Abstract
Despite the historical focus on water quality, it is becoming increasingly evident that physical habitat conditions also significantly affect lake biota. We examined associations between fish and bird assemblages and lake physical habitat based on relatively rapid assessments of Northeast US lakes. Richness of intolerant fish species declined, and that of tolerant fish species increased across regional gradients of increasing shoreline human development and decreasing abundance and structural complexity of riparian vegetation and littoral cover. Breeding bird assemblages observed in the nearshore littoral and riparian area of these lakes were similarly affected by disturbance and habitat simplification. The percentage of native neotropical migrant birds declined, and the percent of tolerant bird species increased across the same gradients. We conclude that our relatively rapid physical habitat assessments produce biologically relevant metrics useful for evaluating lake physical habitat condition and potential impacts on lake biota in regional and national lake assessments.
In lakes, as in streams, the distribution and abundance of lentic organisms are influenced by physical, chemical, hydrological, and biological attributes that collectively comprise habitat. There is growing recognition of the importance of near-shore physical habitat structure for understanding differences in lake biotic assemblages (Allen et al. Citation1999, Whittier et al. Citation2002b, USEPA Citation2009). Information concerning the multiple dimensions of physical and chemical habitat is necessary to interpret biological information and assess ecological condition in lakes. Evaluation of near-shore habitat structure is particularly important because the physical and chemical exchange, nutrient cycling, and energy dissipation that occur there are especially vulnerable to anthropogenic perturbation (Schindler and Scheuerell Citation2002, Strayer and Findlay Citation2010, Hampton et al. Citation2011).
Littoral structure and complexity have long been known to influence lake fish populations and assemblages (e.g., Eschmeyer Citation1936, Tarzwell Citation1936). Littoral woody structure (large woody debris, or LWD) and aquatic macrophytes provide refuge from predation and affect nutrient cycling and littoral production (Wege and Anderson Citation1979, Lynch and Johnson Citation1989, Savino and Stein Citation1989). Many sport fish selectively use complex habitat structure and cover in lakes (Sass et al. Citation2006), and the maintenance of diverse fish assemblages requires heterogeneity in littoral physical habitat structure and cover (Tonn and Magnuson Citation1982, Eadie and Keast Citation1984, Benson and Magnuson Citation1992, Taillon and Fox Citation2004). Similarly, riparian and littoral habitat complexity are associated with increased diversity in periphyton and macroinvertebrate assemblages (Smokorowski et al. Citation2006, Brauns et al. Citation2007, Butler and deMaynadier Citation2008, Remsburg and Turner Citation2009).
Riparian faunas are also influenced by near-shore terrestrial and aquatic habitat structure in lakes (e.g., O’Connor et al. Citation2000). More bird taxa inhabit less-modified near-shore zones that have greater riparian vegetation complexity (MacArthur and MacArthur Citation1961) and more abundant and diverse littoral aquatic macroinvertebrate prey (Larsen et al. Citation2010). The anthropogenic simplification of riparian vegetation structure and composition favors habitat generalists and invasive alien bird species (O’Connor et al. Citation2000, Bryce et al. Citation2002).
Habitat complexity, expressed as the density of riparian trees and littoral coarse wood, is greatly reduced where there is a large amount of lakeshore residential development (Christensen et al. Citation1996, Francis and Schindler Citation2006, CitationKaufmann et al. 2014b). Whole-lake experiments manipulating coarse wood loadings have shown that the ecological effects of habitat simplification can be severe and difficult to reverse (e.g., Sass et al. Citation2006, Citation2012). Lake shoreline human disturbances are among the most extensive stressors in lakes of the Northeastern US, and they are associated with reduced complexity of riparian and littoral habitat structure (Whittier et al. Citation2002b).
Many aspects of shoreline development can have detrimental effects on fish habitat (Halliwell Citation2007, Citation2008). For example, developed shorelines had decreased woody structure (snag habitat), increased sandy shorelines, increased submerged aquatic macrophyte cover, or decreased emergent and floating-leaf aquatic macrophytes. Such changes reduced habitat complexity in lakes of Vermont (Merrell et al. Citation2009), the Upper Midwest (Radomski and Geoman Citation2001, Jennings et al. Citation2003, Hatzenbeler et al. Citation2004), Maine (Ness Citation2006), and Germany (Brauns et al. Citation2007).
Changes in biotic composition and ecosystem function are associated with reduced habitat complexity caused by human activities along lakeshores. In many of the previous studies, marked reductions in habitat structural complexity deleteriously affected fish and other aquatic biota (e.g., Wagner et al. Citation2006, Taillon and Fox Citation2004, Engel and Pederson Citation1998, Whittier et al. Citation2002a, Citation2002b). Wagner et al. (Citation2006) reported negative effects of residential lakeshore development on littoral fishes resulting from reductions in the use of disturbed and simplified near-shore habitat for nesting, foraging, and refuge. Brauns et al. (Citation2007) reported that taxa richness and diversity of littoral aquatic macroinvertebrates in lowland German lakes were significantly lower in simplified littoral habitats near disturbed shorelines than in more complex natural littoral areas. In Northeast US lakes, shoreline disturbance was associated with reduced species richness of native minnows and increased nonnative piscivorous fish species (Whittier et al. Citation1997a). Jennings et al. (Citation1999) also reported negative effects on fish assemblages as riparian alteration increased in Midwest US lakes.
Kaufmann and Whittier (Citation1997) developed a rapid, semiquantitative approach for evaluating near-shore human disturbances and physical habitat structure, including cover and complexity in littoral and riparian habitats. The US Environmental Protection Agency (EPA) Environmental Monitoring and Assessment Program (EMAP) piloted that approach in its 1992–1994 Northeastern Lake Survey (EMAP-NE). Since then, many elements of the EMAP approach for evaluating lake physical habitat structure were adapted for monitoring by the European Union's Water Framework Directive (Rowan et al. Citation2006) and also were applied in a multi-year study of Brazilian reservoirs (Molozzi et al. Citation2011, Macedo et al. Citation2012). The EPA modified the EMAP methods for use in its 2007 National Lakes Assessment (NLA; USEPA Citation2007, Citation2009, CitationKaufmann et al. 2014a, 2014b); however, the biological relevance of the EPA habitat assessment approach has not been formally evaluated.
