Abstract
This study evaluated the impact of oxygenation on the accumulation of methylmercury (MeHg), total mercury (Hg), iron (Fe), and manganese (Mn) in the anoxic hypolimnion of North Twin Lake, Washington (mean depth = 9.7 m). Within 8 hours of the start of the oxygenation test, mean hypolimnetic total Fe dropped from 833 to 243 μg/L (58% dissolved), and Mn decreased from 119 to 32 μg/L (100% dissolved). After 17 days of oxygenation, mean hypolimnetic total Hg decreased from 0.77 to 0.58 ng/L, and MeHg decreased from 0.20 to 0.10 ng/L. A month after the end of the oxygenation test, MeHg, total Hg, Fe, and Mn concentrations rebounded to pre-oxygenation levels. Metal dynamics were explained by the differing redox characteristics of Fe (fast oxidation kinetics; reduction at lower redox potential) and Mn (slow oxidation kinetics; reduction at higher redox potential) in natural waters, and the tendency for Fe oxides to co-precipitate with reduced Mn, ionic Hg, and MeHg. From a lake management perspective, the study highlights the role that oxidized metals in surfacial sediments play in retaining Hg in profundal sediments and suggests that sorption characteristics of metal oxides could be exploited to manage Hg bioaccumulation in aquatic biota. The study also suggests that for oxygenation to effectively repress the accumulation of redox-sensitive compounds in the hypolimnion, oxygenation systems should (1) start before anoxic conditions are established; (2) operate continuously through the entire stratified season; and (3) maintain oxygen levels at the sediment–water interface to ensure oxygen penetration into surfacial sediments.
Hypolimnetic anoxia is a frequent problem in stratified, nutrient-rich lakes. Summer hypolimnetic oxygen consumption occurs as organic material settles into the isolated profundal zone and decomposes. Oxygen level and redox potential at the sediment–water interface are particularly critical in regulating the flux of dissolved substances out of profundal sediments (Golterman Citation2001). A key concern for lake managers is internal nutrient loading caused by the release of nutrients from anoxic sediments into the water column. Oxygen depletion at the sediment–water interface can result in the release of phosphorus into the water column through a number of mechanisms, including the reduction of phosphorus-rich metal oxides (Boström et al. Citation1988). Anoxic conditions can also cause hypolimnetic enrichment of ammonia as anoxic conditions impede biological nitrification and ammonia assimilation (Beutel Citation2006). Diffusion, entrainment, and fall turnover can transfer accumulated nutrients into the photic zone where they can stimulate primary productivity, increasing the flux of organic matter to the profundal zone and perpetuating anoxia in bottom waters. Internal nutrient loading is especially significant because it can impair water quality and prolong lake recovery after the control of external nutrient loading (Welch and Jacoby Citation2001).
Anoxia has other profound impacts on the ecological functioning of lakes and reservoirs. Depressed oxygen levels and redox potential in the profundal zone of lakes can enhance the sediment release of iron (Fe) and manganese (Mn; Gantzer et al. Citation2009a, Bryant et al. Citation2011a). If the lake is a raw water source, these metals can complicate potable water treatment (Gantzer et al. Citation2009a, Betancourt et al. Citation2010). Ammonia and sulfide, which are extremely toxic to aquatic biota, also tend to accumulate in bottom waters under anoxic conditions (Beutel et al. Citation2001, Beutel Citation2006). Finally, hypolimnetic anoxia can lead to degraded cold-water fish habitat as cool bottom waters become void of oxygen and accumulate toxins (Beutel et al. Citation2001, Christensen and Moore Citation2009).
A growing body of evidence is linking hypolimnetic anoxia with mercury (Hg) accumulation in hypolimnetic waters, and lake managers are in the initial phases of testing and implementing in-lake management strategies to minimize Hg bioaccumulation in lakes and reservoirs (Drury Citation2011, Austin Citation2013, Beutel et al. Citation2013, Matthews et al. Citation2013). Anoxic bottom waters can contain inorganic mercury (Hg(II)) and methylmercury (MeHg), the primary species that accumulate in aquatic biota, at concentrations greater than that of oxic bottom waters (Eckley and Hintelmann Citation2006, Watras Citation2009). The higher levels of MeHg associated with hypolimnetic anoxia can increase exposure of the contaminant to the base of the aquatic food web, thereby enhancing Hg bioaccumulation (Slotton et al. Citation1995, Herrin et al. Citation1998).
Hg contamination in aquatic systems is a significant concern in the United States. Three-fourths of states currently have blanket fish consumption advisories due to elevated Hg in fish tissue, and nearly half of US lake acres and more than one-third of US river miles are subject to Hg-related consumption advisories (USEPA Citation2009). On a global scale, rates of atmospheric Hg deposition have increased 3-fold as a result of cultural post-industrial activities, and an estimated 5–20% of deposited Hg is delivered to aquatic ecosystems where it may accumulate in aquatic biota (Swain et al. Citation2007).
In the absence of point sources, Hg typically enters a lake in the inorganic form via aerial deposition and watershed runoff. Hg(II) can complex with dissolved organic carbon (DOC) and co-precipitate with settling mineral particles rich in Fe and Mn (Chadwick et al. Citation2006, Watras Citation2009). Hg(II) in an anoxic hypolimnion can be methylated by sulfate-reducing bacteria in anoxic profundal waters and sediments (Benoit et al. Citation2003). MeHg can also enter the water column when it is released from metal oxide complexes in surface sediments that dissolve after the onset of anoxia (Chadwick et al. Citation2006, Merritt and Amirbahman Citation2008).
