Abstract
Fincel MJ, Dembkowski DJ, Chipps SR. 2014. Influence of variable rainbow smelt and gizzard shad abundance on walleye diets and growth. Lake Reserv Manage. 30:258–267.
Prey availability influences growth and condition of walleye (Sander vitreus) in large systems. In Lake Oahe, South Dakota, rainbow smelt (Osmerus mordax) and gizzard shad (Dorosoma cepedianum) are primary prey of walleye, but their abundance varies substantially year to year. To evaluate the influence of gizzard shad and rainbow smelt on walleye diets and growth in Lake Oahe, we compared recent estimates of walleye diets and growth in 2008 through 2010 with those from the late 1990s and early 2000s. Walleye diets differed seasonally with increased piscivory in July and October. In 2008, gizzard shad were the dominant prey item of walleye, representing about 60% of the diets by weight; however, by 2009, gizzard shad declined appreciably in the diet (22%) and were completely absent from walleye diets by 2010. Conversely, rainbow smelt abundance represented 12%, 27%, and 90% of walleye diets by weight in 2008, 2009 and 2010, respectively. Changes in growth corresponded to changes in diets, with the slowest growth occurring when gizzard shad were dominant in the diets and increasing growth every year thereafter. Because gizzard shad are only available during short periods (<3 months) in late summer, walleye can only achieve about 50% of their annual maintenance energy requirements from this prey source. Conversely, rainbow smelt, which are available and consumed year round, provide a continuous energy source that contributes to high growth rates. Nonetheless, when abundant, gizzard shad may provide an important subsidy to Lake Oahe walleye during periods of low rainbow smelt abundance.
Prey fish availability is an important factor regulating piscivore growth and survival in large reservoirs (Ney et al. Citation1986, Porath and Peters Citation1997). In the absence of a dominant prey base, piscivores are often forced to consume a wide range of prey types, resulting in increased energy expenditures and reduced growth and survival (Graeb et al. Citation2008). Moreover, changes in prey fish abundance can have dramatic effects on sport fish growth, condition, and/or abundance (Adams et al. Citation1982, Michaletz Citation1998). Thus, understanding the influence of prey availability on piscivore foraging and growth can have important implications for management, particularly in systems characterized by variable prey resources or systems with multiple prey resources.
Walleye (Sander vitreus) is a top piscivore widely distributed in North America. It is a popular sport fish in the United States (Hushak et al. Citation1986) and the most sought game fish in South Dakota (Gigliotti Citation1999). Lake Oahe, a large Missouri River impoundment, is nationally recognized as a trophy walleye fishery. During the mid-1990s, studies found that walleye diets consisted primarily of rainbow smelt (Osmerus mordax, ∼95%), and smelt consumption contributed to some of the fastest walleye growth rates ever reported for the Missouri River (Bryan Citation1995, Graeb et al. Citation2008). Following high entrainment in the late 1990s, however, rainbow smelt population numbers declined appreciably and walleye were forced to consume other prey resources, resulting in depressed growth and significant increases in mortality (Graeb et al. Citation2008).
In 2002, gizzard shad (Dorosoma cepedianum) became established in Lake Oahe and, although variable from year to year (Fincel et al. Citation2013), their numbers have increased considerably. This increasing prey population may be beneficial because walleye seem to exhibit high growth rates when feeding on gizzard shad (Jester and Jensen Citation1972, Hartman and Margraf Citation1992) when compared to invertebrates or spiny-rayed fish species (Slipke and Duffy Citation1997, Quist et al. Citation2002, Graeb et al. Citation2008). Given the expansion of the gizzard shad population in South Dakota, it is unclear what role gizzard shad have on diets and growth of walleye in Lake Oahe.
Lake Oahe is one of the few systems that contains gizzard shad, rainbow smelt, and walleye; thus, it offers a unique opportunity to examine walleye foraging and growth patterns in the presence of both species. Moreover, as projected climate changes begin to affect species in northern latitudes, gizzard shad will likely experience range expansions; thus, identifying effects of gizzard shad on food webs in northern latitudes is needed (Wuellner et al. Citation2008). In this study, we investigated the energetic importance of gizzard shad and rainbow smelt to walleye in Lake Oahe, South Dakota. Specifically, we used walleye diet and growth data from 2008 through 2010 to evaluate the influence of variable prey abundance on walleye energy allocation and growth in a large Missouri River reservoir. We compare these findings to previous work from the mid-1990s when rainbow smelt were the dominant prey fish and the early-2000s when neither gizzard shad nor rainbow smelt were abundant in the reservoir.
