm mesh Wisconsin-style plankton net hauled from 11 m to the surface and preserved with buffered formalin to a final concentration of 4–10%.
To determine differences in reservoir and mesocosm sedimentation rates, 2 polyvinyl chloride (PVC) sediment traps (7.26 cm diameter; 35 cm length; Larsson et al. Citation1986) were suspended in the reservoir and in the middle of each mesocosm at a depth of 10 m. Sedimentation rates were evaluated to determine if any changes in water clarity between the reservoir and mesocosms were due to differences in sedimentation.
Laboratory analyses of samples collected in 2010
Laboratory analyses in 2010 were similar to those used in 2009 (Supplemental Information 1b). A modified second derivative spectroscopy method from Crumpton et al. (Citation1992) and a modified ascorbic-acid method from Eaton et al. (Citation2005) were used to analyze TN and TP, respectively. Both of these methods yielded results similar to samples analyzed concurrently at the Cooperative Chemical Analysis Laboratory at Oregon State University from the US Army Corps of Engineers (USACE) main site (approximately 200 m from the mesocosm dock and representative of general reservoir conditions; ) where USACE samples for general WCR monitoring were analyzed in 2010 (data not shown). The abundance and biovolume of phytoplankton were analyzed following the methods of Lund et al. (Citation1958) and Hillebrand et al. (Citation1999), respectively.
Phytoplankton samples were counted to at least 400 natural units (colonies, filaments, unicells) and identified to the genus level using keys by Prescott (Citation1962), Wehr and Sheath (Citation2003), and Bellinger and Sigee (Citation2010). Because cyanobacteria have phytosynthetic functionality and are typically sampled and analyzed as part of the phytoplankton community, cyanobacteria were included as phytoplankton (Wehr and Sheath Citation2003, Graham et al. Citation2008, Bellinger and Sigee Citation2010). Potential N2- and non-N2-fixers were grouped as per Paerl and Fulton (Citation2006; i.e., N2-fixers = Aphanizomenon, Anabaena, Lyngbya, and Gloeotrichia; non-N2-fixers = Microcystis, Woronichinia, and Merismopedia). Potentially toxic cyanobacteria were grouped as per Graham et al. (Citation2008; microcystin producing taxa = Aphanizomenon, Anabaena, Gloeotrichia, Microcystis, and Lyngbya).
Zooplankton samples were counted and identified using keys by Dodson and Frey (Citation2001) and Williamson (Citation2001). We focused on Daphnia because they were the primary filter feeder by abundance in WCR (Britton J, USACE, unpubl. data). Daphnia size was measured from the top of the head capsule to the inflection of the tailspine using a calibrated ocular micrometer. Microcystin samples were lysed by 3 freeze–thaw cycles, filtered, and analyzed by the US Geological Survey Organic Geochemistry Research Lab in Lawrence, Kansas, using Abraxis ADDA-microcystin enzyme-linked immunosorbent assays (ELISA; detection limit 0.1 μg/L; Abraxis, Warminster, PA). The sedimentation rate was determined by filtering sediment trap samples through an 80 μm mesh concentrating screen, drying material collected on the screen at 105 C for 24 h in preweighed aluminum boats, measuring dry mass, dividing the dried mass by the number of days the traps were deployed, and converting to unit area (Eaton et al. Citation2005).
Statistical analyses
The global microcystin dataset was analyzed by interval maxima regression (IMR) following methods in Graham et al. (Citation2004). Statistical thresholds in the global microcystin dataset were determined by a 2-dimensional Kolmogorov-Smirnov (2DKS) test following methods described by Garvey et al. (Citation1998). To test if water quality conditions differed between the reservoir and the mesocosms in 2010, nonparametric paired Wilcoxon signed rank tests (Ott and Longnecker Citation2010) were used. Each pair of data consisted of the mean for the mesocosms and the reservoir sample for each date. Vertical profile data (temperature, DO, conductivity, and pH) were also compared with paired Wilcoxon tests; pairs consisted of the mean value for each depth from the mesocosms paired with the corresponding reservoir value for the depths between 0 and 11 m. Sampling dates were arranged consecutively. All statistical analyses were completed using R 2.13.2 (R Development Core Team Citation2011).
Results
Global dataset
In the global dataset, TN concentrations ranged from 5 to 26,100 μg/L (mean = 1257, median = 816 μg/L), TP concentrations ranged from 1 to 4865 μg/L (mean = 30, median = 38 μg/L), and the TN:TP ratio ranged from 0.2 to 1160 (mean = 30, median = 22). Nutrient concentrations in the global dataset were therefore representative of the full range of trophic conditions encountered in aquatic ecosystems.
Microcystin was detected in 36% (n = 1828) of samples in the global dataset, and total concentrations ranged from 0.05 to 541 μg/L (mean = 1.0, median = 0.1 μg/L). Of the samples with microcystin detections, 25% had concentrations >1 μg/L. High microcystin concentrations (>20 μg/L) occurred at relatively high TN (>390 μg/L) and TP (>22 μg/L) concentrations (; ), but not when TN:TP ratios were >32 (, and ). Mean and median TN and TP concentrations were higher as microcystin concentrations increased by category, while the mean and median TN:TP ratio values were lower (). The 2DKS test revealed that microcystin concentrations were significantly lower at TN:TP ratios ≥19.3 (; DKS = 0.014, microcystin concentration <0.67 μg/L, p < 0.001, n = 5101); however, microcystin concentrations >6 μg/L still occurred at TN:TP ratios >19.3 in 2% of the samples with detectable microcystin (). In addition, one of the highest microcystin concentrations in the dataset (225 μg/L) occurred at a TN:TP ratio of 32.2, 1.7 times larger than the value identified by the 2DKS test.