A variety of in-lake management strategies are used to ameliorate problems associated with hypolimnetic anoxia (Cooke et al. Citation2005). One strategy that has grown in application is hypolimnetic oxygenation, the use of an engineered system to enhance dissolved oxygen (DO) levels in bottom waters using pure oxygen gas (Beutel and Horne Citation1999, Singleton and Little Citation2006, Moore et al. Citation2012). Hypolimnetic oxygenation systems are designed to maintain adequate DO in the hypolimnion while preserving thermal stratification.
A line diffuser oxygenation system was installed in 2008 in North Twin Lake, Washington (Beutel et al. Citation2011). The lake is an important recreational fishing site that supports a cold-water fishery and has been impaired by poor cold-water habitat resulting from hypolimnetic anoxia (Christensen and Moore Citation2009). A short-term test of the oxygenation system in fall 2008 provided a unique opportunity to evaluate the effects of oxygenation on accumulated Fe, Mn, and Hg in an anoxic hypolimnion. Our hypothesis was that oxygenation would result in a decrease in Fe in the water column with a concurrent decrease in Hg. The likely mechanism for such an outcome would be the oxidation of soluble reduced Fe to particulate oxidized Fe with co-precipitation of Hg and subsequent sedimentation out of the water column. While previous studies have focused on the effect of oxygen addition on hypolimnetic accumulation of nutrients and conventional metals (i.e., Fe and Mn), this study is novel in that it also evaluated the fate of MeHg, a potent and widespread bioaccumulatory toxin in aquatic ecosystems.
Materials and methods
Study site
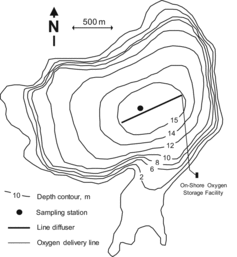
North Twin Lake (mean depth = 9.7 m; maximum depth = 15.2 m; area = 371 ha) is a moderately deep, dimictic, meso-eutrophic lake located on the reservation of the Colville Confederated Tribes in eastern Washington State (). Hypolimnetic oxygen depletion occurs soon after the development of thermal stratification. The entire hypolimnion is commonly anoxic by mid-June and remains so until fall turnover in late October. During the period of hypolimnetic anoxia, cold-water trout reside in a 2 m thick zone in the metalimnion, unable to access the anoxic cold-water habitat below (Christensen and Moore Citation2009). A hypolimnetic oxygenation system, consisting of a 750 m long, fine-bubble diffuser line connected to an on-shore 23 m3 liquid oxygen storage tank, was installed in early fall 2008 to improve the habitat for cold-water fish (Beutel et al. Citation2011). The oxygenation system was tested from 28 August to 14 September 2008.
Water quality monitoring
Extensive water quality monitoring was conducted at a 15 m deep site located approximately 40 m perpendicular to the oxygen diffuser line (). Water quality monitoring for DO, temperature, conductivity, total Fe, total Mn, total Hg, and MeHg was conducted on 13 August, 13 September, and 20 October, representing conditions before, during, and after the oxygenation test, respectively. DO, temperature, and conductivity profiles were measured at 1 m vertical intervals with an MS5 Sonde Hydrolab (Hach Company, CO). Additional monitoring for total and soluble Fe and Mn was performed on 28 August after 8 hr of oxygenation, 3 September, and 13 September. A DO, conductivity, and temperature profile was also measured at 0.5 m vertical intervals using a Zebra-Tech D-Opto optical DO probe (Ocean Instruments, CA) and a SBE-37SI (Sea-Bird Electronics, WA) on 28 August after 8 hr of oxygenation. Samples for all dates were also tested for the presence of sulfide using a serendipitous sniff test (Barbash Citation2002).
Fe and Mn samples were collected at 1 m intervals along a vertical profile using Tygon tubing attached to a Dayton model 1P580E pump. Samples collected for both total and soluble metals were split, with a portion filtered (0.45 μm mesh) for soluble metals and another portion unfiltered for total metals. All Fe and Mn samples were preserved with nitric acid (APHA Citation2005). Profiles for total Hg and MeHg were sampled at 2 m vertical intervals with a Teflon Kemmerer sampler. Samples were stored in acid-washed glass bottles with Teflon-lined caps. Clear glass was used for total Hg and amber glass for MeHg. All Hg sampling protocol followed US Environmental Protection Agency (EPA) methods 1630 and 1631 (USEPA Citation2001, Citation2002). Total Hg samples were preserved with bromine monochloride, and MeHg samples were preserved with trace-metal–grade hydrochloric acid then were stored at 4 C until analysis.
To quantify the spatial distribution of oxygen, vertical DO profiles were measured every 0.5 m along a perpendicular transect at 40, 80, 160, 320, 480, and 640 m from each side of the diffuser using a Zebra-Tech D-Opto optical DO probe (Ocean Instruments) on 28 August just prior to oxygenation, 30 August, 6 September, and 15 September.
Water quality analyses
Fe and Mn in water samples were analyzed using inductively coupled plasma mass spectrometry (ICP-MS; APHA Citation2005). Total Hg samples were analyzed using a Tekran 2600 cold vapor atomic fluorescence spectroscopy (CVAFS) Hg auto analyzer (USEPA Citation2002). MeHg samples were analyzed at Battelle Marine Science Laboratories (Sequim, WA) with EPA method 1630 modular analytical technique, which involves sample distillation, ethylation, purge and trap, thermal desorption, gas chromatography separation, pyrolyzation, and subsequent detection via a Tekran 2500 CVAFS (USEPA Citation2001). The method detection limit was 0.2 ng/L for total Hg and 0.02 ng/L for MeHg. Standard quality control procedures were followed, including calibration blanks, matrix spike samples (acceptable range 70–130%), and ongoing precision recovery samples (acceptable range 75–125%). Mean hypolimnetic water column concentrations for the 15 m deep site for conductivity, Fe, Mn, total Hg, and MeHg were calculated by averaging values from a sampled profile. Values of percent soluble and the Fe to Mn ratio were also based on mean water column concentrations. Volume-weighted concentrations were not used because the hypolimnion exhibited spatial heterogeneity in DO levels, and presumably the metals of interest as well, during the oxygenation test.
