Abstract
Recent studies suggest that nitrogen additions to increase the total nitrogen:total phosphorus (TN:TP) ratio may reduce cyanobacterial biovolume and microcystin concentration in reservoirs. In systems where TP is >100 μg/L, however, nitrogen additions to increase the TN:TP ratio could cause ammonia, nitrate, or nitrite toxicity to terrestrial and aquatic organisms. Reducing phosphorus via aluminum sulfate (alum) may be needed prior to nitrogen additions aimed at increasing the TN:TP ratio. We experimentally tested this sequential management approach in large in situ mesocosms (70.7 m3) to examine effects on cyanobacteria and microcystin concentration. Because alum removes nutrients and most seston from the water column, alum treatment reduced both TN and TP, leaving post-treatment TN:TP ratios similar to pre-treatment ratios. Cyanobacterial biovolume was reduced after alum addition, but the percent composition (i.e., relative) cyanobacterial abundance remained unchanged. A single ammonium nitrate (nitrogen) addition increased the TN:TP ratio 7-fold. After the TN:TP ratio was >50 (by weight), cyanobacterial biovolume and abundance were reduced, and chrysophyte and cryptophyte biovolume and abundance increased compared to the alum treatment. Microcystin was not detectable until the TN:TP ratio was <50. Although both treatments reduced cyanobacteria, only the nitrogen treatment seemed to stimulate energy flow from primary producers to zooplankton, which suggests that combining alum and nitrogen treatments may be a viable in-lake management strategy to reduce cyanobacteria and possibly microcystin concentrations in high-phosphorus systems. Additional studies are needed to define best management practices before combined alum and nitrogen additions are implemented as a reservoir management strategy.
Severe freshwater eutrophication is frequently indicated by the occurrence of food web disrupting and often toxic blooms of cyanobacteria (Schindler and Vallentyne Citation2008, Paerl et al. Citation2011). Cyanobacteria blooms decrease lake and reservoir aesthetics (Paerl et al. Citation2011), cause taste and odor problems in drinking water supplies (Graham et al. Citation2010), and produce a suite of potent toxins (cyanotoxins) linked to poisoning fish (Landsberg Citation2002), wild and domestic animals (Briand et al. Citation2003), and humans (Chorus et al. Citation1999). Because humans are susceptible to cyanotoxins, a variety of in-lake management strategies have been employed to provide relief from cyanobacteria and cyanotoxins. One of the most prominent strategies is the addition of alum because it permanently binds to phosphorus and removes it from the water column by sedimentation (Cooke et al. Citation2005). In addition, alum treatments permanently seal phosphorus below the water–sediment interface, preventing it from being re-mobilized into the water column.
The importance of nutrient ratios in structuring phytoplankton communities has been known for ∼50 years (Redfield et al. Citation1963, Tilman Citation1982, Smith Citation1983). Surveys by Graham et al. (Citation2004), Orihel et al. (Citation2012), and Harris et al. (Citation2014) showed that cyanobacterial biovolume and cyanotoxin (i.e., microcystin) concentrations were highest when TN:TP ratios were <50 (by weight). Thus adding nitrogen (i.e., NH4NO3 or NaNO3) to increase the TN:TP ratio to >50 may be a viable management strategy to reduce the occurrence of cyanobacteria and cyanotoxins when other alternative management options (e.g., P reduction) are not available (Harris et al. Citation2014).
Table 1 Summary of methods used to determine total nitrogen (TN), total phosphorus (TP), microcystin, phytoplankton, zooplankton, and sedimentation rate (sediment). For a detailed explanation of methods see Harris et al. (Citation2014).
Adding nutrients (i.e., nitrogen) to eutrophic systems is counterintuitive, and may meet resistance by the public, lake managers, and regulatory agencies. Additionally, adding nitrogen to increase the TN:TP ratio to >50 in systems with high phosphorus concentrations (e.g., TP > 100 μg/L) could result in extremely high concentrations of nitrite/nitrate and ammonia (Randall and Tsui Citation2002) that would potentially endanger aquatic and terrestrial organisms, including humans (WHO Citation2011). Thus, in systems where TP is sufficiently high that a nitrogen addition that increases the TN:TP ratio to >50 would cause nitrogen toxicity, phosphorus concentrations must be decreased before nitrogen is added. We wanted to experimentally determine (1) if water column phosphorus removal via alum is sufficient to yield a TN:TP ratio >50 in a high TP system, and (2) if addition of alum alone does not yield a TN:TP ratio >50, could a subsequent addition of nitrogen (i.e., NH4NO3) increase the TN:TP ratio to reduce cyanobacterial abundance and biovolume and microcystin concentrations.
Materials and methods
Experimental design
Experiments were conducted in Willow Creek Reservoir (WCR), near Heppner, Oregon (see Harris et al. Citation2014 for study site details). Alum and nitrogen were added to triplicate 3 m diameter, 10 m deep (70.7 m3), cylindrical polyethylene mesocosms. Additional information about design, fabrication, deployment, and location of mesocosms is given in Harris et al. (Citation2014). Only 3 mesocosms were installed due to cost and logistical constraints of large-scale mesocosm experiments. Although this limited the experimental design, Harris et al. (Citation2014) demonstrated that unmanipulated mesocosms generally reflected water quality conditions in the reservoir.
