Abstract
Hatchery-reared fish are stocked widely to enhance recreational fisheries but are often consumed by predators. Stable isotope analyses were used to evaluate tiger muskellunge (northern pike [Esox lucius] × muskellunge [E. masquinongy]) predation on stocked salmonids (Oncorhynchus) relative to naturally reproducing white suckers (Catostomus commersonii), in 5 Colorado reservoirs. Stable isotope analyses coupled with a mixing model using a Bayesian framework indicated that tiger muskellunge primarily consumed stocked salmonids (53–84% by mass). These results suggest that stocking salmonids into systems that contain tiger muskellunge (and potentially other predators) may result in losses of valuable stocked fish. Further, the use of tiger muskellunge or other piscivores as biological control of less desirable species to benefit sympatric salmonid populations may be counterproductive to management goals. Finally, this study demonstrates the potential for managers to use this framework as a tool to identify and evaluate unintended losses of fishes to piscivores in other systems.
Fish are stocked globally for various purposes including providing sport fisheries for anglers and enhancing native species restoration efforts; however, stocked fish are often consumed by predators. For example, rainbow trout (Oncorhynchus mykiss) are stocked widely (Halverson Citation2010), but even rainbow trout >150 mm are consumed by piscivores (Yule et al. Citation2000, Lepak et al. Citation2012b). Kekäläinen et al. (Citation2008) found that a population of ∼1500 northern pike (Esox lucius) >40 cm consumed ∼29% of 40,000 stocked Atlantic salmon (Salmo salar) smolts in a 2.5 km reach of a northern Baltic river over the course of 7 d. In addition, introduced nonnative piscivores are frequently related to declines in native fish populations (Findlay et al. Citation2000, Muhlfeld et al. Citation2008), and native fish restoration efforts can be hindered when nonnative piscivores consume stocked native fish (Karam and Marsh Citation2010).
Hatchery-reared fish are generally not as successful at avoiding predators relative to wild fish; thus, hatchery-reared fish typically experience higher mortality rates when compared to wild fish (Olla et al. Citation1994, Citation1998, Weber and Fausch Citation2003). Mesa et al. (Citation1994) reviewed studies that showed prey fish not previously exposed to predators were more vulnerable to predation relative to those that were because of differences in behavior. These differences are thought to result from hatchery-reared fish having relatively limited exposure to predators (Suboski and Templeton Citation1989, Brown and Smith Citation1998, Ferrari et al. Citation2010).
Although it is has been known for decades that stocked fish are vulnerable to predation (Johnson and Hasler Citation1954), stocking fish in systems where piscivores are present still occurs. For example, Tiger muskellunge (northern pike [Esox lucius] × muskellunge [E. masquinongy]) have been stocked in lakes and reservoirs in 18 US states (Kutcha Citation2004) to suppress undesirable white sucker (Catostomus commersonii) populations (Kerr and Lasenby Citation2001) and to create recreational fisheries (Wingate Citation1986). In Colorado for example, tiger muskellunge have been stocked in more than 40 systems that are surveyed regularly and sustain naturally reproducing white sucker populations. These systems also receive hatchery-reared rainbow trout and other salmonids vulnerable to predation by large predators (J.M. Lepak, Colorado Parks and Wildlife, unpubl. data).
Esocids prefer to consume fusiform, soft-rayed prey species with high energy densities relative to other species (Wahl and Stein Citation1988). Specifically, northern pike have been shown to prefer to consume rainbow trout over white suckers when both were available in small, artificial pond systems (Lepak et al. Citation2012a). Additionally, elevated growth rates and body condition in top predators have been correlated with stocking of soft-rayed prey fish (salmonids) with high energy density and minimal handling time (Marwitz and Hubert Citation1997, Johnson and Martinez Citation2000, Flinders and Bonar Citation2008). These findings suggest that apex predators are taking advantage of energy subsidies in the form of stocked fish.