In this study we evaluate the biological relevance of the EPA lake physical habitat assessment approach by examining associations of biota with habitat metrics derived using this approach. Our objectives were to determine if the taxa richness and structure of fish and near-shore bird assemblages were associated with indices of habitat condition. We expected that simplification of littoral and riparian structure associated with increased human disturbance of lakes would increase the richness of tolerant fish species and decrease the richness of intolerant fish species. Similarly, we expected that human activity and simplification of near-shore habitat would increase the proportion of tolerant birds and decrease the proportion of sensitive native taxa, such as neotropical migrant birds, in the lake near-shore area.
Study sites and methods
Sample lakes
The EMAP-NE was conducted as a pilot survey to test designs and methods for application at regional and national scales (Larsen and Christie Citation1993, Whittier et al. Citation2002b). We examined data from 179 lakes sampled during summers 1992 to 1994 (Larsen and Christie Citation1993, Larsen et al. Citation1994; ). The population of interest for EMAP-NE was all lakes in the Northeast US with an open water surface area of 1–10,000 ha (0.01–100 km2) and a depth ≥1 m. The survey employed a spatially balanced, probability selection of lakes from an explicitly defined sampling frame, the Digital Line Graph (DLG) version of the US Geological Survey 1:100,000 map series. Surveyed water bodies included natural lakes without anthropogenic modifications, ponded wetlands, human-constructed lakes, and run-of-the-river reservoirs. We report findings for the Northeast region as a whole and for 2 subregions: the NE Lowlands and the NE Highlands (). The NE Highlands is an aggregation of the Adirondack Mountains and the Omernik (Citation1987) NE Highlands Level III ecoregions.
Physical habitat observations and metric definitions
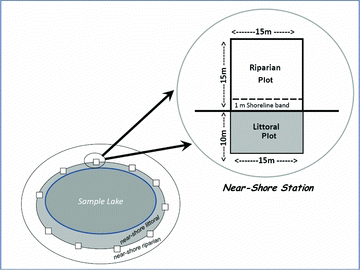
Field crews characterized physical habitat by collecting data at a randomized set of 10 near-shore stations spaced equidistant around each lake (). At each station, crews recorded data within a 10 × 15 m littoral plot, a 15 × 15 m riparian plot, and a 1 m-wide shoreline band (). Cover and structure of riparian vegetation, aquatic macrophytes, littoral habitat features, and substrate were visually estimated (Kaufmann and Whittier Citation1997). Field forms and procedures were designed for rapid recording of data; as a result, measurements and observations at each station required approximately 5 min. Including transit time between stations, the physical habitat component of lake sampling required 1.5–3.5 h on lakes ranging from 7 to 560 ha.
The field methods provided information to quantify 7 dimensions of lake physical habitat in the near-shore zone of lakes: (1) water depth and surface characteristics, (2) substrate size and type, (3) aquatic macrophyte cover and structure, (4) littoral cover for biota, (5) riparian vegetation cover and structure, (6) near-shore human land use and disturbances, and (7) bank characteristics that indicate water level fluctuations and terrestrial-aquatic interaction (Paulsen et al. Citation1991, Kaufmann Citation1993). CitationKaufmann et al. (2014a) describe the calculations we used to reduce data collected at the 10 littoral-riparian stations to a set of metrics describing near-shore habitat characteristics for each sample lake; they also quantify the precision of those metrics.
Composite habitat quality indices
We calculated 5 composite habitat indices as described in detail by CitationKaufmann et al. (2014b) for EPA's NLA. The habitat indices used here were modified slightly from those because the survey on which we based our results, conducted in 1992–1994, used an earlier version of the EPA field measurement protocols that was slightly different from those used in the NLA:
The lakeshore human disturbance index incorporated measures of the extent and intensity of 12 predefined types of near-shore human land use activities (commercial development, buildings, roads/railroads, row crops, orchards, pastures, landfill/trash, lawns, developed parks, utility lines, bulkheads/revetments, and docks/boats), and was scaled from 0 (absence of any human disturbance) to 1 (extremely high disturbance).
The riparian cover index characterized the cover and structural complexity of the 3-layer (canopy, mid, and ground) lakeshore riparian vegetation, including inundated upland or wetland vegetation. Each contributing metric was scaled from 0 to 1, and metrics were averaged, yielding an index that varied from 0 to 1.
The littoral cover index characterized the cover, structure, and variety of the 8 littoral fish cover elements: woody snags, brush, overhanging vegetation, inundated trees, inundated nonwoody vegetation, boulders, rock ledges, anthropogenic structures, and 3 aquatic macrophyte cover types (floating, emergent, and submerged). We incorporated both the amount of cover and the variety of cover types into the littoral cover index because we believe that both influence the number of individual fish present, as well as the richness of species present and the variety of guilds represented. Like the previous indices, the subcomponents and final index were scaled from 0 to 1.
The littoral-riparian complexity index expresses the amount and structural complexity of the combined littoral-riparian zone and was calculated by averaging the riparian and littoral cover indices.
Finally, the overall lakeshore habitat quality index was calculated by averaging the 3 primary indices: riparian cover, littoral cover, and [lack of] lakeshore human disturbance.
The first 4 of these indices are nearly exact analogues of the 4 habitat condition indices used in USEPA's (2009) NLA.
GIS-derived lake and landscape metrics
In addition to the field data, a number of GIS-based landscape metrics were calculated for each lake. Lake elevation and the total drainage area contributing to the lake (based on topography) were extracted from the National Elevation Dataset. Mean annual precipitation at each lake was estimated using PRISM data gridded at a 2 km resolution (Daly and Taylor Citation2002). Percent watershed area with urban, agricultural, and forest cover were derived from the National Land Cover Dataset (Vogelmann et al. Citation2001; available at http://landcover.usgs.gov/natllandcover.php). Population density (individuals/km2) was estimated for the contributing watershed for each lake based on data from the US Census Bureau (Citation1990, Citation2001). We calculated road density (m/ha) from digital road data (TIGER Citation1990) in the drainage area of each lake. Road density serves as an efficient surrogate for catchment-scale anthropogenic disturbances (Kaufmann and Hughes Citation2006).