Mesocosms were installed on 24 June 2011 and removed on 2 October 2011; the reservoir was thermally stratified throughout the experiment. Because we were interested in comparing unmanipulated mesocosms to treated mesocosms, the first 4 weeks (24 Jun–26 Jul 2011) of the experiment were used as a before-manipulation period. To determine if alum treatments can be followed by nitrogen additions to increase the TN:TP ratio, all 3 mesocosms were treated with alum and monitored for approximately 4 weeks (27 Jul–22 Aug 2011). All 3 mesocosms then were treated with nitrogen (as NH4NO3 because of its near neutral pH) and monitored for the remainder of the experiment (23 Aug–2 Oct 2011). Thus, the experiment was broken up into 3 treatment periods: unmanipulated mesocosms, removal of phosphorus via alum, and addition of nitrogen to increase the TN:TP ratio (hereafter referred to as before-manipulation, alum, and N-addition treatments, respectively).
To ensure the addition of alum was nontoxic, laboratory tests were conducted in 0.5 L beakers and 19 L buckets with fresh water from WCR according to Cooke et al. (Citation2005). To determine the acid neutralizing capacity (ANC) of water from WCR, samples for alkalinity analysis were collected on 4 dates before alum addition. Results of these preliminary tests showed that the maximum alum dose required to decrease P while not causing aluminum toxicity or decreasing the pH below 6 was 12.5 mg/L of Al. To treat each mesocosm, 5600 g of alum was applied to each mesocosm after routine sampling on 26 July 2011. This alum treatment only removed phosphorus from the water column and did not prevent internal P-loading events from occurring post-alum treatment (i.e., loading across the thermocline). On 22 August 2011, 70 g of NH4NO3 was added to each mesocosm. To ensure the alum/NH4NO3 additions were homogeneously dispersed, each mesocosm was stirred for approximately 10 minutes with a boat paddle.
Sampling of mesocosms commenced immediately after installation and continued at approximately weekly intervals throughout the experiment. On each sampling occasion, profiles of temperature, pH, dissolved oxygen, and conductivity were collected at 1 m intervals from the water surface to a depth of 11 m, Secchi depth was measured, and water samples were collected from each mesocosm. Samples for the analysis of TN, TP, and microcystin were collected at a depth of 0.5 m. A phytoplankton sample was collected by lowering a 2 L weighted sampler through the photic zone (defined as 2 times Secchi depth) of the water column in each mesocosm. A zooplankton sample was collected using a 0.12 m diameter, 80 μm mesh zooplankton net from a depth of 11 m to the water surface in each mesocosm. Sediment traps were deployed at a depth of 10 m in each mesocosm to analyze the sedimentation rate. Sampling methods and laboratory analyses of TN, TP, microcystin, phytoplankton, zooplankton, and sedimentation rate () are described in detail in Harris et al. (Citation2014). Daphnia biomass was estimated using the cladoceran mass–length relationship of Bottrell et al. (Citation1976).
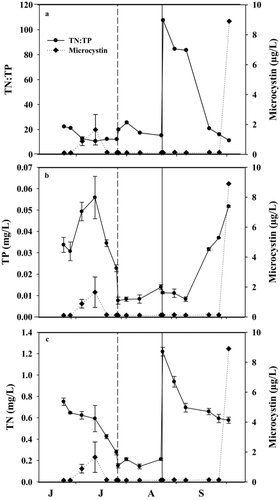
Figure 1 Temporal trends of the (a) total nitrogen to total phosphorus ratio (TN:TP), (b) TP, and (c) TN, in mg/L in the mesocosms in Willow Creek Reservoir, Heppner, OR, during 2011. Microcystin concentrations (μg/L) are presented on the right y-axis. Vertical dashed and solid lines represent the date of additions of alum and nitrogen, respectively. The x-axis labels refer to the first letter of the month starting with June. Exact sample dates are given in .
Statistical analyses
To determine the effects of alum and nitrogen addition, alum treatment results were compared to before-manipulation, and N-addition results were compared to alum treatment using Wilcoxon rank sum tests in R 3.0.1 (Ott and Longnecker Citation2010; R Development Core Team Citation2011). Data from each mesocosm were averaged for each treatment; the 3 mean values (representing each mesocosm) for each treatment were used to make among-treatment comparisons, except Daphnia biomass, which is described below. Although the experimental design did not allow us to account for differences in time or to compare N-addition treatment to before-manipulation, it did simulate a real-world alum and subsequent N-addition treatment.
To compare Daphnia biomass between treatments, each of the 3 mesocosm values were averaged by date and compared with a Wilcoxon rank sum test (Ott and Longnecker Citation2010). Daphnia biomass was averaged by date rather than by mesocosm because some mesocosm samples had low (<30) Daphnia per sample and would have biased biomass estimates.
Results and discussion
Alum treatment to reduce water-column TP
The experimental results show that our water column alum treatment did not significantly change the TN:TP ratio (; ; pre-alum TN:TP = 15, post-alum TN: TP = 19); thus, the alum treatment alone was not sufficient to yield a TN:TP ratio >50. Relative cyanobacterial biovolume was reduced by 24% on average, but relative cyanobacterial abundance only decreased by 4% on average ( and ; ). These results indicate that because the TN:TP ratio was unchanged, cyanobacteria continued to dominate the phytoplankton community (based on abundance).
Table 2 Wilcoxon rank sum tests for chemical, algal, and zooplankton parameters between the before manipulation period (BM) and alum treatment and the alum and N-addition (N-add) treatments. W = Wilcox rank sum test statistic; p = probability value.