Dietary habits inferred from stable isotope analysis were used to evaluate tiger muskellunge predation of stocked salmonids and wild white suckers living in sympatry with tiger muskellunge. Although esocid dietary habits (northern pike primarily) have been evaluated previously in small experimental systems or inferred from diet data with limited temporal scale (e.g., Flinders and Bonar Citation2008, Lepak et al. Citation2012a), tiger muskellunge diets specifically have not been evaluated across multiple (5) reservoirs using stable isotope analysis. This technique is beneficial because isotopic signatures used to characterize diets of large piscivores are integrated across time, and the mixing model is able to account for variability in isotopic signatures of prey sources. This technique is also nonlethal and can be applied with relatively low sample sizes, which is often necessary when studying large predators. For example, McCauley et al. (Citation2012) used this technique to characterize diets of 2 types of reef shark species (n = 53 and 9) and one snapper species (n = 30) in a tropical marine ecosystem. Here, the same technique is used with the objective to characterize the diets of tiger muskellunge in 5 freshwater systems to better understand their interactions with stocked salmonids intended for recreational fisheries.
Study site
Five Colorado reservoirs (Big Creek, Clear Creek, De Weese, Parvin, and Pinewood) were selected as study systems because they had similar species compositions and stocking histories. These systems were typical of most Colorado reservoirs with steep sides, relatively little vegetation (with the exception of some macrophytes near the inlets of Big Creek and Clear Creek reservoirs), and rock and sediment substrates. The primary forage species available in these reservoirs were stocked salmonids and white suckers. All 5 systems supported naturally reproducing white sucker populations and had been stocked intermittently (when available from 1990 through 2009) with tiger muskellunge at varying densities to control white sucker populations considered too abundant and competing for resources with stocked salmonids ().
Table 1 Study reservoir characteristics and tiger muskellunge (TGM) stocking history. The mean annual TGM stocking density represents the average number of TGM stocked per ha from the years when they were available (1990 through 2009) to stock in each system. Because TGM availability was intermittent, the number of years they were available in each system is also provided.
Numbers of tiger muskellunge to satisfy management goals were not available every year during this period, but Big Creek, De Weese, and Pinewood reservoirs were stocked regularly during this period (), while Clear Creek and Parvin reservoirs received tiger muskellunge for 3 consecutive years each (2004–2006 and 2001–2003 respectively). All 5 systems continue to be stocked several times every year with large (>225 mm total length [TL]) salmonids, primarily rainbow trout, cutthroat trout (O. clarkii), and their hybrids (crosses of rainbow trout strains with each other and crosses of rainbow trout with cutthroat trout) to create or enhance recreational fisheries. In addition to large salmonids, all 5 systems experienced stocking of a limited biomass (<20% by weight in all cases) of relatively small (fish <100 mm TL not vulnerable to angling when stocked) salmonids of a variety of species depending on the reservoir, but primarily consisting of rainbow trout, rainbow trout hybrids, kokanee salmon (O. nerka), and brown trout (Salmo trutta).
The large salmonids stocked repeatedly throughout the growing season experienced poor or no growth. Stocked fish generally did not overwinter, with the exception of a small portion (by weight) of small salmonids in Clear Creek Reservoir (kokanee salmon <100 mm) and Parvin Reservoir (rainbow trout <100 mm) that grew to sizes vulnerable to anglers. More specifically, just prior to this study throughout the 2011 growing season, Big Creek, Clear Creek, De Weese, Parvin, and Pinewood reservoirs were stocked with 36, 49, 33, 29, and 139 kg/ha of salmonids, respectively, of which 87, 97, 98, 81, and 99%, respectively, were large salmonids by weight.
Recent surveys of the study reservoirs by Colorado Parks and Wildlife biologists using boat electric fishing, gillnets, and trapnets indicated that rainbow trout and white suckers compose more than 75% of the catch by number in all 5 systems (). Tiger muskellunge were stocked in all 5 systems to control white sucker populations and have been captured regularly during routine monitoring efforts or recreational angling since they were stocked.