Results
Dissolved oxygen, temperature, and conductivity
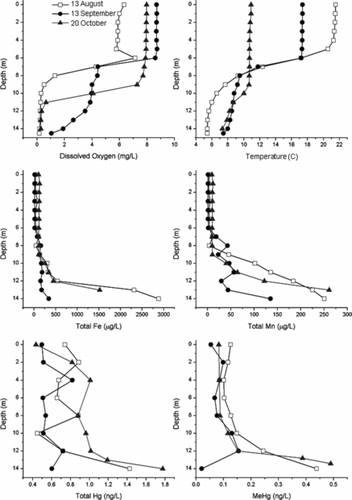
Bimonthly monitoring of DO and temperature showed that thermal stratification started in April, and that hypolimnetic anoxia was fully established by late June. On 13 August, prior to the oxygenation test, hypolimnetic DO was <1 mg/L beneath the 7 m deep thermocline (). Mean conductivity in the hypolimnion was 67 μS/cm with a corresponding peak near the sediments of 81 μS/cm (). Conductivity in the hypolimnion was substantially higher than levels in the epilimnion (mean of 53 μS/cm). On 28 August, approximately 8 hr after oxygenation commenced, hypolimnetic DO was around 1.3 mg/L, approximately 0.5 mg/L higher than pre-oxygenated conditions. The hypolimnetic temperature was around 6.6 C, a slight increase of around 0.2 C from pre-oxygenated conditions. Compared to 13 August, mean conductivity in the hypolimnion dropped to 47 μS/cm, and the peak near the sediments also dropped to 53 μS/cm ().
Table 1 Iron, manganese, conductivity, and sulfide in the hypolimnion of North Twin Lake, WA.
Approximately 2 weeks into the oxygenation test on 13 September, DO and temperature in the hypolimnion increased (). DO levels ranged from 4 mg/L in the upper hypolimnion to 1–3 mg/L in the lower hypolimnion. Hypolimnetic temperature warmed to 8–9 C. Mean conductivity was 63 μS/cm with a peak of 70 μS/cm near the sediments. On 20 October, nearly 1 month after oxygenation was turned off, epilimnetic temperature cooled to around 11 C, and the thermocline declined to 10 m (). The remaining hypolimnion below 11 m was anoxic. Conductivity rebounded to levels higher than pre-oxygenation conditions, with a hypolimnetic mean of 74 μS/cm and a peak near the sediments of 104 μS/cm.
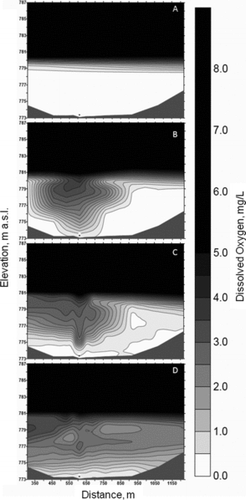
DO isopleths were developed from transects perpendicular to the line diffuser for 28 August (prior to oxygenation), 30 August (2 days into oxygenation), 7 September (9 days into oxygenation), and 15 September (12 h after oxygen was turned off; ). On 28 August the hypolimnion was completely anoxic up to the thermocline (). Two days into oxygenation, an oxygen plume extended roughly 250 m horizontally to both the north and the south of the line diffuser in the upper hypolimnion (). Nine days into the oxygenation test, there was considerable DO migration horizontally to the south (). After 17 days of oxygenation, DO distribution in the hypolimnion was heterogeneous, with DO elevated in the southern upper hypolimnion, some DO in the northern upper hypolimnion, and little or no DO at the sediment–water interface ().
Iron and manganese
Prior to oxygenation in mid-August, mean hypolimnetic water column concentrations were 833 μg/L for total Fe and 119 μg/L for total Mn (; ). Maximum hypolimnetic concentrations above the sediments were 2882 μg/L for total Fe and 250 μg/L for total Mn. Hypolimnetic concentrations of Fe and Mn decreased substantially 8 hr after the start of the oxygenation test. Mean total Fe decreased to 243 μg/L (58% soluble fraction), and mean total Mn decreased to 32 μg/L (100% soluble fraction). Mean hypolimnetic total Fe continued to drop throughout the oxygenation test, to 208 μg/L on day 7 and 173 μg/L on day 17. Soluble fractions of total Fe fluctuated in the range of 30–60% of total. Mean hypolimnetic total Mn ranged from 30–50 μg/L throughout the oxygenation test, with the soluble fraction decreasing from 94% on day 7 to 49% on day 17. Peak concentrations of total Fe and Mn displayed similar patterns as the mean hypolimnetic concentrations.
On 20 October, about a month after the oxygenation test was terminated, anoxia had reestablished within the hypolimnion. Mean hypolimnetic total Fe and Mn concentrations rebounded to pre-oxygenation levels: 642 μg/L for total Fe and 122 μg/L for total Mn (). Peak concentrations above the sediments were approximately half of the pre-oxygenation level for Fe (1519 μg/L) but similar to pre-oxygenation levels for Mn (261 μg/L). The vertical gradient of Fe reached up to 12 m, while the vertical gradient of Mn reached up to the thermocline ().