Figure 2 (a) Relations of total phosphorus (TP) concentration as a function of total nitrogen (TN) concentration; and (b) microcystin concentration as a function of TN:TP ratio. Black points represent points used to estimate the interval maxima regression curve (dash-dot line; see methods).
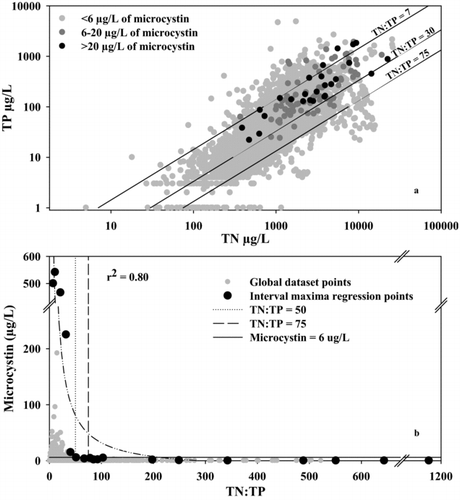
Table 2 Mean ± standard error for the 2010 WCR reservoir and mesocosm samples. Secchi depth (Zsd) in m; all concentrations in μg/L, except sedimentation rate (Sed; g/m2/d).
Although the 2DKS test identified a statistical threshold at a TN:TP ratio of 19.3, microcystin concentrations relevant to human health concerns (>6 μg/L) still occurred above this threshold. For example, when the TN:TP ratio was between 30 and 50, a microcystin concentration of 6 μg/L was exceeded 12 times in 10 lakes; however, when the TN:TP ratio was between 50 and 75, only 7% of the data points exhibited a microcystin concentration of 1–6 μg/L (). Thus, microcystin concentrations that pose a concern to human health (> 6 μg/L) were rare at TN:TP ratios >50 ().
Manipulation of the TN:TP ratio by the addition of nitrogen in mesocosms in 2010
Conditions in the reservoir and mesocosms were similar at the beginning of the experiment. Mean epilimnetic temperature, DO, and pH were similar throughout the experimental period and varied by <0.1 C, 0.2 mg/L, and 0.09, respectively. Mean conductivity did not differ between the reservoir and the mesocosms. Secchi depth was 1.1–1.8 times greater in the mesocosms than the reservoir during most of the experimental period (; paired Wilcoxon test, V = 42, p = 0.02). Secchi depths were greatest at the highest TN:TP ratios.
Figure 3 TN:TP ratio and microcystin concentration (MC) for the (a) reservoir (Res) and; (b) mesocosms (Meso) throughout the 2010 N addition experiment. The solid horizontal line in (b) represents a TN:TP ratio of 75.
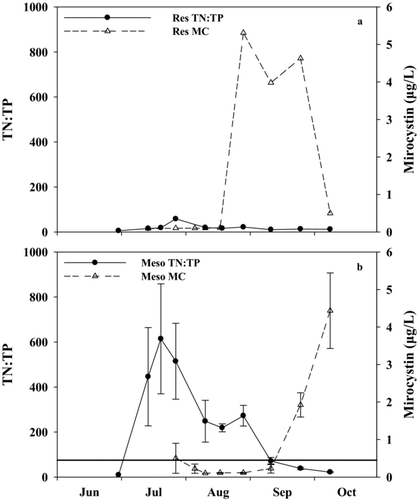
After N addition on 7 July 2010, TN concentrations in the mesocosms (7527 μg/L; ) increased 38-fold relative to the reservoir (199 μg/L; ). Total N concentrations in the mesocosms remained >4000 μg/L after the N addition until the beginning of September, when concentrations were allowed to decrease (; paired Wilcoxon test, V = 55, p = 0.002). Total P was similar in the reservoir and the mesocosms throughout the study (; paired Wilcoxon test, V = 29, p = 0.92). The TN:TP ratio in the mesocosms was generally 10 times higher than the reservoir after N was added until the end of the experiment ( and ; mean reservoir TN:TP ratio = 18, mean mesocosm TN:TP ratio = 190; paired Wilcoxon test, V = 55, p = 0.002). After September, the TN:TP ratio declined steadily from 75 to 25 until the end of the experiment ().
In 2010, the reservoir phytoplankton community was dominated by cyanobacteria (>50% by biovolume) during the entire experimental period, while the mesocosms were dominated by chlorophytes (>50% by biovolume) after the N addition ( and ). An exception to this occurred when cyanobacteria increased by 60% for less than 2 weeks in the mesocosms, about 3 weeks after N was added initially (), due to the periphytic cyanobacterium species Lyngbya that became detached from the mesocosm walls. Total phytoplankton biovolume was not significantly different in the reservoir and the mesocosms throughout the experiment (; paired Wilcoxon test, V = 33, p = 0.63), although on 2 dates it was higher in the mesocosms (). The biovolume of chlorophytes was higher (59% on average; ; paired Wilcoxon test, V = 53, p = 0.006) and the biovolume of cyanobacteria was lower (55% on average) in the mesocosms compared to the reservoir (; paired Wilcoxon test, V = 1, p = 0.004).
Figure 4 Percent phytoplankton biovolume of the (a) reservoir and (b) mesocosms in 2010. The solid and dashed vertical lines in (b) represent when N was added and when the TN:TP ratio fell below 75, respectively. Chrysophytes = Chryso; Dinoflagellate = Dino; Cryptophytes = Crypto; Chlorophytes = Chloro; Cyanobacteria = Cyano; Cyanobacteria, Potential Microcystin Producer = PMP.