Table 2 Reservoir sampling efforts. The most recent effort (provided in hours) for various gear types (EF = night boat electric fishing, GN = overnight gillnet set, TN = overnight trapnet set) are provided for each study reservoir by season and year. Species composition of the catch (RBT = rainbow trout, WHS = white sucker, and Other = all other species captured; e.g., tiger muskellunge, brown trout, and kokanee salmon) is provided as a percentage of the total number of fish caught. Sample size (n), mean length (mm) and standard deviations (SD) for rainbow trout (RBT) and white suckers (WHS) are presented for the fish sampled during these surveys.
Materials and methods
Sample collection
Tiger muskellunge (n = 44) were collected from all 5 study reservoirs by boat electric fishing and gillnet from May to November 2011. In Pinewood Reservoir, additional tiger muskellunge (n = 2) were collected in April 2012. All tiger muskellunge were weighed (g) and measured (mm), and a sample of muscle tissue was taken with a stainless steel fillet knife from anterior-dorsal musculature (directly behind the head and above the lateral line) for stable isotope analyses (SIA). All fish tissue samples were frozen and stored at −20 C until SIA were conducted.
Prey fish (rainbow trout, white suckers, cutthroat trout, and kokanee salmon) were primarily collected by boat electric fishing, gillnet, and trapnet from May to November 2011 for SIA. In addition to these collections, 2 opportunities arose to collect supplementary prey fish samples while in the field. First, 17 small (<100 mm TL) rainbow trout were collected from the stomachs of tiger muskellunge sampled from Parvin Reservoir in November 2011. These rainbow trout were primarily intact but had partially digested fins and skin that were not used for SIA. Given that digestive enzymes had not made contact with the internal muscle tissues used for analyses, no bias was expected. Second, 11 rainbow trout and 10 white suckers were collected in Pinewood Reservoir in April 2012 using gillnets. Prey fish were weighed (g), measured (mm) and sacrificed for SIA. Anterior-dorsal musculature tissue (directly behind the head and above the lateral line) was taken from rainbow trout and white suckers with a stainless steel fillet knife. All samples were stored at −20 C until SIA were conducted.
Stable isotope analyses
Individual fish tissue samples collected were analyzed for stable isotopes δ13C and δ15N to estimate tiger muskellunge diet composition over approximately the past year, which enabled us to evaluate a time frame containing multiple stockings of potential forage (salmonids) as well as constant occurrence of white sucker. This time frame is an estimate based on stable isotope tissue turnover rates for relatively slow-growing fish (Hesslein et al. Citation1993). Stable isotopes provide a means to quantify multiple sources (e.g., hatchery-derived vs. produced within a particular system) of energy assimilated by an organism from their diets integrated across time, which is an improvement over intermittent stomach content data that represents a short-term indication of diet unless intensive and repetitive sampling is conducted (Peterson and Fry Citation1987). Further, stable isotope signatures can be used in the study reservoirs to differentiate prey species produced within the systems themselves versus those originating from the hatchery, largely due to the marine signature of the feed consumed by the hatchery-reared fish. Muscle tissue samples were dried ≥48 h at 60 C and then homogenized prior to analysis. Stable isotope analyses were performed using a Carlo Erba NC2500 elemental analyzer interfaced to a Thermo Finnigan MAT Delta Plus at Cornell University's Boyce Thompson Stable Isotope Facility (Ithaca, NY).