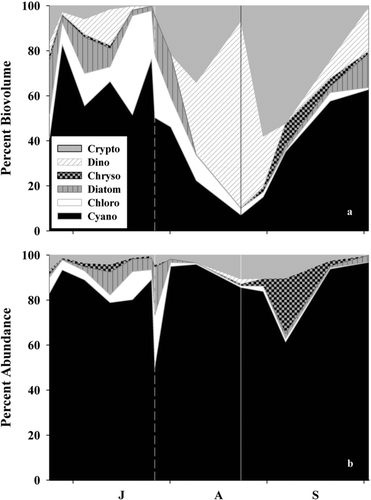
Figure 2 Temporal trends of (a) relative phytoplankton biovolume and (b) abundance (as cells/mL) in the mesocosms during 2011. Vertical dashed and solid lines represent the date of additions of alum and nitrogen, respectively. Cryptophytes = Crypto; Dinoflagellate = Dino; Chrysophytes = Chryso; Chlorophytes = Chloro; Cyanobacteria = Cyano. X-axis labels refer to the first letter of the month starting with July. For exact sample dates see .
Note that TP and TN decreased in the last 3 sampling periods in the before-manipulation period (pre-alum treatment; and ); the initial TP and TN concentrations in the before-manipulation period were related to a rain-on-snow event in the upper portion of the WCR watershed that caused sediment- and nutrient-rich water to enter the reservoir. By early July, however, the reservoir became strongly thermally stratified (resistance to thermal mixing sum >100; data not shown) and spring winds diminished, causing sediment and nutrients ( and ) to sink out of the epilimnion in the reservoir and mesocosms. A similar decrease in TN and TP due to stratification and nutrient loss from the epilimnion was observed in the reservoir, indicating that the mesocosms reflected reservoir processes and nutrient concentrations (Harris et al. Citation2014). Additionally, alum treatment of the mesocosms decreased TP and TN concentrations 2–3-fold lower than the reservoir, indicating that the sharp decrease in TP and TN in the alum treatment portion of the mesocosm study was due to our water column alum treatment, and not natural decreases due to reservoir processes.
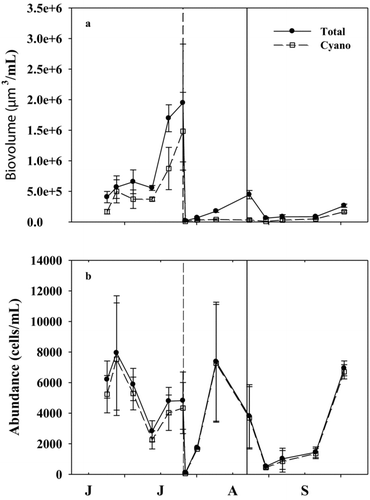
On average, the water column alum treatment reduced biovolume and abundance of phytoplankton by 91 and 43%, respectively ( and ; ). The decrease in phytoplankton biovolume after addition of alum was generally sustained throughout the alum portion of the study, likely because of the sustained decrease in TP (65% reduction; ; ). This finding reflects the accepted practice of adding alum to reduce phosphorus and phytoplankton biovolume as a lake management strategy (Kennedy et al. Citation1987, Barko et al. Citation1990, Jacoby et al. Citation1994, Welch and Cooke Citation1999, Huser et al. Citation2011). Relative reductions of TN (46% reduction; ; ) and TP were similar, consequently, the TN:TP ratio in the alum treatment was similar to the before-manipulation period (; ).
Figure 3 Temporal trends of (a) total phytoplankton (Total) and cyanobacterial (Cyano) biovolume and (b) abundance throughout the experiment in the mesocosms during 2011. Vertical dashed and solid lines represent the date of additions of alum and nitrogen, respectively. X-axis labels refer to the first letter of the month starting with June. Exact sample dates are given in .
Because the TN:TP ratio remained unchanged, cyanobacteria continued to have a competitive advantage over other phytoplankton taxa, which explains the similar relative cyanobacterial abundance between pre- and post-alum treatments (; ). Evidence for this phenomenon is already present in the literature. For example, all 19 alum-treated lakes reviewed by Welch and Cooke (Citation1999) had initial reductions in absolute and relative cyanobacterial biovolume, but results for long-term (>1 year) reductions of relative cyanobacterial biovolume were mixed. Additionally, one of the only alum treatment studies to report both TN and TP concentrations showed that the TN:TP ratio remained largely unchanged after a whole-lake treatment with alum (Jacoby et al. Citation1994). If the addition of alum does not increase the TN:TP ratio, as suggested by this study and supported by Jacoby et al. (Citation1994), cyanobacteria may still dominate the phytoplankton community. Based on the results of this study, managers contemplating the addition of alum to reduce phosphorus should execute pilot studies that incorporate alum effects on nitrogen to ensure that a TN:TP ratio capable of altering dominance by cyanobacteria is achieved.