Fish and riparian bird assemblage sampling and analysis
Field crews sampled fish assemblages overnight in July and August at a set of randomly selected sites stratified by macrohabitat, based on level of human activity, quantity and type of cover, and substrate (Baker et al. Citation1997, Whittier et al. Citation1997b). The potential number of fish species present in a given lake increases with lake size (Tonn and Magnuson Citation1982, Whittier et al. Citation1997a, Citation1997b, Vaux et al. Citation2000); therefore, depending on lake size, 3 to 26 sites were selected. Gear included pelagic gill net sets, littoral trap net and minnow trap sets, evening seining, and littoral gill net sets in large lakes. In addition, crews used best professional judgment to locate 1–2 sites based on microhabitat (e.g., stream inlet, macrophyte patch, and rock outcrop). These lake-size–adjusted field sampling methods were designed to capture >80% of the lake species pool and adequate numbers of individuals for stable estimates of proportional abundances (Whittier and Kincaid Citation1999, Vaux et al. Citation2000). Data for all gear were combined into a single lake value for each fish assemblage metric (Baker et al. Citation1997, Whittier et al. Citation1997a, Citation1997b).
Field crews surveyed riparian breeding birds from 0.5 h before sunrise to 4 h after sunrise on days with little wind or precipitation between late May and early July (Baker et al. Citation1997, Allen et al. Citation1999). Observations were made from canoes at stations every 200 m along a transect 10 m from and parallel to the lake shore. Crews recorded all terrestrial and aquatic individuals seen or heard within a radius of approximately 100 m around the station during a 5 min period. For lake perimeters >5 km, 24 stations were allocated in proportion to the extent of lake shore habitat types.
We examined the associations of 2 fish assemblage metrics and 2 riparian breeding bird assemblage metrics with the 5 near-shore habitat indices and catchment road density. We classified fish and riparian bird species according to their tolerance to general human disturbance (Hughes et al. Citation1993, Whittier and Hughes Citation1998) and their feeding habits, habitat preferences, life history, and reproductive guild membership (Hughes et al. Citation1993, O’Connor et al. Citation2000). Note that only native taxa were included as intolerant, and the total taxa list included species not classified as tolerant or intolerant. We wanted to show the associations (and possible causal effect) of habitat condition on tolerant and intolerant fishes and birds. Among the 4 possible metrics for each of these (richness vs., % of taxa, and richness vs.% of individuals), we chose the form of the biotic assemblage metric that showed the clearest association with habitat and disturbance.
We chose 2 fish assemblage metrics: richness of native intolerant species and richness of tolerant species. To account for species–area relationships, these fish metrics were expressed as residuals from regressions predicting species richness as a function of log10 lake surface area. Both metrics are commonly used for assessing the condition of lotic ecosystems, either alone (Whittier et al. Citation2007, Segurado et al. Citation2011) or in multimetric indices (Roset et al. Citation2007, Whittier et al. Citation2007).
We characterized bird assemblages in our analysis with 2 assemblage metrics: the proportion of total individual birds observed that are native neotropical migrant species, and the proportion of total bird species that are tolerant of anthropogenic disturbances. These bird assemblage proportional metrics did not require adjustment for lake or basin size. O’Connor et al. (Citation2000) found similar bird assemblage metrics to be responsive to human disturbances around lakes. Croonquist and Brooks (Citation1993), Bryce et al. (Citation2002), and Bryce (Citation2006) also used similar metrics to assess the condition of riparian bird assemblages along streams.
Results
Associations between biota and physical habitat
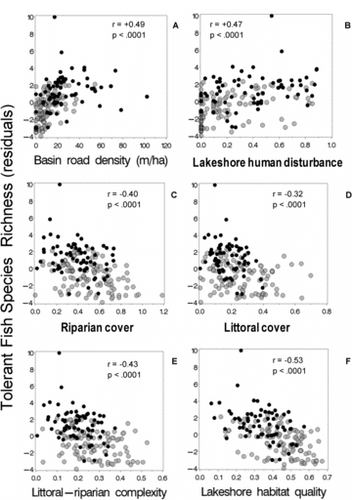
After scaling the fish assemblage metrics for lake size (surface area), no other natural landscape attributes were strongly correlated with either biotic or physical habitat indices in the EMAP-NE, except for negative correlations between elevation and the tolerant fish (r = −0.50; Bonferroni-adjusted p < 0.02) and tolerant bird indices (r = −0.40; Bonferroni-adjusted p < 0.02; ). Basin road density was consistently related to the habitat and biotic indices as well as basin-scale land uses (). Road density was moderately correlated with near-shore human disturbance (r = 0.58; Bonferroni-adjusted p < 0.02) and the lakeshore habitat quality index (r = −0.55; Bonferroni-adjusted p < 0.02).
Table 1 Correlations of EMAP Northeast Lake Survey biotic metrics and habitat indices with catchment land use and geoclimatic variables. Spearman r with absolute values >0.30 have p < 0.0001 (Bonferroni-adjusted p < 0.0015); those between 0.25 and 0.30 have p < 0.001 (adjusted p < 0.15). Parentheses denote r values before watershed area correction. Data from US EPA's EMAP probability survey of 185 lakes during the spring (birds) and summer (fish and habitat) seasons of 1992–1994 (179 lakes with complete set of variables used here). Correlations >|0.40| are bold.
Fish assemblages
In agreement with our expectations, we found higher intolerant fish taxa richness and smaller proportions of tolerant generalist species in relatively undisturbed lakes with complex physical habitat. The number of intolerant fish species (adjusted for lake area) was weakly associated with catchment road density (r = −0.29) near-shore human disturbance (r = −0.26), with riparian cover (r = 0.21), littoral cover (r = 0.09), combined littoral-riparian habitat complexity (r = 0.18), and the lakeshore habitat quality index (r = 0.27) (). In the NE Highlands, higher than expected numbers of intolerant fish species were found only in relatively undisturbed lakes with high littoral-riparian habitat cover and complexity (). Although intolerant fish species in NE Lowlands lakes showed slight increases with littoral cover complexity (), few were observed in those lakes, regardless of habitat complexity or near-shore disturbance levels.