Isotopic standards were materials routinely calibrated against internationally approved reference materials provided by the International Atomic Energy Association. These standards were selected historically based on their ability to sufficiently characterize different quality control and assurance metrics and ensure data quality and comparability across laboratories. Values presented for each standard here are n (sample size); δ13C, the corrected isotope delta value (in parts per thousand) of 13C measured against the primary reference scale of Vienna Pee Dee Belmnite; δ15N, the corrected isotope delta value (in parts per thousand) of 15N measured against the primary reference scale of Atmospheric Air; C, the elemental proportion of the sample that is carbon in parts per hundred; and N, the elemental proportion of the sample that is nitrogen in parts per hundred.
Standards used for normalization correction were brown trout (n = 43, δ13C = −25.58‰, δ15N = 17.31‰, 48.53% C, 12.91% N) and corn (n = 43, δ13C = −11.66‰, δ15N = 0.90‰, 45.29% C, 2.01% N). Standards used to determine isotopic precision were mink (n = 65, δ13C = −25.58‰, δ15N = 11.34‰, 49.99% C, 13.42% N) and rice (n = 11, δ13C = −29.16‰, δ15N = 1.02‰, 39.64% C, 4.17% N). A methionine standard (n = 32, δ13C = −27.63‰, δ15N = −4.67‰, 41.04% C, 9.64% N) was used to determine instrument linearity during analyses. The standard error from the mean of each standard never exceeded 0.1‰ (range = 0.01–0.08‰) during isotopic runs to determine sample δ13C and δ15N signatures.
Samples that vary widely from a C:N ratio of 3.32 require adjustments in δ13C to account for lipid content and differential carbon stable isotope fractionation if lipid extractions are not performed (Post et al. Citation2007). Thus, the following correction (Equationequation 1(1) ) was applied to each individual sample:
(1)
where δ13Cnormalized = normalized δ13C, δ13Cmeasured = measured δ13C, and C:N in the ratio of carbon to nitrogen in the sample. This correction accounts for the differential fractionation of δ13C between muscle and lipid tissues.
Estimation of tiger muskellunge diet composition
Once fish tissue stable isotopic signatures were established, a mixing model (MixSIR; Semmens and Moore Citation2008) was used to estimate the proportion (set to sum to 100%) of different prey species composing tiger muskellunge diets in each reservoir. Briefly, MixSIR is a graphical user interface in a MATLAB (MathWorks) platform that carries out stable isotope mixing models using sampling–importance–resampling (an algorithm used to obtain a random sample from a target distribution) to develop a posterior probability of the proportion of a given prey species in tiger muskellunge diets.
Stable isotope analyses used to estimate diet proportions have limitations related to uncertainty of isotopic signatures; however, MixSIR can explicitly account for uncertainty in isotopic values when estimating the contributions of prey sources to an isotope mixture and characterize uncertainty in the estimates of source contributions based on underlying uncertainty in the mixture and source isotopic signatures (Semmens and Moore Citation2008). Default values for isotopic trophic fractionation (the increase in heavy isotope in an organism with an increase of one trophic level) of 0.4% for δ13C and 2.3‰ for δ15N for aquatic systems (McCutchan et al. Citation2003) were used within the model to estimate diet composition. Inputs included the mean and standard deviation of δ13C and δ15N signatures for each prey item sampled pooled by species for each reservoir, and δ13C and δ15N signatures for tiger muskellunge grouped by reservoir (one estimate for each reservoir).
For each reservoir estimate, 1,000,000 iterations were run, a number that seemed appropriate because in all cases there were >1000 posterior draws (44,822–203,048), no duplicate draws in the posterior chain, and the ratio between the posterior at the best draw and the total posterior density was <0.01. These metrics ensure that the resulting histogram surface has converged on the true posterior likelihood surface, that the number of iterations run was high enough to appropriately develop the posterior distribution, and that the resulting distribution has plausible geometry. For further detail consult Semmens and Moore (Citation2008).