Total mercury and methylmercury
Total Hg and MeHg levels on 13 August, prior to oxygenation, were elevated in bottom waters (; ). Total Hg was around 0.9 ng/L in the upper hypolimnion and 1.4 ng/L near the sediments. MeHg was 0.1 ng/L in the upper hypolimnion but increased below 12 m to a peak concentration of 0.44 ng/L near the sediments. After 17 days of oxygenation, total Hg and MeHg decreased in the hypolimnion. Mean hypolimnetic total Hg dropped from 0.77 to 0.58 ng/L, and mean hypolimnetic MeHg dropped from 0.20 to 0.10 ng/L. The ratio of MeHg to total Hg decreased slightly after oxygenation, indicating that MeHg was more effectively lost from the water column. MeHg concentration was especially low near the sediments after oxygenation, decreasing from 0.44 to 0.02 ng/L. As with Fe and Mn, total Hg and MeHg concentration in bottom waters returned to pre-oxygenation levels 1 month after the end of the oxygenation test (). By 20 October, mean hypolimnetic concentrations of total Hg were 1.14 ng/L with a peak of 1.78 ng/L above the sediments. Mean hypolimnetic concentrations of MeHg were 0.27 ng/L with a peak above the sediments of 0.49 ng/L.
Table 2 Total mercury and methylmercury in the hypolimnion of North Twin Lake, WA.
Discussion
Dissolved oxygen, temperature and conductivity
Oxygenation had an immediate and profound effect on hypolimnetic water quality at the study site. Approximately 8 hr after oxygenation, hypolimnetic DO was around 1.3 mg/L, approximately 0.5 mg/L higher than pre-oxygenated conditions, indicating that oxygen had begun to accumulate in the hypolimnion early in the test. Mean hypolimnetic conductivity and peak conductivity at the sediment 8 hr after oxygenation also dropped relative to pre-oxygenation conditions, and this drop corresponded with substantial drops in total Fe and Mn in bottom waters. Approximately 2 weeks into the oxygenation test, DO levels had increased further (1–4 mg/L), and mean hypolimnetic temperature was around 2 C warmer than before oxygenation. Bottom water warming is commonly observed in the early stages of hypolimnetic oxygenation operations due to mixing induced between the hypolimnion and warmer waters of the lower metalimnion (Gantzer et al. Citation2009b).
In the early days of oxygenation (), DO showed a typical oxygen distribution for the initial operation of a line diffuser, in which a bubble plume rises from the diffuser, entrains and oxygenates upwelling water, then releases the oxygenated water horizontally into the upper hypolimnion (Singleton et al. Citation2007). Nine days into the oxygenation test, there was considerable DO migration horizontally to the south (). Even after 17 days of oxygenation, DO distribution in the hypolimnion was heterogeneous, with DO elevated in the southern end and little or no DO near the sediments ().
Field observations suggest that the pattern of DO distribution in the upper hypolimnion was driven by internal seiches. Internal seiches, wind-driven standing waves at the thermocline with relatively large amplitudes (cm to m) and long periods (hours to days), can result in substantial advection of dissolved compounds near the pelagic thermocline of lakes (Preusse et al. Citation2010). As storms or high winds moved through the area, generally moving from north to south, elevated DO in the upper hypolimnion of North Twin Lake was observed to smear north one day and south the next day. On low-wind days, uniform oxygen distribution was observed on either side of the diffuser.
Modeling using a line bubble plume model (Singleton et al. Citation2007) suggests that the observed spatial pattern of DO during and at the end of the oxygenation test was affected by a pre-existing DO deficit in the hypolimnion. The model estimated that 20% of the hypolimnion was circulated daily by the bubble plume; thus, the hypolimnion was exchanged through the oxygen plume every 5 days, or around 3 times during the oxygenation test. The 5-day estimate is a minimum because it is a nominal volume, and short-circuiting of water near the bubble plume limits the amount of far-field water actually entrained into the bubble plume.
Typically it can take weeks for a uniform flow pattern to establish within a hypolimnion after start-up of line diffuser oxygenation systems (Bryant et al. Citation2011b). The bubble plume model predicted a hypolimnetic oxygen input rate of 4300 kg/d over the course of the test, a value that would theoretically result in a final average hypolimnetic DO of 5.7 mg/L at the end of the 17 day test, assuming no oxygen demand in the hypolimnion. The measured average hypolimnetic DO was 1.9 mg/L at day 14 of the test, however, which is equivalent to a total hypolimnetic oxygen demand of 2300 kg/day, which was likely the result of the oxygen demand of reduced compounds (e.g., Fe, Mn, sulfide, methane, and organic matter) that accumulated in the hypolimnion prior to the oxygenation test. Based on the observed rate of DO accumulation, it would have taken approximately 30 days to reach an average hypolimnetic DO content of 5 mg/L, a level suitable for cold-water fish and more likely to result in oxygen penetration into profundal sediments. This finding reaffirms that oxygenation systems should be started before anoxic conditions are established for oxygenation to most effectively repress the accumulation of redox-sensitive compounds in hypolimnetic waters.