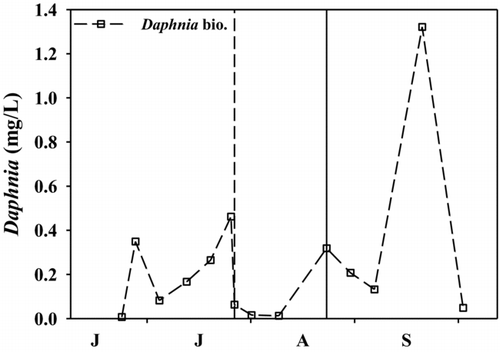
Although alum treatments may reduce relative cyanobacterial biovolume, they may result in an inedible phytoplankton community, leaving zooplankton without a sufficient food source (Kennedy et al. Citation1987, Barko et al. Citation1990, Jacoby et al. Citation1994). In this study, the phytoplankton community during the before-manipulation phase was dominated (>50% total biovolume) by cyanobacterial genera Anabaena, Aphanizomenon, and Lyngbya ( and ). These genera are inedible, of low nutritional value, and potentially toxic to zooplankton (Holm and Shapiro Citation1984, Christoffersen et al. Citation1990, DeMott and Müller-Navarra Citation1997). After addition of alum, the phytoplankton community was dominated by nontoxic and inedible Ceratium (dinoflagellate) and potentially toxic Pseudanabaena (cyanobacteria; ). Daphnia biomass decreased 7-fold on average during the alum treatment (; ). Although the initial decrease in Daphnia biomass may have been due to the alum treatment, the continued low Daphnia biomass may be reflective of shifts in phytoplankton community composition (); therefore, in lakes and reservoirs where addition of alum does not change the TN:TP ratio, the efficiency of the flow of energy from phytoplankton to higher trophic levels (e.g., zooplankton) may not change.
Figure 4 Daphnia biomass (mg/L) throughout the experiment in the mesocosms during 2011. Vertical dashed and solid lines represent the date of additions of alum and nitrogen, respectively. X-axis labels refer to the first letter of the month starting with June. Exact sample dates are given in .
WCR did not have high microcystin concentrations during the before-manipulation period and alum-treatment portions of the study (all reservoir samples were <1 μg/L; Britton J, USACE, unpubl. data), and concentrations were similarly low in the experimental mesocosms (–). Peak microcystin concentration in the before-manipulation period (3.1 μg/L) was about 16 times larger than peak concentration (0.2 μg/L) in alum-treated mesocosms (–); however, both concentrations were low from a lake management perspective. Suppression of high microcystin concentrations via alum additions therefore cannot be conclusively determined given the relatively low microcystin concentrations during the alum phase.
A subsequent addition of nitrogen to increase the TN:TP ratio
Adding nitrogen to the mesocosms increased the TN:TP ratio to >50 (7-fold increase; ; ) and decreased relative cyanobacterial biovolume and abundance by 7 and 14%, respectively ( and ; ), while reducing total phytoplankton biovolume and abundance by 35 and 7%, respectively ( and ; ). Total phytoplankton biovolume remained low until TN:TP ratios were <75, at which time total phytoplankton biovolume steadily increased until the end of the experiment (3.4-fold increase in phytoplankton biovolume after the TN:TP ratio was <75; and ). However, total phytoplankton biovolume at the end of the experiment was still 2.5-fold lower than in WCR (average relative cyanobacterial biovolume throughout N-addition treatment in WCR was 85%, mesocosm average was 35%; Britton J, USACE, unpubl. data), which was dominated by cyanobacteria throughout N-addition treatment (). The biovolume of cyanobacteria was 3-fold lower than the alum treatment after the addition of nitrogen (). After the initial reduction, relative cyanobacterial biovolume steadily increased as TN:TP ratios decreased throughout N-addition treatment.
Increasing the TN:TP ratio via nitrogen addition may have caused a reduction of inedible and toxic phytoplankton taxa (i.e., dinoflagellates and filamentous cyanobacteria) and an increase in edible and non toxic (by relative abundance; ) phytoplankton taxa for zooplankton. After nitrogen was added, relative biovolume of dinoflagellates decreased by 68% and cryptophytes increased by 56% (). Additionally, chrysophytes increased after the addition of nitrogen and represented 14 and 25% of relative biovolume and abundance, respectively, when the TN:TP ratio was >75 ( and ). Given that cryptophytes and chrysophytes are a more nutritional food source for zooplankton than dinoflagellates and cyanobacteria (Gulati and Demott Citation1997), the increase in Daphnia biomass (13-fold higher on average; ; ) may have been due to the shift in the phytoplankton community.
The increase in Daphnia biomass probably resulted in increased water column filtering, which subsequently led to greater Secchi depths (23 Aug–6 Sep) compared to the alum treatment (; Daphnia biomass in WCR did not increase during this time, data not shown). Because sedimentation rates were similar to pre-N-addition conditions (), the increase in Daphnia biomass during N-addition treatment may indicate that the efficiency of the flow of energy increased from phytoplankton to zooplankton (Stockner Citation1987, Stocker and MacIsaac 1996, Ashley et al. Citation1997). In addition, once the TN:TP ratio decreased and cyanobacteria started to regain dominance in the mesocosms, the sedimentation rate increased. The increase in the sedimentation rate at the end of the mesocosm experiment may indicate that the efficiency of the flow of energy decreased from phytoplankton to zooplankton; however, this result is confounded given the internal phosphorus loading during that time in the experiment.
Table 3 Mean ± standard error for Secchi depth and sedimentation rate (Sed) in Willow Creek Reservoir in 2011.