Results
Tiger muskellunge (n = 46) ranging from 658 to 1110 mm TL (TL mean = 885 mm; standard deviation = 108 mm) were collected from the study reservoirs for stable isotope analyses (). A variety of large stocked rainbow trout, cutthroat trout, and their hybrids (crosses of rainbow trout strains with each other and crosses of rainbow trout with cutthroat trout) and fish stocked at small sizes (kokanee salmon in Clear Creek Reservoir and rainbow trout in Parvin Reservoir) were collected for SIA as input for the mixing model ().
Table 3 Number (n) and size of fishes (TGM = tiger muskellunge and WHS = white suckers) collected for stable isotope analysis and mixing model input by study reservoir. Mean total lengths (TL) in mm and standard deviations (SD) of TL are provided. Salmonids presumed to be stocked at <100 mm TL (all kokanee salmon from Clear Creek Reservoir and rainbow trout <100 mm TL from Parvin Reservoir) were designated as small salmonids while all others were designated as large salmonids. “—” indicates that no fish were collected for stable isotope analysis.
Based on mixing model results, posterior probabilities of estimated proportions of prey species consumed by tiger muskellunge indicated that overall in every study reservoir, tiger muskellunge were consuming more salmonids (median = 53–84% of diet by mass; ) than white suckers (median = 16–47% diet by mass; ). Posterior probabilities of estimated proportions of tiger muskellunge diets by species (explicitly accounting for uncertainty in prey δ13C and δ15N signatures and isotopic fractionation by trophic level) are an indication of estimate precision. For example, there was little overlap in the posterior probabilities of estimated proportions of large salmonids and white suckers consumed by tiger muskellunge collected in Big Creek Reservoir (, panel a), making that estimate more precise relative to the estimated proportions of large salmonids and white suckers consumed by tiger muskellunge collected in De Weese Reservoir (, panel c) where the estimate overlap was more pronounced.
Table 4 Mixing model estimates (25th confidence interval [CI], median and 75th CI) of the percent prey item (WHS = white suckers) composition of tiger muskellunge diets by study reservoir. Total median stocked salmonid consumption is the sum of large plus small salmonid median estimates. Salmonids presumed to be stocked at <100 mm TL (all kokanee salmon from Clear Creek Reservoir and rainbow trout <100 mm from Parvin Reservoir) were designated as small salmonids while all others were designated as large salmonids. “—” indicates that no fish were collected for modeling purposes.
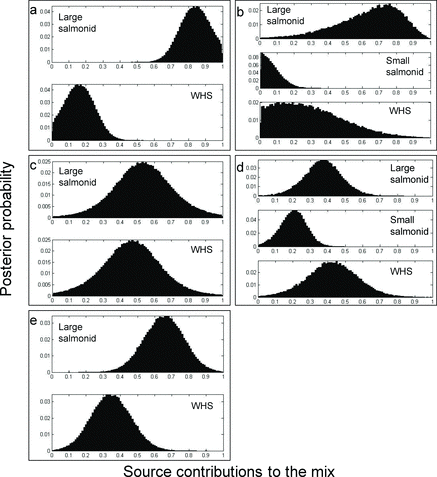
The overlap in the posterior probabilities of estimated proportions of large salmonids and white suckers consumed by tiger muskellunge in Pinewood Reservoir (, panel e) was somewhere in between. This inherent uncertainty in the model estimates can be evaluated by visually comparing the overlap in posterior probabilities of estimated proportions of prey items consumed by tiger muskellunge in each system () and by comparing overlap in the confidence intervals (25th and 75th percentiles) provided (). According to the mixing model results, however, the majority of the biomass of prey being consumed by tiger muskellunge in every reservoir was stocked salmonids, with large salmonids (rather than smaller rainbow trout and kokanee salmon) being the more prevalent prey items ().
Discussion
Mixing model results indicated that tiger muskellunge sampled in this study consumed more stocked salmonids than wild prey species (i.e., white suckers) in all 5 study systems whether stocked salmonids or white suckers were the dominant species captured during routine sampling efforts. This supports the findings of a smaller-scale study conducted by Lepak et al. (Citation2012a) who found that esocids (northern pike) preferentially consumed rainbow trout relative to white suckers in replicated pond systems, whether rainbow trout and white suckers were present in equal numbers (50:50) or rainbow trout were less abundant (20:80).