Iron and manganese
Vertical distribution of iron and manganese prior to oxygenation
Fe and Mn displayed contrasting vertical distributions prior to the oxygenation test. In mid-August, a steep Fe gradient extended from the bottom to 12 m, while the Mn gradient extended from the bottom to about 8 m (). This observation can be explained based on 2 contrasting redox characteristics of Fe and Mn in natural waters: Mn(IV) is generally reduced at higher redox potential than Fe(III); and Mn(II) is generally oxidized much slower than Fe(II). Because Mn(IV) is reduced at a higher redox potential, the efflux of soluble Mn(II) from sediments likely started before Fe(II), and with time, soluble Mn(II) diffused higher into the anoxic hypolimnetic water column relative to Fe(II). Also enhancing the upward diffusion of soluble Mn(II) was its resistance to re-oxidation back to particulate Mn(IV), which would have been lost from the water column via sedimentation.
Internal recycling dynamics can also enhance the spatial differentiation of Fe and Mn in the water column (Davison Citation1993, Dellwig et al. Citation2012). Particulate metal oxides, either from fluvial inputs or produced internally, continuously settle from surface waters through a vertical redox gradient in the hypolimnion. Because Mn(IV) is reduced at a higher redox potential, particulate Mn(IV) is reduced to soluble Mn species, such as Mn(III) and Mn(II), in the upper hypolimnion, where it accumulates in the relatively stable reduced form. In contrast, because Fe(III) is reduced at lower redox potential, particulate Fe(III) can settle deeper into the anoxic hypolimnetic water column. After having settled into deep and highly reduced bottom waters, particulate Fe(III) can be reduced to soluble Fe(II) and accumulate in bottom waters.
Two other phenomena may account for the sharp decrease in Fe at a depth of 12 m. One is the abiotic reduction of particulate Mn(IV) by soluble Fe(II). When settling Mn(IV) comes in contact with Fe(II), Mn(IV) can be reduced abiotically to Mn(II) by stripping an electron from Fe(II), resulting in freshly oxidized Fe(III), which in its new particulate form settles out of the water column. As a result, Fe(II) can be inhibited from migrating up into the water column in the presence of Mn(IV). This mechanism was identified by Hongve (Citation1997) as the key sink for Fe in the water column of meromictic Lake Nordbytjernet, Norway.
Another potential loss mechanism for Fe(II) in deep hypolimnetic waters is through Fe(II) sulfide (FeS) precipitation. As sulfide concentration increases, hydrogen sulfide (HS−) can bind with Fe(II), forming particulate FeS (Murray Citation1995). Based on solubility relationships presented in Beutel (Citation2000), including an Fe(II) activity coefficient of 0.72 and FeS solubility product of 3.0, the potential for FeS formation in the hypolimnion of North Twin Lake can be assessed. Assuming a typical Fe(II) concentration (3000 μg/L) and pH (6.5), FeS would form at sulfide concentrations of around 0.3 mg/L. Sulfide was detected via a serendipitous sniff test in water samples near the bottom observed prior to oxygenation (), suggesting that FeS formation may have been a sink for Fe(II) in bottom waters of North Twin Lakes prior to oxygenation.
Loss of iron and manganese after oxygenation
The rapid loss of Fe from the water column with the onset of oxygenation, while dramatic, followed conventional Fe oxidation kinetics. Fe(II) is sensitive to abiotic oxidation and rapidly oxidizes when exposed to oxygen. The half-life of Fe(II) in air-saturated solutions at pH 7 has been reported to range from 27 to 380 min (Davison Citation1993). Based on estimates of initial and final Fe(II) concentrations (830 and 140 μg/L, respectively; ), and the initial 8 hr duration of oxygenation, the half-life for Fe(II) in North Twin Lake was 190 min, which falls within the range of laboratory observations. Unlike Fe(II), abiotic oxidation of Mn(II) is slow in natural waters, and this mechanism likely acted as a negligible sink for Mn during the first few hours of the oxygenation test. Typical half-lives of Mn(II) in natural waters are on the order of months (Davison Citation1993). In studies that have documented relatively rapid removal of Mn(II) from lake waters over days to weeks, loss was attributed to ubiquitous chemolithotrophic Mn-oxidizing microorganisms in aquatic sediments (Wehrli et al. Citation1995, Beutel et al. Citation2008, Bryant et al. Citation2012).
The probable mechanism for the Mn loss observed in the hypolimnion of North Twin Lake during the first few hours of the oxygenation test was Mn(II) co-precipitation with Fe(III), a reaction known to occur in natural waters (Davison Citation1993, Hongve Citation1997). Mn loss may have been enhanced in North Twin Lake by the relatively high Fe to Mn ratio in the water column prior to oxygenation of 7.0; typical waters have an Fe to Mn ratio around 1 (Davison Citation1993). A large quantity of oxidized Fe may have overwhelmed the relatively low quantity of Mn(II). Only a fraction of Fe(III) needed to bind with Mn(II) to result in a substantial loss of Mn from the water column. Fe(III)-mediated Mn(II) removal is also consistent with an observed lack of particulate Mn in the water column.
A key difference between pre- and post-oxygenation concentrations of Fe and Mn was illustrated by the Fe to Mn ratio, which decreased from 7.0 before the oxygenation test to 5.3 one month after the end of the test (). Faster recovery of Mn enrichment, as reflected by a lower Fe to Mn ratio and relatively low peak Fe concentration, is consistent with the tendency of Mn(IV) reduction to occur before Fe(III) reduction upon the depletion of oxygen at the sediment–water interface.