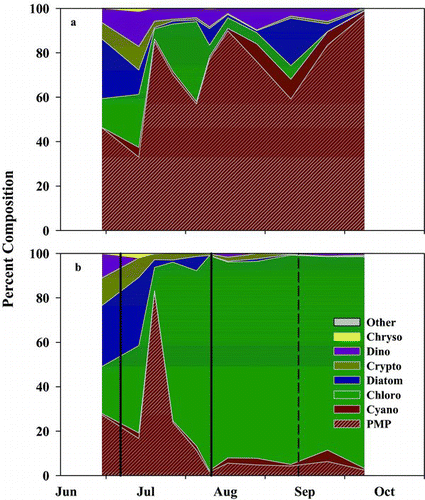
Figure 5 (a) Total phytoplankton, (b) chlorophyta, and (c) cyanobacterial (cyano) biovolume in the reservoir (Res) and mesocosms (Meso) in 2010. Solid and dashed vertical lines represent when N was added and when the TN:TP ratio fell below 75 in the mesocosms, respectively. Error bars represent standard error.
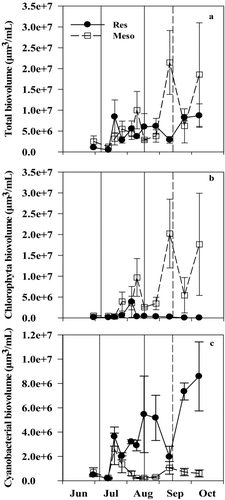
Figure 6 Biovolume of (a) potential N2-fixing, (b) non-N2-fixing, and (c) potential microcystin producing cyanobacteria in the reservoir (Res) and mesocosms (Meso) in 2010. Solid and dashed vertical lines represent when N was added and when the TN:TP ratio fell below 75, respectively. Error bars represent standard error.
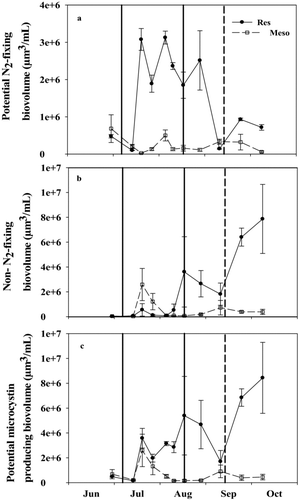
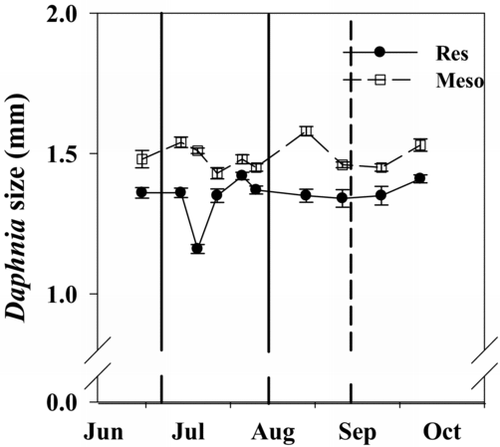
Figure 7 Daphnia size in the reservoir (Res) and the mesocosms (Meso) in 2010. Solid and dashed vertical lines in (b) represent when N was added and when the TN:TP ratio fell below 75, respectively. Error bars represent standard error.
All genera of phytoplankton present in the reservoir also were present in the mesocosms but not vice versa. For example, Dictyosphaerium and Spirogyra (Chlorophyta) were completely absent from the reservoir samples, while Quadrigula, Cosmarium, and Scenedesmus (Chlorophyta) were rarely (<12 cells of each) counted in the reservoir samples; however, these genera were abundant in the mesocosms when the TN:TP ratio was >75 (). Chrysophytes, cryptophytes, and dinoflagellates only contributed a small fraction to the overall phytoplankton community biovolume in both the reservoir and mesocosms and varied throughout the study ( and ).
Potential N2-fixing (generally Aphanizomenon), non-N2-fixing (generally Woronichinia or Microcystis), and potential microcystin producing cyanobacteria were lower (on average 12, 24, and 18%, respectively) in the mesocosms after the N addition relative to the reservoir (–; paired Wilcoxon tests, V = 1, 19, and 1, p = 0.004, 0.43, and 0.004, respectively). On average, potential N2-fixing, non-N2-fixing, and potential microcystin producing cyanobacteria represented 68, 4, and 68% of phytoplankton biovolume in the reservoir, respectively, and 16, 2, and 16% in the mesocosms. Microcystin was detectable in the reservoir from mid-August to early October and peaked on 28 August (5.31 μg/L; ). Microcystin was generally only detected in the mesocosms when TN:TP ratios were <75. The only exception occurred in mid-July, during the Lyngbya bloom (). Once TN:TP ratios were <75, microcystin concentrations increased to a maximum concentration of 4.4 μg/L ().
Daphnia size ranged from 1.16 to 1.42 mm in the reservoir and 1.43 to 1.58 mm in the mesocosms. The mean body size of Daphnia in the mesocosms was larger (1.49 mm) than in the reservoir (1.35 mm; ; paired Wilcoxon test, V = 55, p = 0.005). Sedimentation rates were approximately 2-fold lower in the mesocosms compared to the reservoir when the TN:TP ratio was >75 (); however, sedimentation rates did not differ between the reservoir and the mesocosms over the entire course of the experiment (; Wilcoxon test, V = 12, p = 0.84).