Predator sample size was small in this study, but results were similar across reservoirs in that tiger muskellunge consumed more stocked salmonids (small and large combined) than white suckers. It is noteworthy that the proportion of stocked salmonids consumed in the study systems is conservative. Despite repeated stocking during the growing season, it is possible that salmonids begin to uptake some energy after stocking and could be mistaken for wild fish isotopically over time. As a result, estimates of tiger muskellunge consumption of stocked salmonids could be biased low. Further, although prey species aside from catostomids and salmonids are relatively rare in the study systems, and because they would have an isotopic signature consistent with in-lake production (i.e., more similar to in-lake white suckers than hatchery-reared fish), these prey species when consumed would produce estimates of white sucker consumption by tiger muskellunge that are biased high. Thus, in this respect, our estimates of the benefits of tiger muskellunge as consumers of white suckers might be higher than estimated in the study systems. Given these findings, additional system-specific investigations may be warranted to evaluate if unintended losses of stocked salmonids (or other desirable species) to predation represents an impediment to achieving management objectives.
In previous studies of esocid predation on stocked salmonids, individual predators consumed approximately 0.5 to 1 prey fish per day (Kekäläinen et al. Citation2008, Lepak et al. Citation2012a, Citation2012c). Assuming a consumption rate of 0.5 fish per day per tiger muskellunge, and that target densities of tiger muskellunge were achieved (approximately 4 to 20 individuals per ha; ), the tiger muskellunge populations in the 5 study reservoirs could consume approximately 20,000 (e.g., Parvin Reservoir) to more than a half million (e.g., Clear Creek and De Weese reservoirs) fish annually. Although qualitative, this estimate, combined with the estimated proportions of forage species consumed by tiger muskellunge in this study (medians = 53–84% of diet by mass of stocked salmonids), provides some basis to evaluate the magnitude of the consumption potential of these predators. This is speculative and would vary widely depending on the ecosystem and characteristics like species composition and habitat; however, more detailed calculations to quantify unintended losses of sport fish to predators and the economic impact of those losses have been conducted and were found to present significant challenges to fishery managers (e.g., Johnson and Martinez Citation2000). Thus, when relevant, managers should consider these potential losses when making decisions that influence the food web structure of fish communities.
Note that these results are based on systems where stocked salmonids and naturally reproducing white suckers were available as forage; however, these prey species have the potential to behave differently and inhabit different areas within a system. Further, the life histories of the prey are different (hatchery-reared vs. naturally reproducing), and these characteristics influence how they interact with predators (e.g., ability to recognize and avoid predators, foraging behavior; Mesa et al. Citation1994). Thus, these differences must be considered when making comparisons between these species with respect to their interactions with predators. In addition, the forage (and predator) species present in other systems should be considered when evaluating predator–prey interactions in contexts similar to those presented in this study. For example, proportional salmonid consumption by predators might be lower in systems where salmonids are naturally reproducing (i.e., having had some exposure to predators in the wild) compared to the systems evaluated here.
Esocids (Marwitz and Hubert Citation1997, Flinders and Bonar Citation2008, Lepak et al. Citation2012a, Citation2012c) and other piscivores like walleye (Sander vitreus; Yule et al. Citation2000, Baldwin et al. Citation2003, Lepak et al. Citation2012b) are consuming stocked fish, which is expected given that hatchery-reared fish have relatively limited experience with predators (Suboski and Templeton Citation1989, Brown and Smith Citation1998, Ferrari et al. Citation2010). To address this, researchers have suggested stocking relatively large fish to reduce losses to piscivores (e.g., Flinders and Bonar Citation2008); however, tiger muskellunge (and other relatively large predators) can consume stocked fish that are large, and large fish are generally more costly to produce. Thus, size of fish intended for stocking should be considered in the context of species and size of predators present when attempting to reduce losses of stocked fish to predators (Mittelback and Persson 1998).