The N-addition may have been a factor in the increase of zooplankton biomass, albeit in our experiments the increase was primarily due to a single sample date (). A similar shift was observed in nitrogen addition experiments conducted by Harris et al. (Citation2014). Additionally, whole-lake additions of nitrogen in a reservoir with TP concentrations similar to those of our alum-treated mesocosms (TP ∼ 10–15 μg/L; e.g., Dworshak Reservoir, ID) have shown similar trophic level effects. In years in which nitrogen was added to Dworshak Reservoir, Daphnia and kokanee salmon (Oncorhynchus nerka) biomass were 2-fold higher compared to years without addition of nitrogen (IDFG Citation2012). Therefore, as exemplified by this study, Harris et al. (Citation2014), and whole-lake nitrogen additions in Dworshak Reservoir, increasing the TN concentration, and hence the TN:TP ratio, may shift the phytoplankton community to a more nutritional food source for zooplankton, which increases the amount of energy transferred to higher trophic levels and indirectly increases water clarity (reflected by deeper Secchi depth from 23 Aug to 6 Sep; ).
Despite studies by Downing et al. (Citation2001) and Watson et al. (Citation1997) indicating that the concentration of TP determines phytoplankton community composition, our data suggest that the TN:TP ratio may be a stronger determinant of phytoplankton community composition than TP alone, especially in eutrophic systems (Smith Citation1983). Surveys by Graham et al. (Citation2004), Orihel et al. (Citation2012), and the global dataset reported in Harris et al. (Citation2014) show that cyanobacterial biovolume is suppressed when TN:TP ratios are >50, further supporting results from our experiment. Our experimental addition of nitrogen was conducted during alum-induced, low phosphorus concentrations (TP = 10–15 μg/L pre-N-addition), suggesting that regardless of trophic status (i.e., phosphorus concentration), adding nitrogen to increase the TN:TP ratio may increase the efficiency of energy transfer through the food web by shifting the phytoplankton community composition to one that is more edible and of higher nutritional value to zooplankton than compositions found at lower TN:TP ratios (Carpenter et al. Citation1985, Citation2001).
These results are similar to those observed for nitrogen additions at a whole-lake scale (i.e., Dworshak Reservoir, ID; IDFG Citation2012). Although reducing phosphorus is key to reducing cyanobacterial and other phytoplankton biovolume in general (Schindler Citation1977, Smith and Schindler Citation2009), adjusting in-lake TN:TP ratios by addition of nitrogen can also reduce cyanobacterial biovolume. Unlike alum treatments that limit higher trophic levels by reducing phytoplankton in general, the addition of nitrogen may offer lake managers a way to increase water transparency and reduce cyanobacterial blooms by increasing energy flow from the primary producers to higher trophic levels. As shown by this experiment, if increasing the TN:TP ratio by adding nitrogen would cause nitrate/ammonia toxicity, alum treatment(s) may be used to decrease phosphorus such that a subsequent nitrogen addition could be used to increase the TN:TP ratio.
Microcystin concentrations were low (<1 μg/L) throughout the N-addition treatment, with the exception of the final sampling date when the concentration of microcystin was 8.9 μg/L (–). Microcystin was never >1 μg/L when TN:TP ratios were >50 in N-treated mesocosms (). Although microcystin concentration was never high (>20 μg/L used as the threshold for high microcystin concentrations; Chorus and Bartram Citation1999) in the mesocosms, microcystin concentrations did exceed the Oregon Department of Health Services (ODHS; >8 μg/L) limit for reservoir closure when the TN:TP ratio fell below 50 (). Additionally, WCR had extremely high concentrations of microcystin (maximum microcystin >500 μg/L; Britton J, USACE, unpubl. data) throughout N-addition treatment. Thus, microcystin concentration was suppressed in the mesocosms relative to the reservoir, particularly when TN:TP ratios were >50, a finding similar to results reported in Harris et al. (Citation2014), which show that microcystin was not detected when the TN:TP ratio was >75. In Harris et al. (Citation2014), however, alum was not used before nitrogen was added; thus, TP was much higher (∼30 μg/L higher). Therefore, in systems with lower concentrations of TP (e.g., mesotrophic) or eutrophic systems that have been treated with alum to reduce TP, adding nitrogen to increase the TN:TP ratio to >50 does not seem to stimulate microcystin production or exacerbate eutrophy.
Conclusion
Phosphorus reduction is essential to reduce the long-term eutrophication of freshwaters (Schindler Citation1977, Citation2012, Smith and Schindler Citation2009). In both Harris et al. (Citation2014) and this study, total phytoplankton biovolume was generally proportional to the concentration of phosphorus, which is consistent with the findings of numerous mesocosm and whole-system studies (Jeppesen et al. Citation2005, Citation2007, Sondergaard et al. Citation2005, Smith and Schindler Citation2009, Moore and Christensen Citation2009, Moore et al. Citation2009, Schindler Citation2012). Thus, the current paradigm of reducing phosphorus to reduce phytoplankton biomass in general is supported by our studies. However, both Harris et al. (Citation2014) and this study show that even if total phytoplankton biovolume is proportional to the concentration of phosphorus, the TN:TP ratio seems to control the percent composition of cyanobacterial biovolume. Therefore, in freshwater systems that consistently receive phosphorus-rich allochthonous inputs (low TN:TP ratio) the addition of nitrogen could potentially serve as a short-term, in-lake management solution to reduce the occurrence of cyanobacterial blooms while watershed improvements are made to reduce phosphorus loads.