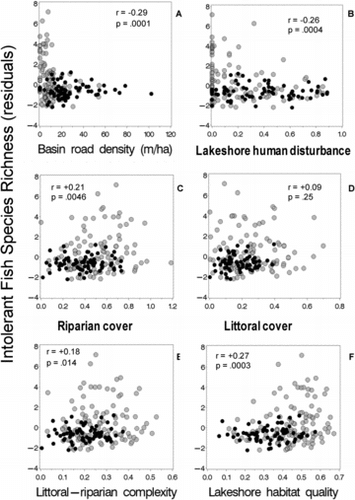
Also in agreement with our expectations, the number of generally tolerant fish species (adjusted for lake area) was positively correlated with road density and near-shore human disturbance (r = 0.49 and 0.47, respectively; and ), and negatively correlated with riparian cover, littoral cover, and littoral-riparian habitat complexity (r = −0.40 to −0.43; –). The weakest association between tolerant fish species and habitat was with littoral cover (r = −0.32) for all lakes combined (). The strongest association with tolerant fish species was with the lakeshore habitat quality index in the Northeastern United States (r = −0.53), and this moderate decline in tolerant species with habitat quality was evident in both the NE Highlands and NE Lowlands ecoregions (compare grey and black symbols in ).
Riparian breeding bird assemblages
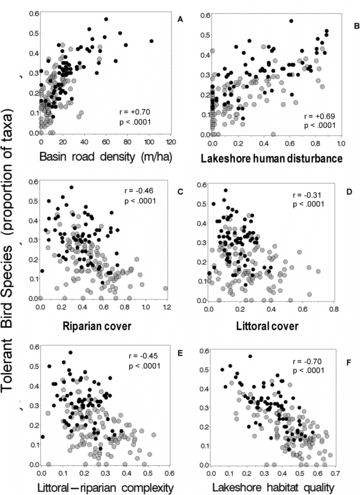
In agreement with our expectations, the percent of neotropical migrants in the bird counts were higher (generally >50%) at lakes with low levels of human disturbances and high levels of habitat complexity, and lower (generally <20%) in disturbed lakes with low habitat complexity. The proportion of neotropical migrant birds declined with increasing road density (r = −0.44) and near-shore human disturbances (r = −0.50; and ). The proportion of neotropical migrants was positively (weakly) correlated with both littoral and riparian cover (r = 0.29 and 0.25; and ) and with combined littoral-riparian habitat complexity (r = 0.33; ). The strongest and regionally most consistent association with neotropical migrant birds was with the lakeshore habitat quality index (r = 0.51) and was evident in both NE Highlands and NE Lowlands ecoregions ().
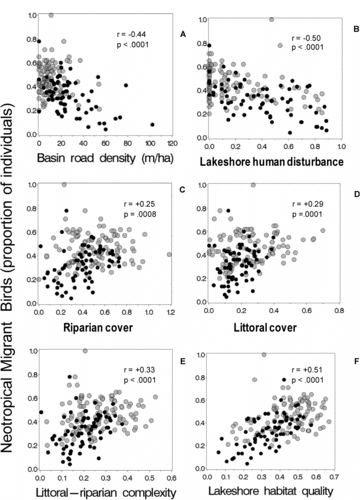
Also as expected, the proportion of bird species tolerant to human disturbance was strongly correlated with road density and near shore disturbance (r = 0.69 and 0.70; and ). Tolerant birds were moderately negatively correlated with the habitat cover and complexity indices that omitted direct measures of human disturbance (r = −0.45 to −0.31, –) and strongly negatively correlated with the lakeshore habitat quality index (r = −0.70). Associations between tolerant bird species and lakeshore habitat indices were consistently evident in both NE Highlands and NE Lowlands ecoregions (). Among the 4 biotic assemblage metrics we examined, the proportion of bird taxa tolerant to human disturbance had the strongest, most linear, and most regionally consistent association with the 5 near-shore habitat indices (compare panels B–F across –).
Discussion
We developed the lake physical habitat field methods and defined habitat metrics and indices to assess many of the habitat features important to faunal assemblages, including riparian birds and lentic fish and macroinvertebrates. The associations we observed between lake fauna and habitat indices in the EMAP-NE data indicate that those indices provide useful explanatory information regarding habitat suitability for fish and birds. Similarly, our human disturbance index was correlated with increases in the proportions or richness of fish and bird taxa tolerant of human disturbance and decreases in taxa intolerant of human disturbances. Those correlations indicate that the index quantifies aspects of human activities and physical habitat alterations relevant to those assemblages.
Of the 5 habitat and disturbance metrics we evaluated, the 4 biological metrics were most strongly correlated with the lakeshore habitat quality index that combined littoral and riparian cover complexity with near-shore disturbance data. The lakeshore habitat quality index was also related to disturbances at a larger scale, with moderate correlations with basin-scale road density, human population density, and percent urban land use (). Thus, if the aim is to use a single metric of lake physical habitat condition to link biological responses with basin-scale human pressures, an ecoregionally adjusted version of this overall lakeshore habitat quality index would be our recommendation. Since Karr's (Citation1981) introduction of the Index of Biotic Integrity (IBI), multimetric indices have been found useful for assessing and reporting on biological condition at multiple scales and on multiple continents (Hughes and Oberdorff Citation1999, Roset et al. Citation2007). The same may be true for physical habitat assessment and reporting, but further studies are needed to affirm these patterns.
Although fish and birds were not assessed in the EPA's NLA, other biotic assemblages sampled in that assessment were associated with near-shore physical habitat condition. The relative risk for impairment of phytoplankton and zooplankton taxa richness was approximately 3 times greater in lakes that had poor physical habitat condition than in lakes with fair or good condition, as measured by the NLA habitat condition indices (USEPA Citation2009, Van Sickle Citation2013). The relative risk to planktonic assemblages from near-shore habitat degradation was greater than that for excessive nutrients (USEPA Citation2009), implying that littoral features such as aquatic macrophytes, snags, and coarse substrates increase littoral habitat complexity and provide important substrate and cover for phyoplankton and zooplankton, just as they do for fish. Furthermore, lake riparian and littoral cover and complexity can buffer anthropogenic nutrients, sediments, and toxic inputs from upland areas just as they do along streams (Carpenter and Cottingham Citation1997, Strayer and Findlay Citation2010).