Methods
Study area
Lake Oahe is the second largest impoundment on the Missouri River, extending from Bismarck, North Dakota, to Pierre, South Dakota. At normal pool, the South Dakota portion of Lake Oahe has a surface area of approximately 150,000 ha, with a mean depth of approximately 19 m and a maximum depth of 67 m. The lower reservoir thermally stratifies in the summer and maintains an oxygenated hypolimnion, and this coldwater habitat encompasses about 48,000 ha at operating pool. Lake Oahe is most productive in the upper South Dakota portion of the lake (total phosphorus 0.04–0.1 mg/L), and productivity decreases as you move downstream toward the dam (total phosphorus 0.01 –0.03 mg/L; Fincel Citation2011). The South Dakota portion of Lake Oahe can be classified as mesotrophic to oligotrophic.
Lake Oahe has a diverse fish assemblage. Sport fishes include walleye, sauger (Sander canadensis), chinook salmon (Oncorhynchus tshawytscha), northern pike (Esox lucius), channel catfish (Ictalurus punctatus), and smallmouth bass (Micropterus dolomieui). Primary prey resources include rainbow smelt, spottail shiners (Notropis hudsonius), lake herring (Coregonus artedi), white bass (Morone chrysops), yellow perch (Perca flavescens), freshwater drum (Aplodinotus grunniens), emerald shiners (N. atherinoides), white crappie (Pomoxis annularis), gizzard shad, age-0 sport fish, and various invertebrates.
Prey fish assessment
Common Lake Oahe prey fish were collected in August of 2008, 2009, and 2010 using standard seining techniques (net size 30.5 × 2.5 m; Bonar et al. Citation2009). No coldwater prey fish were collected during the standard prey fish collections, so 15 rainbow smelt were obtained by South Dakota Department of Game, Fish and Parks (SDGFP) deep-water gillnet surveys, and 10 Chinook salmon smolts were obtained from the Cleghorn Springs fish hatchery (Rapid City, SD). All prey fish were measured for total length (mm) and weighed (g).
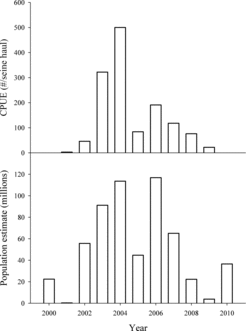
To determine temporal trends in gizzard shad abundance from 2000 to 2010, age-0 gizzard shad were sampled using standard seining techniques. Four quarter-arc hauls were completed at each of 8 shoreline sites spaced equidistant from each other on Lake Oahe, and catch per unit effort (CPUE) was expressed as the number of gizzard shad per seine haul. Additionally, rainbow smelt population estimates were examined during the same time (2000–2010). Rainbow smelt population estimates were generated using hydroacoustic sonar by running 20 transects throughout Lake Oahe and extrapolating densities to surrounding volumes of water to derive lakewide population estimates. For specifics regarding sampling procedures for gizzard shad and rainbow smelt, refer to Nelson-Stastny (Citation2001), Adams et al. (Citation2009), or Fincel et al. (Citation2012).
Walleye collection
Walleye were collected in May, July, and October from 2008 through 2010 in Lake Oahe. Six sites were chosen at equal distances to encompass the South Dakota portion of the lake (). Because walleye feed predominantly during crepuscular periods (Forney Citation1977), experimental mesh gillnets were set just before sunrise and retrieved within 2 h. Captured walleye were measured for total length (TL; mm) and weight (g), then immediately placed on ice. Stomachs were removed within 1 h, and stomach contents were preserved in ethanol for identification in the laboratory. Sagittal otoliths were removed from walleye, sectioned, polished, and used for age determination. Each otolith was aged independently by 2 experienced readers. Any discrepancies in walleye age were reviewed by a third reader.
Walleye diets
In the laboratory, stomach contents of walleye were identified to species when possible, enumerated, and weighed for wet mass. Because prey digestion was minimized by short-term gillnet sets, unidentifiable prey represented <6% of total diets (by weight). We calculated the percent composition by weight for prey items in individual walleye stomachs and averaged this by year class to use as input in a bioenergetics model.
Prey and predator energy density
Prey fish were dried at 60 C for 72 h and energy densities determined via bomb calorimetry by drying whole fish, pulverizing fish to powder, and using a Parr model 1108 oxygen bomb calorimeter (Parr Instrument Company, Moline, IL). After removing stomachs and otoliths, walleye were weighed to obtain a wet weight and then dried at 60 C to obtain dry weight. Dry-to-wet weight ratio of each fish was calculated and used to estimate energy density (J/g wet weight) as reported by Hartman and Brandt (Citation1995). Mean walleye energy densities from each sampling period were used as input parameters in the bioenergetics models.