Lake and reservoir managers should add nitrogen only if nitrate/ammonia toxicity is not a threat to aquatic organisms and humans (see WHO Citation2011 and Randall and Tsui Citation2002 for terrestrial and aquatic organismal limits) and when other management options do not exist (e.g., P reduction to increase the TN:TP ratio). Additionally, increased nitrogen exports from alum and nitrogen-treated lakes that flow into estuaries and the ocean are also undesirable. The amount of nitrogen added to yield the critical N:P ratio of >50 must be carefully determined to minimize negative effects downstream. Removal of the added nitrogen by uptake in downstream rivers, coastal wetlands, and shallow shore regions are well-known methods of nitrogen reduction (Horne and Goldman Citation1994). Moreover, in-lake management solutions are unique to individual lakes, and our observed relations may not justify widespread application of ammonium nitrate to suppress cyanobacteria. Thus, alum and ammonium nitrate treatments may only be applicable for lakes and reservoirs with high phosphorus, such as lakes and reservoirs like WCR or urban impoundments in which phosphorus reduction is beyond reasonable control, that are sufficiently oxygenated to tolerate ammonium nitrate additions, and that have a biological structure capable of utilizing the algae fostered by a shift in nutrient ratios. In a situation of high planktivore density, consumption of algae is unlikely due to the lack of large-bodied zooplankton grazers (Carpenter et al. Citation2001); thus, although improvements in water clarity may not be realized, changes in phytoplankton taxa should still occur with a concomitant reduction in cyanotoxins.
Results from this study indicate that a sequential addition of alum followed by nitrogen to increase the TN:TP ratio to >50 may reduce cyanobacteria and microcystin concentrations when nitrate/ammonia toxicity is a concern. Applying successive chemical treatments in this manner decreased TP concentrations; may have increased the flow of energy to higher trophic levels, possibly further preventing cyanobacterial bloom occurrences; and reduced the probability of high microcystin concentration by increasing the TN:TP ratio temporarily by adding ammonium nitrate (Otten et al. Citation2012, Orihel et al. Citation2012).
Before the addition of nitrogen can be effectively used as a lake and reservoir management practice, several additional requirements must be met, including experiments that (1) address the dosage and frequency of application at a range of N:P ratios and with different nitrogen compounds (e.g., NH4NO3, NaNO3, NO3); (2) examine potential unintended consequences (e.g., the growth of near-shore macrophytes); and (3) determine broad-scale applicability and cost. Note that our alum experiment used alum only to remove phosphorus from the water column and did not prevent the internal phosphorus loading observed during the N-addition treatment. Thus, in a whole-lake treatment, the longevity and effectiveness of the N-addition probably would have been greater if the internal phosphorus loading had been controlled via an alum dosing applied to reduce easily resuspended sediment (Rydin and Welch Citation1999, Rydin et al. Citation2000). Such a dose of alum would achieve both short-term water clarity and long-term control of autochthonous, but not allochthonous, phosphorus concentrations, allowing periodic treatments of N-addition applied by lake managers a greater chance of success in controlling toxic cyanobacterial blooms. Future studies should incorporate mobile-sediment doses of alum and conduct experiments at whole-lake scales. Additionally, because adding a nutrient violates federal and state laws, water managers will need permits before any addition of nitrogen can commence. Given potential economic and recreational losses associated with cyanobacteria-caused lake and reservoir closures (Dodds et al. Citation2009), the addition of nitrogen (increasing TN:TP ratios to >50) could be given more attention as a short-term, in-lake management practice to combat effects of eutrophication while implementing long-term improvements to reduce autochthonous and allochthonous phosphorus inputs.
Funding
Funding and support for this project were provided by the US Army Corps of Engineers, US Geological Survey, and a University of Idaho Jeff Braante grant.
Acknowledgments
We thank Drs. Robert Mahler of the University of Idaho, Reed Green of the US Geological Survey, Alex Horne, Barry Moore, Gertrud Nürnberg, Ken Wagner, and 2 anonymous reviewers for their insightful comments that improved the manuscript. Field and laboratory assistance was provided by C. Adams, T. Caldwell, E. Reams, K. Gilbert, A. Waldo, and Amanda “Mandy” Stone. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Ashley , K A , Thompson , L C , Lasenby , D C , McEachern , L , Smokorowski , K E and Sebastian , D . 1997 . Restoration of an interior lake ecosystem: the Kootenay Lake fertilization experiment . Water Qual Res J Can. , 32 : 192 – 212 .
- Barko , J W , James , W F , Taylor , W D and McFarland , D G . 1990 . Effects of alum treatment on phosphorus and phytoplankton dynamics in Eau Galle Reservoir: a synopsis . Lake Reserv Manage. , 6 : 1 – 18 .
- Bottrell , H H , Duncan , A , Gliwicz , Z M , Grygierek , E , Herzig , A , Hillbright-Ilkowska , A , Kurasawa , H , Larsson , P and Weglenska , T . 1976 . A review of some problems in zooplankton production studies . Norw J Zool. , 24 : 419 – 456 .
- Briand , J F , Jacquet , S , Bernard , C and Humbert , J-F . 2003 . Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems . Vet Res. , 34 : 361 – 377 .
- Carpenter , S R , Cole , J J , Hodgson , J R , Kitchell , J F , Pace , M L , Bade , D , Cottingham , K L , Essington , T E , Houser , J N and Schindler , D E . 2001 . Trophic cascades, nutrients, and lake productivity: whole lake experiments . Ecol Monogr. , 71 : 163 – 186 .