We found that habitat simplification in lakes was associated with anthropogenic disturbance, as reported by Whittier et al. (Citation2002b) and other researchers. Furthermore, extensive and intensive shoreline human activities simplified habitat structure and increased the abundance and richness of tolerant taxa, reducing the richness of intolerant bird and fish taxa and generally reducing native taxa richness. The EMAP-NE physical habitat field protocol and indicators aided us in assessing such associations. Those associations were consistent with the interpretation that complex, multi-layered near-shore riparian vegetation and abundant, complex littoral cover foster native fish and bird assemblage richness.
In a previous study of the same lakes, the number of native minnow species was negatively associated with shoreline disturbance, whereas the number of alien predator fish species increased (Whittier et al. Citation1997a). Likewise, EMAP-NE impoundments had greater shoreline disturbance, more tolerant fish species and individuals, and more nonnative fish species and individuals than did natural lakes (Whittier et al. Citation2002a, Citation2002b). Using similar protocols, Molozzi et al. (Citation2011) found increased proportions of tolerant benthic macroinvertebrate taxa and individuals with increased near-shore anthropogenic disturbance, and Sanches (Citation2011) and Terra and Araujo (Citation2011) reported similar relationships for fish. Lindsay et al. (Citation2002) found that abundance, richness and diversity of birds increased with moderate levels of lakeshore human development in a mixed northern forest. This pattern is relatively common at intermediate levels of disturbance, reflecting the replacement of intolerant taxa by tolerant taxa as food supplies increase with disturbance-associated increases nutrients. Lindsay et al. (Citation2002) further reported that traditional ecological measures of bird assemblages (abundance, richness, and species diversity) failed to reveal subtle but important changes in response to habitat alteration. Their reported decreases in insectivorous and ground-nesting birds (generally intolerant taxa) and increases in ground-feeding seed eaters (tolerant taxa) and deciduous tree nesters with anthropogenic disturbance are similar to our observations on bird assemblages of the near-shore lake environment.
Demonstration of strong associations between biota and habitat indicate potential control of biota by habitat. In most ecosystems there are aspects of physical habitat that exert natural controls and limitations on the composition and abundance of organisms present. Monitoring programs are typically interested in changes in biota that result from anthropogenic alteration of habitat. Although we were able to demonstrate relatively strong correlations between biota and habitat in this survey, observations of weak or null correlations between habitat and biota do not preclude strong causal control by habitat characteristics, which can be explained by at least 3 reasons. First, the lack of variation in some habitat characteristic over time or across the lakes of a region results in reduced statistical correlation between biota and that habitat characteristic. Habitat characteristics that change little over time, or that vary little spatially, may still exert important controls over biota over longer timescales and larger spatial scales (Ligeiro et al. Citation2013). Second, low precision and low regional variation of the biological metrics relative to measurement “noise” also limit their potential correlations with environmental predictor variables (Hughes et al. Citation1998, Kaufmann and Hughes Citation2006, Stoddard et al. Citation2008, CitationKaufmann et al. 2014a). Third, associations with habitat will not be strong or even readily detectable if other types of controls are limiting, which is frequently the case in nature (Mebane et al. Citation2003, Bryce et al. Citation2008, Citation2010, Steel et al. Citation2010, Ligeiro et al. Citation2013). For example, fish in lakes respond to physical habitat, water chemistry, zoogeography, biological interactions, and fishery management (Tonn Citation1990). Quantitative evaluation of physical habitat structure, however, helps discriminate its probable importance from these other limiting factors, as suggested by Platts et al. (Citation1983).
In the strictest sense, “cause and effect” can only be proven on the basis of rigorous experimental results (i.e., randomized treatments with causal agent). Under this strict interpretation, no monitoring of any sort could ever establish cause and effect; however, much of the monitoring by State, Federal and Tribal agencies can help establish causation by strong inference in a weight of evidence approach. Establishing strong inference for causal linkage via monitoring requires 4 criteria (Diamond Citation1986, Lloyd Citation1988): (1) plausible mechanisms for the relationship based on ecological first principles; (2) evidence in the literature supporting these mechanisms; (3) a demonstrated, statistically significant association present in the monitoring results; and (4) alternative mechanisms and explanations must be examined and found unlikely. Our demonstration of correlations between human disturbances and alteration of habitat complexity and biotic assemblage composition contribute to the weight of evidence for causal linkages when coupled with other information.
Acknowledgments
Most EMAP-NE data were collected by student employees of the State University of New York–College of Forestry and Environmental Sciences under the direction of N. Ringler and D. Halliwell. The NLA data were collected by State cooperators, US EPA regional staff, and TetraTech, GLEC and Dynamac crews. Bird data were provided by R. O’Connor and A. Moors (University of Maine, Orono). C. Burch-Johnson prepared the maps and T. Kincaid assisted with statistical analyses and guidance. Our manuscript was improved by reviews from J. Kurtenbach, D. P. Larsen, R. Ozretich, E. McGoff, and C. Hawkins. This article was subjected to the National Health and Environmental Effects Research Laboratory's Western Ecology Division and approved for publication. Approval does not signify that the contents reflect the view of the US EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Funding
Funding for data analysis and manuscript preparation were largely from the US EPA Office of Research and Development in support of the Environmental Monitoring and Assessment Program (EMAP) and the EPA Office of Water’s National Lakes Assessment through cooperative agreement #CR-818606 with Oregon State University and contract #68040019 with ManTech Corporation. Bob Hughes was supported in manuscript preparation during sabbatical in Brazil by grant 00011/09 from Fundacao de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), and Companhia Energetica deMinas Gerais (CEMIG).