Although outside of the scope of this study, piscivores themselves (e.g., tiger muskellunge) may represent an important contribution to the economy, and this value should be considered when interpreting the benefits of various management strategies to meet management goals and maximize effectiveness of fish stocking programs. Despite potential economic contributions of tiger muskellunge to fisheries, these findings indicate that if management objectives are focused on sustaining or improving salmonid fisheries in sympatry with white sucker populations, the use of tiger muskellunge may be counterproductive to management goals.
The definitive approach to avoid unintended losses of sport and/or native fish to predators introduced as biological control agents or other purposes is to not introduce the predators. Similarly, the most effective method to reduce losses of stocked fish to predators is to stock in systems where predators are not present. Large, native predators exist in many managed systems however, and naturally reproducing, nonnative predators continue to be spread across the landscape. Many of these nonnative predators are difficult or impossible to remove or control and have perpetual negative impacts on native species (Findlay et al. Citation2000, Muhlfeld et al. Citation2008, Karam and Marsh Citation2010). Thus, managing systems in the presence of predators (native and introduced) is unavoidable in some cases and may even become more prevalent in the future. In these situations, understanding how to evaluate and quantify the interactions of predators with other species is crucial for managers. This study demonstrates the technique described by Semmens and Moore (Citation2008) as another viable approach for managers to recognize and evaluate food web dynamics in freshwater fisheries.
Acknowledgments
We thank M. Avery, K. Davies, D. Dreiling, M. McGree, W. Pate, G. Policky, G. Schisler, B. Swigle, and their technicians for assistance during the project. We thank the Cornell Stable Isotope Facility for analyses. This manuscript was greatly improved with helpful comments provided by 3 anonymous reviewers, and we are thankful for their assistance.
Funding
Project support was provided by Colorado Parks and Wildlife.
References
- Baldwin CM, McLellan JG, Polacek MC, Underwood K. 2003. Walleye predation on hatchery releases of kokanees and rainbow trout in Lake Roosevelt, Washington. N Am J Fish Manage. 23:660–676.
- Brown GE, Smith RJF. 1998. Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): Conditioning hatchery-reared fish to recognize chemical cues of a predator. Can J Fish Aquat Sci. 55:611–617.
- Ferrari MCO, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: A review and prospectus. Can J Fish Aquat Sci. 88:698–724.
- Findlay CS, Bert DG, Zheng L. 2000. Effect of introduced piscivores on native minnow communities in Adirondack lakes. Can J Zool. 57:570–580.
- Flinders JM, Bonar SA. 2008. Growth, condition, diet, and consumption rates of northern pike in three Arizona reservoirs. Lake Reserv Manage. 24:99–111.
- Halverson A. 2010. An entirely synthetic fish: How rainbow trout beguiled America and overran the world. New Haven (CT ): Yale University Press.
- Hesslein RH, Hallard KA, Ramlal P. 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci. 50:2071–2076.
- Johnson BM, Martinez PJ. 2000. Trophic economics of lake trout management in reservoirs of differing productivity. N Am J Fish Manage. 20:127–143.
- Johnson WE, Hasler AD. 1954. Rainbow trout production in dystrophic lakes. J Wildlife Manage. 18:113–134.
- Karam AP, Marsh PC. 2010. Predation of adult razorback sucker and bonytail by striped bass in Lake Mohave. West N Am Naturalist. 70:117–120.
- Kekäläinen J, Niva T, Huuskonen H. 2008. Pike predation on hatchery-reared Atlantic salmon smolts in a northern Baltic river. Ecol Freshw Fish. 17:100–109.