- Carpenter , S R , Kitchell , J F and Hodgson , J R . 1985 . Cascading trophic interactions and lake productivity . Bioscience. , 35 : 634 – 639 .
- Chorus , I and Bartram , J . 1999 . Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management . World Health Organization , [cited 14 Mar 2013]. Available from: http://www.who.int/water_sanitation_health/resourcesquality/toxcyanbegin.pdf
- Chorus , I , Falconer , I R , Salas , H J and Bartram , J . 1999 . Health risks caused by freshwater cyanobacteria in recreational waters . J Toxicol Env Heal B. , 3 ( 4 ) : 323 – 347 .
- Christoffersen , K , Riemann , B , Hansen , L R , Klysner , A and Sorensen , H B . 1990 . Qualitative importance of the microbial loop and plankton community structure in a eutrophic lake during a bloom of cyanobacteria . Microb Ecol. , 20 : 253 – 272 .
- Crumpton , W G , Isehard , T M and Mitchell , P D . 1992 . Nitrate and organic nitrogen analysis with second derivative spectroscopy . Limnol Oceanogr. , 37 : 907 – 913 .
- Cooke , G D , Welch , E B , Peterson , S A and Nichols , S A . 2005 . Restoration and management of lakes and reservoirs. 3rd ed , Boca Raton, FL : Taylor and Francis .
- DeMott , W R and Müller-Navarra , D C . 1997 . The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions . Freshwater Biol. , 38 : 649 – 644 .
- Dodds , W K , Douska , W W , Eitzmann , J L , Pliger , T J , Pitts , K I , Riley , A J , Schloesser , J T and Thornbrugh , D J . 2009 . Eutrophication of U.S. freshwaters: analysis of potential economic damages . Environ Sci Technol. , 43 : 12 – 19 .
- Dodson , S I and Frey , D G . 2001 . “ Cladocera and other Branchiopoda ” . In Ecology and classification of North American freshwater invertebrates , Edited by: Thorp , J H and Covich , A P . 723 – 786 . Academic Press .
- Downing , J A , Watson , S B and McCauley , E . 2001 . Predicting cyanobacteria dominance in lakes . Can J Fish Aquat Sci. , 58 : 1905 – 1908 .
- Eaton , A D , Clesceri , L S , Rice , E W and Franson , M A . 2005 . Standard methods for the examination of water and wastewater. 21st ed , American Public Health Association, American Water Works Association, Water Environment Federation .
- Graham , J L , Jones , J R , Jones , S B , Downing , J A and Clevenger , T E . 2004 . Environmental factors influencing microcystin distribution and concentration in the Midwestern United States . Water Res. , 38 : 4395 – 4404 .
- Graham , J L , Loftin , K A , Ziegler , A C and Meyer , M T . 2008 . Guidelines for design and sampling for cyanobacterial toxin and taste-and-odor studies in lakes and reservoirs . US Geological Survey Scientific Investigations Report 2008-5038
- Graham , J L , Loftin , K A , Meyer , M T and Ziegler , A C . 2010 . Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States . Environ Sci Technol. , 44 : 7361 – 7368 .
- Gulati , R D and Demott , W R . 1997 . The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities . Freshwater Biol. , 38 : 753 – 768 .
- Harris , T D , Wilhelm , F M , Graham , J L and Loftin , K A . 2014 . Experimental manipulation of TN:TP ratios to suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ mesocosms . Lake Reserv Manage , 30 : 72 – 83 .
- Hillebrand , H , Durselen , C , Kirschtel , D , Pollingher , U and Zohary , T . 1999 . Biovolume calculation for pelagic and benthic microalgae . J Phycol. , 35 : 403 – 424 .
- Holm , N P and Shapiro , J . 1984 . An examination of lipid reserves and the nutritional status of Cladoceran pulex fed Aphanizomenon flos-aquae . Limnol Oceanogr. , 29 : 1137 – 1140 .
- Horne , A and Goldman , C R . 1994 . Limnology. 2nd ed , New York, NY : McGraw-Hill .
- Huser , B , Brezonik , P and Newman , R . 2011 . Effects of alum treatment on water quality and sediment in the Minneapolis Chain of Lakes, Minnesota, USA . Lake Reserv Manage. , 27 : 220 – 228 .
- [IDFG] Idaho Department of Fish and Game . 2012 . Dworshak reservoir nutrient supplementation project update; [cited 31 Aug 2012] . Available from: http://fishandgame.idaho.gov/public/docs/fishReportsNewsletters/clearwaterDworshak12.pdf
- Jacoby , J M , Gibbons , H L , Stoops , K B and Bouchard , D D . 1994 . Response of a shallow, polymictic lake to buffered alum treatment . Lake Reserv Manage. , 10 : 103 – 112 .
- Jeppesen , E , Sondergaard , M , Jensen , J P , Havens , K E , Anneville , O , Carvalho , L , Coveney , M F , Deneke , R , Dokulil , M T Foy , B . 2005 . Lake responses to reduced nutrient loading- an analysis of contemporary long-term data from 35 case studies . Freshwater Biol. , 50 : 1747 – 1771 .
- Jeppesen , E , Sondergaard , M , Lauridsen , T L , Krovang , B , Beklioglu , M , Lammens , E , Jensen , H S , Kohler , J , Ventela , A-M , Tarvainen , M and Tatrai , I . 2007 . Danish and other European experiences in managing shallow lakes . Lake Reserv Manage. , 23 : 439 – 451 .