Statistical analysis
Potential temporal differences in walleye diets among years (2008–2010) were evaluated using a permutational multivariate analysis of variance (PERMANOVA; Anderson Citation2001). The relativized Manhattan distance measure (Faith et al. Citation1987) was applied to fourth-root transformed proportions of individual diet items (MWi), selected because it reduced the skewness of the data better than other transformations. Each value in the data matrix represented the proportion of a given diet item for a given walleye cohort.
Following identification of a significant year-effect, post-hoc multiple comparisons of individual diet items were used to determine species-specific temporal changes. Walleye growth was compared using an analysis of covariance (ANCOVA) to test for differences in length increments between ages 2–3, 3–4, 4–5, 5–6, and 6–7. Due to insufficient sample sizes of age-1 and age-8 and older walleye, we only examined growth patterns of age-2 through age-7 walleye. The PERMANOVA procedure was performed using PRIMER-E statistical software (Clarke and Gorley 2006) with 999 permutations, and the ANCOVA analysis was performed using the SAS statistical software package (SAS 2010). Decision probability for all statistical tests was set at α = 0.10.
Bioenergetics modeling
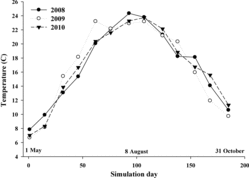
Water temperature data were collected using temperature loggers (Hobo) placed 2–3 m below the water surface at 3 equidistant locations in Lake Oahe. Mean weekly water temperatures were averaged across the reservoir and used as input in the model. In general, seasonal patterns in water temperature were similar from 2008 through 2010; however, due to increasing water levels from above-average rainfall, 2009 showed a faster warming trajectory than 2008 or 2010 ().
We used a bioenergetics model derived from Kitchell et al. (Citation1977) to estimate age-specific consumption by walleye collected from 2008 to 2010 (Hanson et al. Citation1997). We used the energy-balance equation:
(1) where units of G were derived through population measurements; R is a function of fish weight, temperature, diet and activity; and F and U are functions of temperature and diet. We converted the intercept of the allometric function for respiration to joules per gram per day to allow explicit input of differential energy densities of predator and prey (Hanson et al. Citation1997, Lantry et al. Citation2008). We then modeled food consumption based on age-specific growth (initial and final mass, in g), mean weekly water temperature (C), seasonal diet composition of walleye (% wet weight), prey energy density (J/g wet weight), and walleye energy density (J/g wet weight). This walleye bioenergetics model has been applied in a number of field and laboratory studies (Ney Citation1993, Whitledge et al. Citation2006, Lantry et al. Citation2008, Madenjian et al. Citation2010) to quantify prey use.
We used mean monthly length-at-age data for walleye to derive a von Bertalanffy growth function (Gallucci and Quinn Citation1979). Initial and final lengths for each year class were determined using the equation:
(2) where Lt is the length (mm) at time t; L∞ is the mean asymptotic length; K is the growth coefficient; and t0 is a time coefficient at which length would theoretically be zero (). Walleye length was then converted to wet weight using year-specific length–weight regression equations ().
Table 1 Von Bertalanffy and length–weight regression parameters from Lake Oahe walleye in 1994, 2001, and 2008–2010.
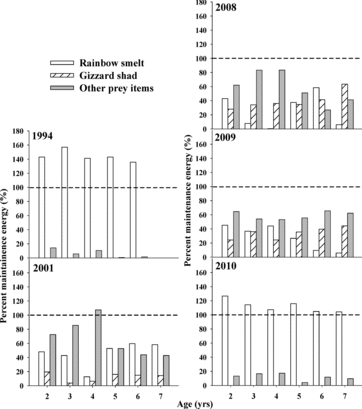
Age-specific von Bertalanffy growth estimates were used as input in the model to predict walleye food consumption from 1 May through 31 October (184 d) each year. The simulation period used here (May–Oct) encompasses the typical walleye growing season in Lake Oahe (Carlander Citation1997, Davis Citation2004). Total consumption of (1) rainbow smelt, (2) gizzard shad, and (3) other prey (as combined categories) generated from the model were compared across years (2008–2010). To evaluate the relative influence of gizzard shad and rainbow smelt on walleye growth, we also compared our results to those reported by Graeb et al. (Citation2008) for 2 time periods: a period characterized by high rainbow smelt use (1994), and a period when both rainbow smelt and gizzard shad were infrequent in the diet (2001).