References
- Allen AP, Whittier TR, Kaufmann PR, Larsen DP, O’Conner RJ, Hughes RM, Stemberger RS, Dixit SS, Brinkhurst RO, Herlihy AT, Paulsen SG. 1999. Concordance of taxonomic richness patterns across multiple assemblages in lakes of the northeastern United States. Can J Fish Aquat Sci. 56:739–747.
- Baker JR, Peck DV, Sutton DW, editors. 1997. Environmental Monitoring and Assessment Program - Surface Waters: field operations manual for lakes. Washington (DC ): US Environmental Protection Agency, Office of Research and Development; EPA/620/R-97/001.
- Benson BJ, Magnuson JJ. 1992. Spatial heterogeneity of littoral fish assemblages in lakes: relation to species diversity and habitat structure. Can J Fish Aquat Sci. 49:1493–1500.
- Brauns M, Garcia XF, Walz N, Pusch MT. 2007. Effects of human shoreline development on littoral macroinvertebrates in lowland lakes. J Appl Ecol. 44:1138–1144.
- Bryce SA. 2006. Development of a bird integrity index: measuring avian response to disturbance in the Blue Mountains of Oregon, USA. Environ Manage. 38:470–486.
- Bryce SA, Hughes RM, Kaufmann PR. 2002. Development of a bird integrity index: using bird assemblages as indicators of riparian condition. Environ Manage. 30:294–310.
- Bryce SA, Lomnicky GA, Kaufmann PR, McAllister LS, Ernst TL. 2008. Development of biologically-based sediment criteria in mountain streams of the western United States. N Am J Fish Manage. 28:1714–1724.
- Bryce SA, Lomnicky SG, Kaufmann PR. 2010. Protecting sediment-sensitive aquatic species in mountain streams through the application of biologically based streambed sediment criteria. J N Am Benthol Soc. 29:657–672.
- Butler RG, deMaynadier PG. 2008. The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). J Insect Conserv. 12:23–36.
- Carpenter SR, Cottingham KL. 1997. Resilience and restoration of lakes. Conserv Ecol. 1(1); . [cited 12 Sept 2011]. Available from: http://www.consecol.org/vol1/iss1/art2
- Christensen DL, Herwig BR, Schindler DE, Carpenter SR. 1996. Impacts of lakeshore residential development on coarse woody debris in north temperate lakes. Ecol Appl. 6:1143–1149.
- Croonquist MJ, Brooks RP. 1993. Effects of habitat disturbance on bird communities in riparian corridors. J Soil Water Conserv. 48:65–70.
- Daly C, Taylor GH. 2002. Development of new spatial grids of R factor and 10-yr EI30 for the conterminous United States. Corvallis (OR ): Oregon State University, Spatial Climate Analysis Service; . Internal EPA Report, NERL-LV.
- Diamond J. 1986. Overview: Laboratory experiments, field experiments, and natural experiments. In: Diamond J, Case TJ, editors. Community Ecology. New York: Harper and Row. p. 3–22.
- Eadie JMA, Keast A. 1984. Resource heterogeneity and fish species diversity in lakes. Can J Zool. 62:1689–1695.
- Engel S, Pederson J. 1998. The construction, aesthetics, and effects of lakeshore development: a literature review. Wisconsin. Madison (WI ): Dept. of Natural Resources, . Report 177; [cited 12 Mar 2014]. Available from: http://digital.library.wisc.edu/1711.dl/EcoNatRes.DNRRep177
- Eschmeyer RW. 1936. Essential considerations for fish management in lakes. In: Proceedings from the North American Wildlife Conference. Washington (DC ): US Government Printing Office. p. 332–229.
- Francis TB, Schindler DE. 2006. Degradation of littoral habitats by residential development: woody debris in lakes of the Pacific Northwest and Midwest, United States. Ambio. 35:274–280.
- Halliwell D. 2007. Lake habitat measures. New England Chapter of the North American Lake Management Society. New England Lake News. 2(2):1–4.
- Halliwell D. 2008. Lake habitat measures. Out of the Blue. Vermont Agency of Natural Resources Department of Environmental Conservation. Water Quality Division newsletter. 33:8–9.
- Hampton, SE, Fradkin SC, Leavitt PR, Rosenberger EE. 2011. Disproportionate importance of nearshore habitat for the food web of a deep oligotrophic lake. Mar Freshw Res. 62:350–358.
- Hatzenbeler GR, Kampa JM, Jennings MJ, Emmons EE. 2004. A comparison of fish and aquatic plant assemblages to assess ecological health of small Wisconsin lakes. Lake Reserv Manage. 20:211–218.
- Hughes RM, Burch-Johnson C, Dixit SS, Herlihy AT, Kaufmann PR, Kinney WL, Larsen DP, Lewis PA, McMullen DM, Moors AK, et al. 1993. Development of lake condition indicators for EMAP 1991 Pilot. In: Larsen DP, Christie SJ, editors. EMAP-Surface Waters 1991 Pilot Report. Washington (DC ): US Environmental Protection Agency, Office of Research and Development; . EPA/620/R-93/003. p. 7–90.
- Hughes RM, Kaufmann PR, Herlihy AT, Kincaid TM, Reynolds L, Larsen DP. 1998. A process for developing and evaluating indices of fish assemblage integrity. Can J Fish Aquat Sci. 55:1618–1631.
- Hughes RM, Oberdorff T. 1999. Applications of IBI concepts and metrics to waters outside the United States and Canada. In: Simon TP, editor. Assessing the Sustainability and Biological Integrity of Water Resources using Fish Assemblages. Boca Raton (FL ): Lewis. p. 79–93.
- Jennings MJ, Bozek MA, Hatzenbeler GR, Emmons EE, Staggs MD. 1999. Cumulative effects of incremental shoreline habitat modification on fish assemblages in north temperate lakes. N Am J Fish Manage. 19:18–27.
- Jennings MJ, Emmons EE, Hatzenbeler GR, Edwards C, Bozek MA. 2003. Is littoral habitat affected by residential development and land use in watersheds of Wisconsin lakes? Lake Reserv Manage. 19:272–279.
- Karr JR. 1981. Assessment of biotic integrity using fish communities. Fisheries. 6(6):21–27.