- Kennedy , R H , Barko , J W , James , W F , Taylor , W D and Godshalk , G L . 1987 . Aluminum sulfate treatment of a eutrophic reservoir: rationale, application methods, and preliminary results . Lake Reserv Manage. , 3 : 85 – 90 .
- Landsberg , JH. 2002 . The effects of harmful algal blooms on aquatic organisms . Rev Fish Sci. , 10 : 113 – 390 .
- Larsson , U , Blomqvist , S and Abrahamsson , B . 1986 . A new sediment trap system . Mar Ecol Prog Ser. , 31 : 205 – 207 .
- Lund , J WG , Kipling , C and Cren , E D . 1958 . The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting . Hydrobiologia. , 11 : 143 – 170 .
- Moore , B C and Christensen , D . 2009 . Newman Lake restoration: a case study. Part I. Chemical and biological responses to phosphorus control . Lake Reserv Manage. , 25 : 337 – 350 .
- Moore , B C , Richter , A C and Christensen , D . 2009 . Newman Lake restoration: a case study. Part II. Microfloc alum injection . Lake Reserv Manage. , 25 : 351 – 363 .
- Orihel , D M , Bird , D F , Brylinsky , M , Chen , H , Donald , D B , Huang , D Y , Giani , A , Kinniburgh , D , King , H Kotak , B . 2012 . High microcystin concentrations occur only at low nitrogen-to-phosphorus ratio in nutrient-rich Canadian lakes . Can J Fish Aquat Sci. , 69 : 1457 – 1462 .
- Ott , R L and Longnecker , M . 2010 . “ An introduction to statistical methods and data analysis. 6th ed ” . Belmont, CA : Brooks/ Cole .
- Otten , T G , Xu , H , Qin , B , Zhu , G and Pearl , H W . 2012 . Spatiotemporal patterns and ecophysiology of toxigenic Microcystis blooms in Lake Taihu, China: implications for water quality management . Environ Sci Technol. , 45 : 3480 – 3488 .
- Paerl , H W , Xu , H , McCarthy , M J , Zhu , G , Qin , B , Li , Y and Gardner , W S . 2011 . Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy . Water Res. , 45 : 1973 – 1983 .
- R Development Core Team . 2011 . R: A language and environment for statistical computing. R Foundation for Statistical Computing , Vienna , , Austria : ISBN 3-900051-07-0 . http://www.R-project.org/
- Randall , D J and Tsui , T KN . 2002 . Ammonia toxicity in fish . Mar Pollut Bull. , 45 : 17 – 23 .
- Redfield , A C , Ketchum , B H and Richards , F A . 1963 . “ The influence of organisms on the chemical composition of seawater ” . In The seas: ideas and observations on progress on the studies of seas , Edited by: Hill , M N . Vol. 2 , 26 – 77 . Interscience, NY : The composition of seawater, comparative and description oceanography .
- Rydin , E , Huser , B and Welch , E B . 2000 . Amount of phosphorus inactivated by alum treatments in Washington lakes . Limnol Oceanogr. , 45 : 226 – 230 .
- Rydin , E and Welch , E B . 1999 . Dosing alum to Wisconsin lake sediments based on in vitro formation of aluminum bound phosphate . Lake Reserv Manage. , 15 : 324 – 331 .
- Schindler , DW. 1977 . Evolution of phosphorus limitation in lakes: natural mechanisms compensate for deficiencies of nitrogen and carbon in eutrophied lakes . Science. , 195 : 260 – 262 .
- Schindler , DW. 2012 . The dilemma of controlling cultural eutrophication of lakes . P Roy Soc B. , 279 : 4322 – 4333 .
- Schindler , D W and Vallentyne , J R . 2008 . The algal bowl: overfertilization of the world's freshwaters and estuaries , Edmonton, AB : University of Alberta Press .
- Smith , VH. 1983 . Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton . Science. , 221 : 669 – 671 .
- Smith , V H and Schindler , D W . 2009 . Eutrophication science: where do we go from here? . Trends Ecol Evol. , 22 : 201 – 207 .
- Sondergaard , M , Jensen , J P and Jeppeson , E . 2005 . Seasonal response of nutrients to reduced phosphorus loading in 12 Danish lakes . Freshwater Biol. , 50 : 1605 – 1615 .
- Stockner , JG. 1987 . “ Lake fertilization: the enrichment cycle and lake sockeye salmon (Oncorhynchus nerka) production ” . In Sockeye salmon (Oncorhynchus nerka) population biology and future management , Edited by: Smith , H D , Margolis , L and Wood , C C . 198 – 215 . Can Spec Pub Fish Aquat Sci .
- Stockner , J G and MacIsaac , E A . 1996 . British Columbia lake enrichment programme: two decades of habitat enhancement for sockeye salmon . Regul Rivers. , 12 : 547 – 561 .
- Tilman , D. 1982 . Resource competition and community structure , Princeton, NJ : Princeton University Press .
- Watson , S B , McCauley , C and Downing , J A . 1997 . Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status . Limnol Oceanogr. , 42 : 827 – 840 .
- Welch , E B and Cooke , G D . 1999 . Effectiveness and longevity of phosphorus inactivation with alum . Lake Reserv Manage. , 15 : 5 – 27 .
- [WHO] World Health Organization . 2011 . Nitrate and nitrite in drinking-water; [cited 28 Feb 2013] . Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite_background.pdf