Discussion
Data from the global dataset and our mesocosm experiments showed that the TN:TP ratio and microcystin concentrations were inversely related and that microcystin concentrations >6 μg/L did not occur when the TN:TP ratio was >50. Thus, manipulating TN:TP ratios by decreasing P or increasing N may be an effective way to reduce microcystin concentrations below thresholds of concern for human health. Additionally, in our experiments increasing the TN:TP ratio shifted the phytoplankton community to Chlorophyta taxa, which were nontoxic, a more nutritional food source for zooplankton, and smaller-sized than the phytoplankton communities found at lower TN:TP ratios. The zooplankton community (primarily Daphnia) in our high TN:TP mesocosms was able to utilize the altered phytoplankton community and, as a result, increased in size (as per Daphnia length). Increased Daphnia size likely resulted in increased filtering efficiency, as reflected by deeper Secchi depths in the mesocosms relative to the reservoir. Although additional studies with careful controls are needed, our study indicates that increasing the TN:TP ratio may be an effective way to reduce cyanotoxin occurrence and concentration, and may increase energy flow through the aquatic food web.
Global dataset
Relationships among TN, TP, TN:TP, and microcystin concentrations in the global dataset were consistent with those found in regional North American datasets (Graham et al. Citation2004, Orihel et al. Citation2012). The global dataset indicated that high microcystin concentrations (>20 μg/L) generally occurred at TN:TP ratios <30 (; , and ), which is similar to the TN:TP ratio threshold originally cited by Smith (Citation1983) to reduce cyanobacteria. Our results are similar to those of Orihel et al. (Citation2012), who showed similar trends and concluded that the probability of high microcystin concentrations increased with decreasing TN:TP ratios. Overall, the global and regional datasets consistently indicate that microcystin concentration is inversely related to the TN:TP ratio (; Graham et al. Citation2004, Orihel et al. Citation2012, Otten et al. Citation2012).
The weight of evidence from the literature and our global dataset suggests that one potential in-lake management strategy to reduce the occurrence of cyanotoxins is the manipulation of the TN:TP ratio. A prominent lake management practice is to reduce P, which can consequently increase the TN:TP ratio (Smith and Schindler Citation2009); however, lake managers who work in systems that may be geologically constrained and naturally high in P (e.g., Zaca Lake, CA; Sarnelle Citation1993) or who are unable to significantly decrease P-rich allochthonous inputs or internal loading (e.g., WCR and reservoirs throughout the Midwestern US; Nürnberg Citation1984, Cooke et al. Citation2011) need a method to increase the TN:TP ratio when the ability to regulate the availability of P is absent or ineffective. Therefore, although P reduction is the only long-term solution to recover waterbodies from eutrophy (Schindler Citation2012), adding N may be an option for lake managers to increase TN:TP ratios and reduce the occurrence of cyanobacteria and associated cyanotoxins in systems where other alternatives (e.g., P reduction) are not possible.
Response of cyanobacteria and microcystin concentration to high TN:TP ratios during 2010
Our experimental results indicate that increasing the TN:TP ratio to >75 by N addition greatly reduced the biovolume of potential N2- and non-N2-fixing cyanobacteria without increasing total phytoplankton biovolume (, , and ). Potential N2- and non-N2-fixing cyanobacteria increased after the TN:TP ratio decreased to <75, suggesting that the TN:TP ratio may control phytoplankton community composition. TP concentrations increased in our mesocosms and the reservoir near the end of the experiment (), and may have contributed to the increase of total phytoplankton and cyanobacterial biovolume (Watson et al. Citation1997). Although total phytoplankton and cyanobacterial biovolume increased after TP concentrations increased, the percent composition of cyanobacteria did not substantially increase in the mesocosms or the reservoir (), suggesting that total phytoplankton biovolume was generally proportional to TP concentrations (Schindler Citation2012) and that the TN:TP ratio controlled the percent composition of cyanobacteria. In whole-lake manipulations, phytoplankton biomass was always proportional to the addition of P, not N (Schindler et al. Citation2008, Smith and Schindler Citation2009). These studies found that when N additions were stopped, N2-fixing cyanobacteria increased to overcome the deficiency of N imposed by the continued addition of P. Overall, these results suggest that increasing the TN:TP ratio above 75 suppresses both N2- (Schindler et al. Citation2008) and non-N2-fixing cyanobacteria (Graham et al. Citation2004) without increasing total phytoplankton biovolume.
Because our experiment was conducted to determine broad changes in the phytoplankton community, the cellular mechanisms driving the observed patterns were not studied. One plausible explanation is that the addition of N prevented depletion of nitrate concentrations, which has been correlated to cyanobacterial dominance in freshwater systems. Whole-lake N addition in a hypereutrophic lake did not prevent cyanobacterial dominance of the phytoplankton community, suggesting that relatively low nitrate concentrations may not consistently trigger cyanobacterial blooms (Lathrop Citation1988). Although the mechanistic factors driving this pattern cannot be determined from this study, increasing the TN:TP ratio seems to be an effective way to reduce cyanobacteria below concentrations that may trigger reservoir closure (>100,000 cells/mL; ). Further exploration of the mechanistic and confounding factors, such as climate and light, driving the observed TN:TP thresholds for cyanobacterial dominance and cyanotoxin production is required before this practice can be implemented as a management tool.