Mass balances for iron and oxygen
A mass balance estimate for the first 8 hours of the oxygenation test suggests that oxygen delivery rates were high enough to account for the mass of Fe(II) lost from the water column, presumably through oxidation and subsequent settling. Average water column hypolimnetic Fe(II) concentrations decreased from around 830 μg/L, the total Fe level on 13 August when the hypolimnion was completely anoxic, to 140 μg/L (total Fe of 240 μg/L, 58% dissolved) after the first 8 hr of the oxygenation test (). The portion of water that moved through the bubble plume during the first 8 hr of the oxygenation test was approximately 7% of the hypolimnion (20% per day for one-third of a day), or 900,000 m3. Combining the observed drop in Fe and volume of water that flowed through the bubble plume system yields an estimated loss of 620 kg of Fe. Given that 1 mole of DO is required to oxidize 4 moles of Fe(II) (Cooke et al. Citation2005), or 0.14 kg of DO for every 1 kg of Fe(II), 90 kg of DO would be needed to account for the Fe(III) oxidized in the hypolimnion. The bubble plume model predicted an oxygen addition rate of 4300 kg/d,, or 1430 kg after 8 hr. Little DO accumulated in the hypolimnion, indicating that DO was rapidly consumed by reduced compounds in the water column. DO increased by only 0.5 mg/L in the water column 8 hr into the oxygenation test, which was equivalent to 45 kg in the water that flowed through the oxygenation system. The mass balance suggests that around 1400 kg of oxygen was consumed in the first 8 hr of the oxygen test, which was an order of magnitude higher than the estimated demand for Fe(II) oxidation.
Total mercury and methylmercury
Mercury in hypolimnetic waters
Hg levels in North Twin Lake prior to oxygenation, including the enrichment of bottom waters with total Hg and MeHg, were characteristic of those observed in other lakes. Hg levels in anoxic hypolimnia in lakes without intensive Hg contamination typically range from 0.1 to 5.0 ng/L for total Hg and 0.01 to 0.8 for MeHg (Watras Citation2009). The sources of Hg to the hypolimnetic waters in North Twin Lake are many and complex. Sources include freshly deposited Hg from direct atmospheric deposition, Hg in watershed runoff, and previously settled Hg in surficial sediments.
Sulfide, a key facilitator of Hg accumulation in anoxic waters, is a byproduct of biological sulfate reduction and a strong ligand for both inorganic Hg and MeHg (Watras Citation2009). Sulfide can strip Hg from settling metal oxides and other seston before it settles onto the sediments (Morel et al. Citation1998). MeHg, in addition to being scavenged from settling seston by sulfide, can also be produced via in situ methylation of Hg(II) by sulfate-reducing bacteria within the water column and in surficial sediments (Benoit et al. Citation2003). Freshly deposited Hg(II) is particularly available for methylation (Chadwick et al. Citation2006). Sediments can be a source of both Hg(II) and MeHg to the hypolimnion through the reduction of oxyhydroxides of Fe(III) and Mn(IV) with subsequent diffusion of newly dissolved Hg species to the overlying water (Chadwick et al. Citation2006, Merritt and Amirbahman Citation2008).
The observed drop in Hg in the hypolimnetic water column during the oxygenation test was likely the result of co-precipitation with Fe(III) and subsequent sedimentation. A number of researchers have implicated Fe and Mn oxides as sinks for water column Hg (Chadwick et al. Citation2006, Merritt and Amirbahman Citation2008). Precipitation of Fe(III) and Mn(IV) in the form of metal oxides can provide a surface for adsorption of a number of metals including Hg, arsenic, copper, zinc, and nickel (Davison Citation1993). In Davis Creek Reservoir, Nebraska, freshly precipitated Mn oxides removed aqueous MeHg from the water column during fall turnover (Gill and Bruland Citation1992, Slotton et al. Citation1995). Unlike North Twin Lake, the water column of Davis Creek Reservoir was rich in Mn relative to Fe. Gill and Bruland (Citation1992) reported a correlation between increased Mn(IV) in surface waters and an increase in particulate-bound Hg, indicating co-precipitation of dissolved Hg with particulate Mn (VI). Hurley et al. (Citation1994) also found a strong correlation between Fe and Hg in 4 northern Wisconsin seepage lakes, where increases in Fe depositional fluxes correlated positively with both Hg(II) and MeHg depositional fluxes from hypolimnetic waters. Chadwick et al. (Citation2006) also reported strong interactions between dissolved species of Hg and DOC in lake waters and the formation of Fe(III)-DOC-Hg complexes under oxic conditions in hypolimnetic waters. A recent study by Matthews et al. (Citation2013) also found that temporal patterns of Fe and MeHg in anaerobic bottom waters of Onondaga Lake, New York, were tightly coupled, suggesting that dissolution of Fe oxides in surfacial sediments was the regulating mechanism controlling MeHg levels in bottom waters.
Implications for lake management
The rapid return to anoxic conditions in the hypolimnion of North Twin Lake after the end of the oxygenation test apparently resulted in the reduction of recently deposited Fe(III), with a concurrent release of Hg back into hypolimnetic waters. Similar dynamics were observed at Round Lake, Minnesota, where the addition of nitrate poised the oxidation–reduction potential and repressed MeHg accumulation in bottom waters (Austin 2013). Once nitrate was exhausted, MeHg concentrations rapidly returned to pre-treatment levels.
The tight coupling of Fe and MeHg levels in relation to the redox status of bottom waters indicates that, to fully repress accumulation of redox-sensitive compounds in bottom waters, hypolimnetic oxygenation must be operated throughout the duration of thermal stratification. In addition, for metal oxides to exert control on the efflux of redox-sensitive compounds from sediment, oxygenation must maintain oxygen levels at the sediment–water interface that promote oxygen penetration into surfacial sediments. Maintenance of oxygenated conditions is especially important late in the season because fall turnover will mix nutrients and Hg accumulated in bottom waters into biologically active surface waters where they can exacerbate eutrophication or bioaccumulate in aquatic biota.