- Kaufmann PR. 1993. Physical habitat. In: Hughes RM, editor Stream Indicator and Design Workshop. Corvallis (OR ): US Environmental Protection Agency; . EPA/600/R-93/138. p. 59–69.
- Kaufmann PR, Hughes RM. 2006. Geomorphic and anthropogenic influences on fish and amphibians in Pacific Northwest coastal streams. In: Hughes RM, Wang L, Seelbach PW, editors. Landscape influences on stream habitat and biological assemblages. . American Fisheries Society Symposium 48; Bethesda (MD). p. 429–455.
- Kaufmann PR, Hughes RM, Van Sickle J, Whittier TR, Seeliger CW, Paulsen SG. . 2014a. Lake shoreline and littoral habitat structure: a field survey method and its precision. Lake Reserv Manage. . 30:157–176.
- Kaufmann PR, Peck DV, Paulsen SG, Seeliger CW, Hughes RM, Whittier RR, Kamman NC. . 2014b. Lakeshore and littoral physical habitat structure in a national lake assessment. Lake Reserv Manage.. 30:192–215.
- Kaufmann PR, Whittier TR. 1997. Habitat assessment. In: Baker JR, Peck DV, Sutton DW, editors. Environmental Monitoring and Assessment Program – Surface Waters: field operations manual for lakes. Washington (DC ): US Environmental Protection Agency, Office of Research and Development; . EPA/620/R-97/001. p. 5–1 to 5–26.
- Larsen DP, Christie SJ, editors. 1993. EMAP-Surface Waters 1991 Pilot Report. Washington (DC ): US Environmental Protection Agency, Office of Research and Development; . EPA/620/R-93/003.
- Larsen S, Sorace A, Mancini L. 2010. Riparian bird communities as indicators of human impacts along Mediterranean streams. Environ Manage. 45:261–273.
- Larsen DP, Thornton KW, Urquhart NS, Paulsen SG. 1994. The role of sample surveys for monitoring the condition of the Nation's lakes. Environ Monit Assess. 32:101–134.
- Ligeiro R, Hughes RM, Kaufmann PR, Macedo DR, Firmiano KE, Ferreira WR, Oliveira D, Melo AS, Callisto M. 2013. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertbrate taxa richness. Ecol Indic. 25:45–57.
- Lindsay AR, Gillum SS, Meyer MW. 2002. Influence of lakeshore development on breeding bird communities in a mixed northern forest. Biol Conserv. 107:1–11.
- Lloyd EA. 1988. The structure and confirmation of evolutionary theory. New York: Greenwood Press.
- Lynch WE Jr, Johnson DL. 1989. Influences of interstice size, shade, and predators on the use of artificial structures by bluegills. N Am J Fish Manage. 9:219–225.
- MacArthur RH, MacArthur JW. 1961. On bird species diversity. Ecology. 42:351–364.
- Macedo DR, Ligeiro R, Ferreira WR, Junqueira NT, Sanches BO, Silva DRO, Alves CBM, Hughes RM, Kaufmann PR, Pompeu PS, et al. 2012. Parâmetros biológicos e de habitats físicos para avaliação de bacias no Sudeste do Brasil. Ação Ambiental. 47:15–18.
- Mebane CA, Maret TR, Hughes RM. 2003. An index of biological integrity (IBI) for Pacific Northwest rivers. T Am Fish Soc. 132:239–261.
- Merrell K, Howe E, Warren S. 2009. Examining shorelines, littorally. Lakeline. 29:1.
- Molozzi J, França JS, Araujo TLA, Viana TH, Hughes RM, Callisto M. 2011. Diversidade de habitats físicos e sua relação com macroinvertebrados bentônicos em reservatórios urbanos. Iheringia Sér Zool. 101:191–199.
- Ness KL. 2006. The effects of shoreline development on lake littoral and riparian habitats: are shoreline protection regulations enough? [masters thesis]. [Orono (ME )]: University of Maine.
- O’Connor RJ, Walls TE, Hughes RM. 2000. Using multiple taxonomic groups to index the ecological condition of lakes. Environ Monit Assess. 61:207–228.
- Omernik JM. 1987. Ecoregions of the conterminous United States. Ann Assoc Am Geogr. 77:118–125.
- Paulsen SG, Larsen DP, Kaufmann PR, Whittier TR, Baker JR, Peck DV, Mcgue J, Stevens D, Stoddard J, Hughes RM, et al. 1991. Environmental Monitoring and Assessment Program (EMAP) – Surface Waters monitoring and research strategy. Washington (DC ): US Environmental Protection Agency, Office of Research and Development; . EPA/600/3-91/022.
- Platts WS, Megahan WF, Minshall GW. 1983. Methods for evaluating stream, riparian and biotic conditions. Ogden (UT ): US Forest Service, Intermountain Forest and Range Experiment Station, Gen. Tech. Rep. INT-138.
- Radomski P, Geoman TJ. 2001. Consequences of human lakeshore development on emergent and floating-leaf vegetation abundance. N Am J Fish Manage. 21:41–46.
- Remsburg AJ, Turner MG. 2009. Aquatic and terrestrial drivers of dragonfly (Odonata) assemblages within and among north-temperate lakes. J N Am Benthol Soc. 28:44–56.
- Roset N, Grenouillet G, Goffaux D, Pont D, Kestemont P. 2007. A review of existing fish assemblage indicators and methodologies. Fish Manage Ecol. 14:393–405.
- Rowan JS, Carwardine J, Duck RW, Bragg OM, Black AR, Cutler JEJ, Soutar I, Boon PJ. 2006. Development of a technique for Lake Habitat Survey (LHS) with applications for the European Union Water Framework Directive. Aquat Conserv. 16:637–657.
- Sanches BO. 2011. Variações espaciais na estrutura da comunidade de peixes do reservatório de Nova Ponte (Rio Araguari, bacia do alto Paraná) [dissertation]. [Belo Horizonte (Brazil )]: Pontifícia Universidade Católica de Minas Gerais.
- Sass GG, Carpenter SR, Gaeta JW, Kitchell JF, Ahrenstorff TD. 2012. Whole-lake addition of coarse woody habitat: response of fish populations. Aquat Sci. 74:255–266.