The concentration of microcystin was lower when the TN:TP ratio was experimentally increased >75 ( and ). These findings are comparable to those found in natural systems (Graham et al. Citation2004, Wu et al. Citation2006, Orihel et al. Citation2012, and our global dataset). With the exception of mid-July microcystin detection, microcystin concentrations were below the limit of detection in the mesocosms until TN:TP ratios decreased to <75 (). Donald et al. (Citation2011) also observed an initial increase in microcystin concentration when N was added to small (∼3000 L), closed bottom mesocosms at Wascana Lake, Saskatchewan, Canada. Similar to the current study, microcystin concentrations decreased after 2–3 weeks. Other small mesocosm studies at Wascana Lake showed that adding N (as urea) increased microcystin concentrations during late August–early September (Finlay et al. Citation2010); however, these studies (1) did not increase the TN:TP ratio to >75, (2) had small spatial and temporal scales relative to our mesocosm study, and (3) used closed-bottom mesocosms, which have given spurious results when compared to whole-lake results (Schindler Citation1998). Additionally, Otten et al. (Citation2012) showed that high (>50) TN:TP ratios favor nontoxic strains of non-N2-fixing cyanobacteria, indicating that at high TN:TP ratios cyanobacteria are primarily nontoxin-producing species. Although adding N may increase the microcystin concentration initially, potential microcystin producers may not be able to compete with Chlorophyta taxa in high TN:TP environments; therefore, management strategies that focus on increasing the N:P ratio, such as decreasing P or increasing N, may be applied to reduce toxic cyanobacterial blooms.
Daphnia length did not differ between the mesocosms and the reservoir in our control year (Supplemental Information ), suggesting that differences in Daphnia length observed between the mesocosms and the reservoir in our experimental year () were due to the N-addition treatment. The larger Daphnia observed in the N-treated mesocosms likely occurred because increasing the TN:TP ratio shifted the phytoplankton community from large, surface-blooming cyanobacteria to smaller-sized Chlorophyta taxa, creating a more physically manageable and nutritional food source for zooplankton (Holm and Shapiro Citation1984, Gliwicz and Lampert Citation1990). The similarity in sedimentation rates between the treated mesocosms and the reservoir () suggests that the increased water transparency (as per Secchi depth; ) observed in the mesocosms was most likely the result of increased zooplankton filtering and a shift away from surface-blooming cyanobacteria.
Additionally, by exchanging cyanobacteria for chlorophytes as the dominant food resource for zooplankton, the flow of energy through the food web may have been increased (Carpenter et al. Citation1985, Citation2001). In the mesocosms, this increase was reflected in larger zooplankton size relative to the reservoir (). Thus, additions of N that increase the TN:TP ratio may indirectly increase (1) water transparency and (2) the efficiency of the flow of energy from the primary producers to higher trophic levels. These changes will only occur, if the waterbody has a biological structure capable of utilizing a more nutritional and smaller-sized phytoplankton community. This study did not explore the effect of predatory interactions, particularly planktivory. Size-selective predation by planktivores would be aided by higher water clarity and large-bodied zooplankton; therefore, to fully understand trophic-level interactions of whole-lake N additions more research is needed.
Whole-lake management approaches that use N additions to increase the TN:TP ratio have found system-specific dosages to reduce cyanobacterial biovolume and microcystin concentration. Preliminary studies of a whole-lake N addition on a mesotrophic reservoir (Dworshak Reservoir, ID) have shown that N additions that maintain the TN:TP ratio observed during winter months reduce cyanobacterial biovolume to <5% of the total phytoplankton community in summer (IDFG Citation2012). Adding N at the whole-lake scale in Dworshak Reservoir had similar trophic level effects to those observed in this study (IDFG Citation2012; Brandt D, Advanced Eco-Solutions, Feb 2012, pers. comm.). Practical application of this management strategy will need to include pilot studies and further experimental studies to determine mechanistic factors, unintended consequences, influences of other confounding factors, and system-specific TN:TP ratios needed to prevent cyanobacterial blooms and cyanotoxins.
Although increasing the TN:TP ratio by N addition is counterintuitive to reducing toxic cyanobacterial blooms, this management strategy may be applicable when no other management alternatives exist. Adding N to increase the TN:TP ratio, without causing N toxicity, may be an option for managers trying to reduce human health risks associated with cyanotoxins in systems where P is beyond control. In these systems, reducing the occurrence of toxic cyanobacterial blooms may also reduce ecotourism-related economic losses associated with health advisories that close recreational reservoirs. Adding N to increase the TN:TP ratio may also reduce the downstream transport of reservoir-produced cyanotoxins, which pose a serious threat to the health of aquatic and terrestrial organisms downstream. Cyanotoxins originating from reservoirs with toxic cyanobacterial blooms have caused human health concerns and death to marine mammals >160 km downstream (Khan et al. Citation2010, Miller et al. Citation2010, Graham et al. Citation2012).
Mitigating cyanobacterial blooms by manipulating the TN:TP ratio via N addition may cause unintended consequences. For example, adding N may cause waterbody N concentrations to surpass N toxicity thresholds for aquatic and terrestrial health. Human health guideline limits for drinking water are 50,000 μg/L as NO3 − ion and 3000 μg/L as NO2 − ion (WHO Citation2011); human health guidelines for drinking water for ammonia are not defined because NH3/NH4 + is only toxic if intake surpasses the capacity to detoxify, and toxicity is dependent on pH, temperature, and species present (WHO Citation2003). Guidelines for freshwater animals are species-specific and are found in Camargo et al. (Citation2005) and Randall and Tsui (Citation2002) for NO3 − /NO2 − and NH3/NH4 +, respectively. Adding N to reservoirs may also cause the transport of N-enriched water downstream, which may cause undesirable effects in marine environments. Additionally, N additions may only be applicable in lakes and reservoirs where P reduction is beyond reasonable control and in lakes and reservoirs that are sufficiently oxygenated and have a biological structure capable of utilizing an algal community fostered by a shifted N:P ratio.