Another implication of this study is that metal oxide content in surfacial sediment needs to be adequate to act as a sink for Hg. This concept is well documented for phosphate binding in lake sediment. For example, Schauser et al. (Citation2006) report that a redox-sensitive phosphorus to Fe ratio >5 is indicative of sediment saturated with phosphorus, which will release phosphorus independent of redox potential at the sediment–water interface. Saturated sediments may also be weak barriers to the efflux of Hg from underlying sediments. This mechanism may not be important, however, because concentrations of ionic Hg and MeHg are generally orders of magnitude lower than phosphate concentrations in sediment, and very little sorption capacity of metal oxides in surfacial sediment would be required to bind dissolved Hg compounds.
Results also suggest that metal addition to sediment may enhance sediment retention of Hg. Metal salts of Fe and aluminum have been used in lakes and reservoirs to repress phosphate release from anaerobic sediments through the formation of metal oxide floc at the sediment–water interface with high sorption capacity (Cooke et al. Citation2005). Aluminum is most commonly used because its oxide form in not redox sensitive. These strategies may have some applicability to Hg control in aquatic systems; however, preliminary work with Hg-contaminated lake sediment showed no inhibition of MeHg efflux from anaerobic sediments covered with a floc of aluminum oxide originating from the addition of buffered sodium aluminate (Beutel et al. Citation2013). Additional work is needed from a lake management perspective regarding how sorption characteristics of metal oxides can be exploited to minimize Hg bioaccumulation in lakes and reservoirs.
Funding
This project was funded in part by the Confederated Tribes of the Colville Indian Reservation, the National Science Foundation, and the Agouron Institute (Pasadena, CA).
Acknowledgments
We thank Dr. Ed Shallenberger and staff of the Colville Confederated Tribes Department of Fish and Wildlife for assistance with field monitoring; Mr. Mark Mobley and staff of Mobley Engineering for assistance with field monitoring; Dr. Gary Gill and Ms. Brenda Lasorsa of the Battelle Marine Sciences Laboratory for assistance with mercury analytical efforts; and the anonymous reviewers for their constructive comments on the manuscript. The views expressed herein are solely those of the authors and do not represent the official policies or positions of any supporting agencies.
References
- [APHA] American Public Health Association. 2005. Standard methods for examination of water and wastewater, 21st ed. Washington (DC): American Public Health Association.
- Austin D. 2013. Suppression of mercury methylation by hypolimnetic liquid calcium nitrate amendment: Results from a field demonstration project in the State of Minnesota, USA. 11th International Conference on Mercury as a Global Pollutant; July 2013; Edinburgh, Scotland.
- Barbash J. 2002. Methods for collecting data on hydrogen sulfide in ground water. . United States Geological Survey Technical Memorandum. September 2002.
- Benoit J, Gilmour C, Heyes A, Mason R, Miller C. 2003. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Cai Y, Braids OC, editors. Biogeochemistry of environmentally important trace element. Washington (DC): American Chemistry Society. p. 262–297.
- Betancourt C, Jorge F, Suarez R, Beutel M, Gebremariam S. 2010. Manganese sources and cycling in a tropical eutrophic water supply reservoir, Paso Bonito Reservoir, Cuba. Lake Reserv Manage. 26:217–226.
- Beutel M. 2000. Dynamics and control of nutrient, metal and oxygen fluxes at the profundal sediment-water interface of lakes and reservoirs [dissertation]. [Berkeley (CA)]: University of California.
- Beutel M. 2006. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol Eng. 28:271–279.
- Beutel M, Dent S, Duvil R, Moore B, Drury D. 2013. Effects of oxygen and nitrate on mercury cycling in natural and contaminated lakes and reservoirs in the Western USA. 11th International Conference on Mercury as a Global Pollutant; July 2013; Edinburgh, Scotland.
- Beutel M, Horne A. 1999. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv Manage. 15(4):285–297.
- Beutel M, Horne A, Roth J, Barratt N. 2001. Limnological effects of anthropogenic desiccation in a large, saline lake, Walker Lake, Nevada. Hydrobiologia. 446:91–105.
- Beutel M, Leonard T, Dent S, Moore B. 2008. Effects of aerobic and anaerobic conditions on P, N, Fe, Mn, and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Res. 42:1953–1962.
- Beutel M, Moore B, Gantzer P, Shallenberger E. 2011. Ease the squeeze in Twin Lakes – The Colville Confederated Tribes work to enhance a trout fishery and improve water quality in reservation lakes. Lakeline. 30(4):31–37.
- Boström B, Andersen J, Fleischer S, Jansson M. 1988. Exchange of phosphorus across the sediment-water interface. Hydrobiologia. 179:229–244.
- Bryant L, Gantzer P, Little J. 2011b. Increased sediment oxygen uptake caused by oxygenation-induced hypolimnetic mixing. Water Res. 45(12):3692–2703.
- Bryant L, Hsu-Kim H, Gantzer P, Little J. 2011a. Solving the problem at the source: Controlling Mn release at the sediment-water interface via hypolimnetic oxygenation. Water Res. 45:6381–6392.
- Bryant L, Little J, Burgmann H. 2012. Response of sediment microbial community structure in a freshwater reservoir to manipulations in oxygen availability. FEMS Microbiol Ecol. 80:248–263.
- Chadwick S, Babiarz C, Hurley J, Armstrong D. 2006. Influences of iron, manganese, and dissolved organic carbon on the hypolimnetic cycling of amended mercury. Sci Total Environ. 368:177–188.