- Kerr SJ, Lasenby TA. 2001. Esocid stocking: An annotated bibliography and literature review. Peterborough (ON ): Ontario Ministry of Natural Resources Fish and Wildlife Branch.
- Kutcha, J. 2004. Tiger trials. In-Fisherman. 29:34–39.
- Lepak JM, Fetherman ER, Pate WM, Craft CD, Gardunio EI. 2012a. An experimental approach to determine esocid prey preference in replicated pond systems. Lake Reserv Manage. 28:224–231.
- Lepak JM, Hooten MB, Johnson BM. 2012b. The influence of external subsidies on diet, growth and Hg concentrations of freshwater sport fish: Implications for management and fish consumption advisories. Ecotoxicology. 21:1878–1888.
- Lepak JM, Kinzli KD, Fetherman ER, Pate WM, Hansen AG, Gardunio EI, Cathcart CN, Stacy WL, Underwood ZE, Brandt MM, et al. 2012c. Manipulation of growth to reduce mercury concentrations in sport fish on a whole-system scale. Can J Fish Aquat Sci. 69:122–135.
- Marwitz TD, Hubert WA. 1997. Trends in relative weight of walleye stocks in Wyoming reservoirs. N Am J Fish Manage. 17:44–53.
- McCauley DJ, Young HS, Dunbar RB, Estes JA, Semmens BX, Micheli F. 2012. Assessing the effects of large mobile predators on ecosystem connectivity. Ecol Appl. 22:1711–1717.
- McCutchan JH Jr, Lewis WM Jr, Kendall C, McGrath CC. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 102:378–390.
- Mesa MG, Poe TP, Gadomski DM, Petersen JH. 1994. Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. J Fish Biol. 45(Suppl):81–96.
- Mittelbach GG, Persson L. 1998. The ontogeny of piscivory and its ecological consequences. Can J Fish Aquat Sci. 55:1454–1465.
- Muhlfeld CC, Bennett DH, Steinhorst RK, Marotz B, Boyer M. 2008. Using bioenergetics modeling to estimate consumption of native juvenile salmonids by nonnative northern pike in the upper Flathead River system, Montana. N Am J Fish Manage. 28:636–648.
- Olla BL, Davis MW, Ryer CH. 1994. Behavioral deficits in hatchery-reared fish: Potential effects on survival following release. Aquacult Fish Manage. 25(Suppl 1):19–34.
- Olla BL, Davis MW, Ryer CH. 1998. Understanding how the hatchery environment represses or promotes the development of behavioral survival skills. Bull Mar Sci. 62:531–550.
- Peterson BJ, Fry B. 1987. Stable isotopes in ecological studies. Annu Rev Ecol Syst. 18:293–320.
- Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. 2007. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 152:179–189.
- Semmens BX, Moore JW. 2008. MixSIR: a Bayesian stable isotope mixing model, Version 1.0. Available from: http://conserveriugocafe.org/user/brice.semmens/MixSIR
- Suboski MD, Templeton JJ. 1989. Life skills training for hatchery fish: Social-learning and survival. Fish Res. 7:343–352.
- Wahl DH, Stein RA. 1988. Selective predation by three esocids: The role of prey behavior and morphology. T Am Fish Soc. 117:142–151.
- Weber ED, Fausch KD. 2003. Interactions between hatchery and wild salmonids in streams: Differences in biology and evidence for competition. Can J Fish Aquat Sci. 60:1018–1036.
- Wingate PJ. 1986. Philosophy of muskellunge management. In: Hall GE, editor. Managing muskies: A treatise on the biology and propagation of muskellunge in North America. Bethesda (MD ): Am Fish Soc. Special Publication. p. 199–202.
- Yule DL, Whaley RA, Mavrakis PH, Miller DD, Flickinger SA. 2000. Use of strain, season of stocking, and size at stocking to improve fisheries for rainbow trout in reservoirs with walleye. N Am J Fish Manage. 20:10–18.