Although P reduction is essential to reduce long-term eutrophication of freshwater systems (Schindler Citation2012), the results of this study show that the addition of N could be explored as a potential short-term, in-lake management strategy to reduce cyanobacteria and associated cyanotoxins. Nutrient abatement has been enforced for decades, and much effort has been invested to reduce nonpoint sources of nutrients, yet eutrophication of aquatic ecosystems continues to be a global problem (Paerl et al. Citation2011, Sprague et al. Citation2011). Reservoirs have been created even when preconstruction environmental impact statements predict a high likelihood of eutrophy and cyanobacterial blooms after impoundment (e.g., WCR; Funk Citation1973). Given the regional importance of WCR and similar reservoirs (WCR is the only reservoir/lake within a 60 mile radius), in the absence of other management options (i.e., P reduction) additions of N that increase the TN:TP ratio to >50 without causing N toxicity may be a viable option to reduce toxic cyanobacterial blooms that pose a concern for human health and to protect the health of in-lake and downstream organisms if the system suffers from low TN:TP allochthonous inputs.
Although adding N is counterintuitive to reduce cyanobacterial blooms and cyanotoxins, the results of this study suggest that increasing the TN:TP ratio by adding N may (1) shift the phytoplankton community from cyanobacteria (found at low TN:TP ratios) to chlorophytes (found at high TN:TP ratios), providing zooplankton with a more physically manageable and nutritional food source, (2) suppress microcystin concentration, and (3) shift the phytoplankton community from an energy (carbon) sink to a carbon source for higher trophic levels (Stockner J, University of British Columbia, adjunct professor, Oct 2011, pers. comm.). Therefore, although additional experimental studies with careful controls need to be conducted to define best management practices and identify potential unintended consequences of adding N at a whole-lake scale, this study indicates that manipulating the TN:TP ratio may be a viable option to limit cyanobacterial blooms and associated toxins in lakes and reservoirs when management options for P control are not available.
Funding
Funding and support for this project was provided by the US Army Corps of Engineers, US Geological Survey, and a University of Idaho Jeff Braante grant.
Supplemental Material
Supplemental data for this article can be accessed on the http://dx.doi.org/10.1080/10402381.2013.876131.
Supplementary Material
Download MS Word (113.8 KB)Acknowledgments
We thank Robert Mahler, Alex Horne, Ken Wagner, and Gertrud Nürnberg for their reviews and C. Adams, T. Caldwell, E. Reams, K. Gilbert, A. Waldo, R. Gonzalez, and J. Amante for field and laboratory assistance. We also thank D. Bigham, A. Ghadouani, J. Jacoby, J. Jones, and R. Zurawell for contributing to our global dataset, 3 anonymous reviewers for comments that improved the manuscript greatly, and John Stockner for input into the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Adams , C. 2012 . Phosphorus, Daphnia, and the recreating public: a multi-disciplinary study of Willow Creek Reservoir, Heppner, Oregon. [master's thesis] . [Moscow (ID)]: University of Idaho
- Bellinger , E G and Sigee , D C . 2010 . Freshwater algae: identification and use as bioindicators , Wiley-Blackwell .
- Bennett , E M , Carpenter , S R and Caraco , N F . 2001 . Human impact on erodible phosphorus and eutrophication: a global perspective . Bioscience. , 51 : 227 – 234 .
- Bigham , D L , Hoyer , M V and Canfield , DE. Jr. 2009 . Survey of toxic algal (microcystin) distribution in Florida lakes . Lake Reserv Manage. , 25 : 264 – 275 .
- Camargo , J A , Alonso , A and Salamanca , A . 2005 . Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates . Chemosphere. , 58 : 1255 – 1267 .
- Canfield , D E , Phlips , D and Duarte , C M . 1989 . Factors influencing the abundance of blue–green algae in Florida lakes . Can J Fish Aquat Sci. , 46 : 1232 – 1237 .
- Carpenter , SR. 2008 . Phosphorus control is critical to mitigating eutrophication . P Natl Acad Sci USA. , 105 : 11039 – 11040 .
- Carpenter , S R , Cole , J J , Hodgson , J R , Kitchell , J F , Pace , M L , Bade , D , Cottingham , K L , Essington , T E , Houser , J N and Schindler , D E . 2001 . Trophic cascades, nutrients, and lake productivity: whole lake experiments . Ecol Monogr. , 71 : 163 – 186 .
- Carpenter , S R , Kitchell , J F and Hodgson , J R . 1985 . Cascading trophic interactions and lake productivity . Bioscience. , 35 : 634 – 639 .
- Chorus , I and Bartram , J . 1999 . Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management . World Health Organization. ,
- Crumpton , W G , Isehard , T M and Mitchell , P D . 1992 . Nitrate and organic nitrogen analysis with second derivative spectroscopy . Limnol Oceanogr. , 37 : 907 – 913 .
- Cooke , G D , Welch , E B and Jones , J R . 2011 . Eutrophication of Tenkiller Reservoir, Oklahoma, from nonpoint agricultural runoff . Lake Reserv Manage. , 27 : 256 – 270 .
- Dodson , S I and Frey , D G . 2001 . “ Cladocera and other Branchiopoda ” . In Ecology and classification of North American freshwater invertebrates , Edited by: Thorp , J H and Covich , A P . 723 – 786 . Academic Press .
- Donald , D B , Bogard , M J , Finlay , K and Leavitt , P R . 2011 . Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters . Limnol Oceangr. , 56 : 2161 – 2175 .
- Eaton , A D , Clesceri , L S , Rice , E W and Franson , M A . 2005 . Standard methods for the examination of water and wastewater. 21st ed , American Public Health Association, American Water Works Association, Water Environment Federation .