- Christensen D, Moore B. 2009. Using stable isotopes and multiple-source mixing model to evaluate fish dietary niches in a mesotrophic lake. Lake Reserv Manage. 23:167–175.
- Cooke G, Welch E, Peterson S, Nichols S. 2005. Restoration and management of lakes and reservoirs. . 3rd ed. Boca Raton (FL): CRC Press.
- Davison W. 1993. Iron and manganese in lakes. Earth-Sci Rev. 34:119–163.
- Dellwig O, Schnetger B, Brumsack H, Grossart H, Umlauf L. 2012. Dissolved reactive manganese at pelagic redoxclines (part II): Hydrodynamic conditions for accumulation. J Mar Syst. 90:31–41.
- Drury D. 2011. Methyl mercury production and control in lakes and reservoirs contaminated by historic mining activities in the Guadalupe River Watershed. . Progress report from the Santa Clara Valley Water District to the California Regional Water Quality Control Board. 92 p.
- Eckley C, Hintelmann H. 2006. Determination of mercury methylation potentials in the water column of lakes across Canada. Sci Total Environ. 368:111–125.
- Gantzer P, Bryant L, Little J. 2009a. Controlling soluble iron and manganese in a water-supply reservoir using hypolimnetic oxygenation. Water Res. 43:1285–1294.
- Gantzer P, Bryant L, Little J. 2009b. Effect of hypolimnetic oxygenation on oxygen depletion rates in two water-supply reservoirs. Water Res. 43:1700–1710.
- Gill B, Bruland K. 1992. Mercury speciation and cycling in a seasonally anoxic freshwater system: Davis Creek Reservoir. . Electric Power Research Institute (EPRI). 80 p.
- Golterman H. 2001. Phosphate release from anoxic sediments or ‘What did Mortimer really write?’ Hydrobiologia. 450:99–106.
- Herrin R, Lathrop R, Gorski P, Andren A. 1998. Hypolimnetic methylmercury and its uptake by plankton during fall destratification: A key entry point of mercury into lake food chains. Limnol Oceanogr. 43(7):1476–1486.
- Hongve D. 1997. Cycling of iron, manganese, and phosphate in a meromictic lake. Limnol Oceanogr. 42(4):635–647.
- Hurley J, Watras C, Bloom N. 1994. Distribution and flux of particulate mercury in four stratified seepage lakes. In: Watras C, Huckabee J. editors. Mercury pollution: Integration and synthesis. New York: Lewis. p. 69–81.
- Matthews D, Babcock D, Nolan J, Prestigiacomo A, Effler S, Driscoll C, Todorova S, Kuhr K. 2013. Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake, NY. Environ Res. 125:52–60.
- Merritt K, Amirbahman A. 2008. Methylmercury cycling in estuarine sediment pore waters (Penobscot River estuary, Maine, USA). Limnol Oceanogr. 53(3):1064–1075.
- Moore B, Cross B, Beutel M, Dent S, Preece E. 2012. Newman Lake restoration: A case study. Part III. Hypolimnetic oxygenation. Lake Reserv Manage. 28:311–327.
- Morel F, Kraepiel A, Amyot M. 1998. The chemical cycle and bioaccumulation of mercury. Ann Rev Ecol Syst. 29:543–566.
- Murray T. 1995. The correlation between iron sulfide precipitation and hypolimnetic phosphorus accumulation during one summer in a softwater lake. Can J Fish Aquat Sci. 52:1190–1194.
- Preusse M, Peeters F, Lorke A. 2010. Internal waves and the generation of turbulence in the thermocline of a large lake. Limnol Oceanogr. 55:2353–2365.
- Schauser I, Chorus I, Lewandowski J. 2006. Effects of nitrate on phosphorus release: Comparison of two Berlin lakes. Acta Hydrochim Hydrobiol. 34:325–332.
- Singleton V, Gantzer P, Little J. 2007. Linear bubble plume model for hypolimnetic oxygenation: Full-scale validation and sensitivity analysis. Water Resour Res. 43(2):w02405, . doi;10.1029/2005WR004836
- Singleton V, Little J. 2006. Designing hypolimnetic aeration and oxygenation systems – A review. Environ Sci Technol. 40:7512–7520.
- Slotton D, Reuter J, Goldman C. 1995. Mercury uptake patterns of biota in a seasonally anoxic northern California reservoir. Water Air Soil Poll. 80:841–850.
- Swain E, Jakus P, Rice G, Lupi F, Maxson P, Pacyna J, Penn A, Spiegel S, Veiga M. 2007. Socioeconomic consequences of mercury use and pollution. Ambio. 36(1):45–61.
- [USEPA] United States Environmental Protection Agency. 2001. Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. Washington (DC): EPA-821-R-01-020.
- [USEPA] United States Environmental Protection Agency. 2002. Method 1631, revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Washington (DC): EPA-821-R-02-019.
- [USEPA] United States Environmental Protection Agency. 2009. National listing of fish advisories. Washington (DC): EPA-823-F-09-007.
- Watras C. 2009. Mercury pollution in remote freshwater lakes. In: Likens G, editor. Encyclopedia of inland waters. New York: Elsevier. p. 100–109.
- Welch E, Jacoby J. 2001. On determining the principle source of phosphorus causing summer algal blooms in western Washington lakes. Lake Reserv Manage. 17(1):55–65.
- Wehrli B, Friedl G, Manceau A. 1995. Reaction rates and products of manganese oxidation at the sediment-water interface. In: Huang C, O’Melia C, Morgan J, editors. Aquatic chemistry: Interfacial and interspecies processes. Advances in chemistry series. San Francisco (CA): American Chemical Society. Vol. 244. p. 111–134.