- Sass GG, Kitchell JE, Carpenter SR, Hrabik TR, Marburg AE, Turner MG. 2006. Fish community and food web responses to a whole-lake removal of coarse woody habitat. Fisheries. 31:321–330.
- Savino JR, Stein RA. 1989. Behavior of fish predators and their prey: habitat choice between open water and dense vegetation. Environ Biol Fish. 24:287–293.
- Schindler DE, Scheuerell MD. 2002. Habitat coupling in lake ecosystems. Oikos. 98:177–189.
- Segurado P, Santos JM, Pont D, Melcher A, Garcia Jalon D, Hughes RM, Ferreira MT. 2011. Estimating species tolerance to human perturbation: expert judgment versus quantitative approaches. Ecol Indic. 11:1623–1635.
- Smokorowski KE, Pratt TC, Cole WG, McEachern LJ, Mallory EC. 2006. Effects on periphyton and macroinvertebrates from removal of submerged wood in three Ontario lakes. Can J Fish Aquat Sci. 63:2038–2049.
- Steel A, Hughes RM, Schmutz S, Muhar S, Poppe M, Trautwein C, Fukushima M, Shimazaki H, Young J, Feist B, Fullerton A, Sanderson B. 2010. Meeting the challenges of landscape scale riverine research: a review. Living Rev Landscape Res. 4:1–60.
- Stoddard JL, Herlihy AT, Peck DV, Hughes RM, Whittier TR, Tarquinio E. 2008. A process for creating multi-metric indices for large-scale aquatic surveys. J N Am Benthol Soc. 27:878–891.
- Strayer DL, Findlay SEG. 2010. Ecology of freshwater shore zones. Aquat Sci. 72:127–163.
- Taillon D, Fox MG. 2004. The influence of residential and cottage development on littoral zone fish communities in a mesotrophic north temperate lake. Environ Biol Fish. 71:275–285.
- Tarzwell CM. 1936. Lake and stream improvement in Michigan. In: Proceedings from the North American Wildlife Conference. Washington (DC ): US Government Printing Office. p. 429–434.
- Terra BF, Araujo FG. 2011. A preliminary fish assemblage index for a transitional river—reservoir system in southeastern Brazil. Ecol Indic. 11:874–881.
- TIGER. 1990. Modified topologically integrated geographic encoding and referencing system; [cited Apr 2011]. . Available from: http://www.census.gov/geo/www/tiger
- Tonn WM. 1990. Climate change and fish communities: a conceptual framework. T Am Fish Soc. 119:337–352.
- Tonn WM, Magnuson JJ. 1982. Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology. 63:1149–1166.
- US Census Bureau. 1990. TIGER Line Maps: 1990 U.S. Counties. Washington (DC ): US Department of Commerce, US Census Bureau.
- US Census Bureau. 2001. US Census Bureau Online Information. Washington (DC ): US Department of Commerce, US Census Bureau; . [cited 17 Apr 2011]. . Available from: http://www.census.gov/main/www/cen2000.html
- [USEPA] US Environmental Protection Agency. 2007. Survey of the nation's lakes. Field operations manual. Washington (DC ): US Environmental Protection Agency; . EPA 841-B-07-004.
- [USEPA] US Environmental Protection Agency. 2009. National Lakes Assessment – a collaborative survey of the Nation's lakes. Washington (DC ): USEPA Office of Water; . EPA 841-R-09-001.
- Van Sickle J. 2013. Estimating the risks of multiple, covarying stressors in the National Lakes Assessment. Freshwater Sci. 32:204–216.
- Vaux PD, Whittier TR, DeCesare G, Kurtenbach JP. 2000. Evaluation of a backpack electrofishing unit for multiple lake surveys of fish assemblage structure. N Am J Fish Manage. 20:168–179.
- Vogelmann JE, Howard SM, Yang L, Larson CR, Wylie BK, Van Driel JN. 2001. Completion of the 1990's national land cover data set for the conterminous United States. Photogram Eng Remote Sens. 67:650–662.
- Wagner T, Jubar AK, Bremigan MT. 2006. Can habitat alteration and spring angling explain largemouth bass nest success? T Am Fish Soc. 135:843–852.
- Wege GJ, Anderson RO. 1979. Influence of artificial structures on largemouth bass and bluegills in small ponds. In Johnson DL, Stein RA, editors. Response of fish to habitat structure in standing water. Bethesda (MD ): American Fisheries Society Special Publication 6. p. 59–69.
- Whittier TR, Halliwell DB, Paulsen SG. 1997a. Cyprinid distributions in Northeast USA lakes: evidence of regional-scale minnow biodiversity losses. Can J Fish Aquat Sci. 54:1593–1607.
- Whittier TR, Hughes RM. 1998. Evaluation of fish species tolerances to environmental stressors in lakes of the northeastern United States. N Am J Fish Manage. 18:236–252.
- Whittier TR, Hughes RM, Lomnicky GA, Peck DV. 2007. Fish and amphibian tolerance values and an assemblage tolerance index for streams and rivers in the western USA. T Am Fish Soc. 136:254–271.
- Whittier TR, Kincaid TM. 1999. Introduced fish in Northeastern USA lakes: regional extent, dominance, and effect on native species richness. T Am Fish Soc. 128:769–783.
- Whittier TR, Larsen DP, Peterson SA, Kincaid TM. 2002a. A comparison of impoundments and natural drainage lakes in the northeast USA. Hydrobiologia. 470:157–171.
- Whittier TR, Paulsen SG, Larsen DP, Peterson SA, Herlihy AT, Kaufmann PR. 2002b. Indicators of ecological stress and their extent in the population of northeastern lakes: a regional-scale assessment. BioScience. 52:235–247.
- Whittier TR, Vaux P, Merritt GD, Yeardley RB. 1997b. Fish Sampling. in Baker JR, Peck DV, Sutton DW. (editors). Environmental Monitoring and Assessment Program -Surface Waters: field operations manual for lakes. Washington, (DC ): US Environmental Protection Agency, Office of Research and Development; . EPA/620/R-97/001. p. 6–1 to 6–57.