- Finlay , K , Patoine , A , Donald , D B , Bogard , M J and Leavitt , P R . 2010 . Experimental evidence that pollution with urea can degrade water quality in phosphorus–rich lakes of the Northern Great Plains . Limnol Oceanogr. , 55 : 1213 – 1230 .
- Funk , WH. 1973 . Willow Creek Reservoir water quality study. Final report to the U.S. Army Corps of Engineers, Walla Walla District, WA , Pullman, WA : Environmental Engineering Laboratories, Washington State University .
- Garvey , J E , Marschall , E A and Wright , R A . 1998 . From star charts to stoneflies: detecting relationships in continuous bivariate data . Ecology. , 79 : 442 – 447 .
- Gliwicz , Z M and Lampert , W . 1990 . Food thresholds in Daphnia species in the absence and presence of blue-green filaments . Ecology. , 71 : 691 – 702 .
- Graham , J L , Jones , J R , Jones , S B , Downing , J A and Clevenger , T E . 2004 . Environmental factors influencing microcystin distribution and concentration in the Midwestern United States . Water Res. , 38 : 4395 – 4404 .
- Graham , J L , Jones , J R and Jones , S B . 2006 . Microcystin in Midwestern lakes . LakeLine , 26 : 32 – 35 .
- Graham , J L and Jones , J R . 2009 . Microcystin in Missouri reservoirs . Lake Reserv Manage. , 25 : 253 – 263 .
- Graham , J L , Loftin , K A , Ziegler , A C and Meyer , M T . 2008 . Guidelines for design and sampling for cyanobacterial toxin and taste–and–odor studies in lakes and reservoirs . US Geological Survey Scientific Investigations Report , : 2008 – 5038 .
- Graham , J L , Loftin , K A and Kamman , N . 2009 . Monitoring recreational freshwaters . LakeLine , 29 : 18 – 24 .
- Graham , J L , Zielger , A C , Loving , B L and Loftin , K A . 2012 . Fate and transport of cyanobacteria and associated toxins and taste-and-odor compounds from upstream reservoir releases in the Kansas River, Kansas, September and October 2011 . US Geological Survey Scientific Investigations Report , : 2012 – 5129 .
- Hillebrand , H , Durselen , C , Kirschtel , D , Pollingher , U and Zohary , T . 1999 . Biovolume calculation for pelagic and benthic microalgae . J Phycol. , 35 : 403 – 424 .
- Holm , N P and Shapiro , J . 1984 . An examination of lipid reserves and the nutritional status of Cladoceran pulex fed Aphanizomenon flos–aquae . Limnol Oceanogr. , 29 : 1137 – 1140 .
- Hudnell , HK. 2013 . An alternative approach to regaining designated uses of clean water act section 303(d) impaired waters . Fla Water Resour J. , 65 : 20 – 26 .
- [IDFG] Idaho Department of Fish and Game . 2012 . Dworshak reservoir nutrient supplementation project update. [cited 31 Aug 2012] . Available from: http://fishandgame.idaho.gov/public/docs/fishReportsNewsletters/clearwaterDworshak12.pdf
- Khan , J , Bowater , L and Corum , S . 2010 . Middle Klamath River toxic cyanobacteria trends. 2009: Aquatic Ecosystems Sciences LLC technical memorandum; [cited 15 Dec 2012] . Available from: http://www.klamathwaterquality.com/documents.html
- Larsson , U , Blomqvist , S and Abrahamsson , B . 1986 . A new sediment trap system . Mar Ecol Prog Ser. , 31 : 205 – 207 .
- Lathrop , RC. 1988 . Evaluation of whole-lake nitrogen fertilization for controlling blue-green algal blooms in a hypereutrophic lake . Can J Fish Aquat Sci. , 45 : 2061 – 2075 .
- Levine , S N and Schindler , D W . 1999 . Influence of nitrogen to phosphorus supply ratios and the physicochemical conditions on cyanobacteria and phytoplankton species composition in the Experimental Lakes Area, Canada . Can J Fish Aquat Sci. , 56 : 451 – 466 .
- Lund , J WG , Kipling , C and Cren , E D . 1958 . The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting . Hydrobiologia. , 11 : 143 – 170 .
- Manly , BFJ. 2009 . Statistics for environmental science and management. 2nd ed , Boca Raton, FL : Chapman and Hall .
- Miller , M A , Kudela , R M , Mekebri , A , Crane , D B , Oates , S C , Tinker , M T , Staedler , M , Miller , W A , Toy–Choutka , S Dominik , C . 2010 . Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters . PLOS ONE. , 5 : e12576
- Nürnberg , GK. 1984 . The prediction of internal phosphorus load in lakes with anoxic hypolimnia . Limnol Oceanogr. , 29 : 111 – 124 .
- [ODHS] Oregon Department of Health Services . 2008 . 2008 Blue-green health advisories; [cited 15 Apr 2011] . Available from: http://public.health.oregon.gov/HealthyEnvironments/Recreation/HarmfulAlgaeBlooms/Pages/new2008.aspx
- Orihel , D M , Bird , D F , Brylinsky , M , Chen , H , Donald , D B , Huang , D Y , Giani , A , Kinniburgh , D , King , H Kotak , B . 2012 . High microcystin concentrations occur only at low nitrogen–to–phosphorus ratio in nutrient–rich Canadian lakes . Can J Fish Aquat Sci. , 69 : 1457 – 1462 .
- Ott , R L and Longnecker , M . 2010 . An introduction to statistical methods and data analysis. 6th ed , Belmont, CA : Brooks/Cole .
- Otten , T G , Xu , H , Qin , B , Zhu , G and Pearl , H W . 2012 . Spatiotemporal patterns and ecophysiology of toxigenic Microcystis blooms in Lake Taihu, China: implications for water quality management . Environ Sci Technol. , 45 : 3480 – 3488 .
- Paerl , H W and Fulton , R S . 2006 . “ Ecology of harmful cyanobacteria ” . In Ecology of harmful algae , Edited by: Granéli , E and Turner , J T . 95 – 109 . Springer-Verlag Berlin Heidelberg .
- Paerl , H W and Scott , J T . 2010 . Throwing fuel on the fire: synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms . Environ Sci Technol. , 44 : 7756 – 7758 .
- Paerl , H W , Xu , H , McCarthy , M J , Zhu , G , Qin , B , Li , Y and Gardner , W S . 2011 . Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N and P) management strategy . Water Res. , 45 : 1973 – 1983 .
- Peters , R H and Downing , J A . 1984 . Empirical analysis of zooplankton filtering and feeding rates . Limnol Oceanogr. , 29 : 763 – 784 .
- Pick , F R and Lean , D RS . 1987 . The role of macronutrients (C, N, P) in controlling cyanobacterial dominance in temperate lakes . New Zeal J Mar Fresh. , 21 : 425 – 434 .
- Prescott , GW. 1962 . Algae of the western great lakes area. Revised eds , Dubuque, IA : William C. Brown Company .
- R Development Core Team . 2011 . R: A language and environment for statistical computing. R Foundation for Statistical Computing . Vienna, Austria. ISBN 3-0900051-07-0; http://www.R–project.org/
- Randall , D J and Tsui , T KN . 2002 . Ammonia toxicity in fish . Mar Pollut Bull. , 45 : 17 – 23 .
- Sarnelle , O. 1993 . Herbivore effects on phytoplankton succession in a eutrophic lake . Ecol Monogr. , 63 : 129 – 149 .
- Sarnelle , O. 1997 . Daphnia effects on microzooplankton: comparisons of enclosure and whole–lake responses . Ecology. , 78 : 913 – 928 .
- Sarnelle , O. 2007 . Initial conditions mediate the interaction between Cladoceran and bloom–forming cyanobacteria . Limnol Oceangr. , 52 : 2120 – 2127 .
- Schindler , DW. 1998 . Replication versus realism: the need for ecosystem–scale experiments . Ecosystems. , 1 : 323 – 334 .
- Schindler , DW. 2012 . The dilemma of controlling cultural eutrophication of lakes . P Roy Soc B. , 279 : 4322 – 4333 .
- Schindler , D W , Hecky , R E , Findlay , D L , Stainton , M P , Parker , B R , Paterson , M J , Beaty , K G , Lyng , M and Kasian , S EM . 2008 . Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37– year whole–ecosystem experiment . P Natl Acad Sci USA. , 105 : 11254 – 11258 .
- Schindler , D W and Vallentyne , J R . 2008 . The algal bowl: overfertilization of the world's freshwaters and estuaries , Edmonton, AB : University of Alberta Press .
- Smith , VH. 1983 . Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton . Science. , 221 : 669 – 671 .
- Smith , V H and Schindler , D W . 2009 . Eutrophication science: where do we go from here? . Trends Ecol Evol. , 22 : 201 – 207 .
- Sprague , L A , Hirsch , R M and Aulenbach , B T . 2011 . Nitrate in the Mississippi River and its tributaries, 1980 to 2008: are we making progress? . Environ Sci Technol. , 45 : 7209 – 7216 .
- Stockner , J G and Shortreed , K S . 1988 . Response of Anabaena and Synechococcus to manipulation of nitrogen: phosphorus ratios in a lake fertilization experiment . Limnol Oceanogr. , 33 : 1348 – 1361 .
- [USACE] US Army Corps of Engineers . 2007 . “ Long-term withdrawal of irrigation water, Willow Creek Lake, Morrow County, Oregon: draft environmental assessment ” . Portland, OR : USACE, Portland District .
- [USEPA] US Environmental Protection Agency . 2007 . National lakes assessment (NLA); [cited 15 May 2011] . Available from: http://water.epa.gov/type/lakes/lakessurvey_index.cfm
- Watson , S , McCauley , E and Downing , J A . 1997 . Patterns in phytoplankton taxonomic composition across temperate lakes and differing nutrient status . Limnol Oceanogr. , 42 : 487 – 495 .
- Wehr , J D and Sheath , R G . 2003 . Freshwater algae of North America: ecology and classification , San Diego, CA : Academic Press .
- Wetzel , RG. 2001 . Limnology: lake and river ecosystems. 3rd ed , San Diego, CA : Academic Press .
- Williamson , CE. 2001 . “ Copepoda ” . In Ecology and classification of North American freshwater invertebrates , Edited by: Thorp , J H and Covich , A P . 787 – 822 . San Diego, CA : Academic Press .
- [WHO] World Health Organization . 2003 . Ammonia in drinking-water; [cited 28 Feb. 2013] . Available from: http://www.who.int/water_sanitation_health/dwq/ammonia.pdf
- [WHO] World Health Organization . 2011 . Nitrate and nitrite in drinking-water; [cited 28 Feb. 2013] . Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite_background.pdf
- Wu , S K , Xie , P , Liang , G D , Wang , S B and Liang , X M . 2006 . Relationships between microcystins and the environmental parameters in 30 subtropical shallow lakes along the Yangtze River, China . Freshw Biol. , 51 : 2309 – 231 .