Because water temperature, fish size, and prey quality can affect the relationship between growth and consumption, we calculated net consumption (kcal) by subtracting maintenance energy requirements incurred over the growing season (184 d) from gross annual food consumption (kcal) generated from observed growth rates (Hewett and Kraft Citation1993). Maintenance energy requirements were obtained by assuming there was no growth, so that initial and final age-specific weights were equal. A positive value for net consumption indicates the amount of surplus energy available for growth after accounting for maintenance costs. A negative value implies that fish did not obtain sufficient energy to meet minimum maintenance requirements (i.e., they lost weight over the sampling interval). To compare age-specific energy intake among years (1993, 2001, 2008–2010), we expressed gross annual consumption for each prey category (i.e., rainbow smelt, gizzard shad, or other) as a percentage of total maintenance cost. This index allowed us to make relative comparisons of prey-specific consumption standardized to the maintenance requirement of the fish.
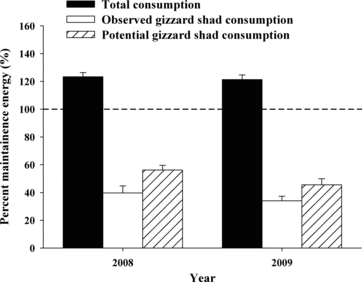
In Lake Sharpe, South Dakota, Wuellner et al. (2010) found that gizzard shad were consumed by walleye as early as June and represented a significant portion of walleye diets (>80%) from August through October. Because our walleye diet sampling may not have coincided with peak density of appropriate-sized gizzard shad for walleye consumption (e.g., Aug), the energetic contribution of gizzard shad could have been underestimated. We therefore modeled potential gizzard shad consumption by applying the observed diet composition in October to 1 August (day 93 of simulation), which likely provided a liberal estimate of gizzard shad use, to mirror the similar time of use found in downstream reservoirs.
Results
Prey fish abundance
Gizzard shad appeared in Lake Oahe standard surveys in 2001 and quickly reached peak abundance of 500 age-0 gizzard shad per seine haul in 2004 (). Following the peak, numbers began to decline owing to colder and longer winters (ice cover >100 d) in 2007, 2008, and 2009. Gizzard shad CPUE dropped from 76/seine haul in 2008 to 22/seine haul in 2009, and no shad were collected in 2010. Rainbow smelt numbers were lowest in 2001, with estimates <1 million individuals. Like gizzard shad, rainbow smelt rebounded quickly and reached population estimates of more than 100 million individuals in 2004 and 2006. Rainbow smelt numbers then dropped to approximately 22 million, 4 million, and 37 million in 2008, 2009, and 2010, respectively.
Walleye diets and growth
We collected 836 walleye from 2008 to 2010, the majority (∼95%) age-2 to age-7. The total number of age-2 to age-7 walleye that had food items in their stomachs was 206 (79.5%), 208 (75.1%), and 210 (70.0%) in 2008, 2009, and 2010, respectively. Gizzard shad were present in walleye diets in 2008 and 2009 but only during the summer and fall sampling periods. In contrast, gizzard shad were completely absent from walleye diets in 2010 (). Conversely, rainbow smelt were present in walleye diets in every season except spring 2008. Invertebrates, primarily ephemerid mayflies, were an important diet item in spring 2008 and 2009 but not in 2010. Nine other fishes were observed in walleye diets, including Chinook salmon, white bass, channel catfish, spottail shiner, emerald shiner, yellow perch, lake herring, freshwater drum, and white crappie.
Table 2 Percent composition (wet weight) of the diets of walleye collected from Lake Oahe in 1994, 2001, and 2008–2010.
Overall, there was a difference in walleye diets among years (F2,618 = 43.8; P < 0.001). The proportion of gizzard shad and rainbow smelt in walleye diets was generally congruent with their relative abundance in the reservoir. Gizzard shad were most prevalent in the diets in 2008 and decreased significantly every year (F2,618 = 32.9; P < 0.001), whereas rainbow smelt were infrequent in the diets in 2008 and significantly increased in occurrence every year thereafter (F2,618 = 114.4; P < 0.001).
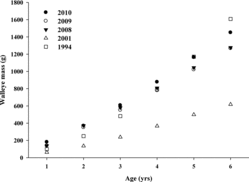
When examining size-at-age relationships, we found no differences in the slope of length increments between 2008 and 2009 (F1,7 = 0.06; P = 0.82), 2009 and 2010 (F1,7 = 0.23; P = 0.65), or 2008 and 2010 (F1,7 = 0.79; P = 0.41). There were also no significant differences in intercepts of length increments between 2008 and 2009 (F1,7 = 0.41; P = 0.54) or 2009 and 2010 (F1,7 = 1.42; P = 0.27). The intercepts of incremental growth trajectories varied between 2008 and 2010 (F1,7 = 4.73; P = 0.07), however, suggesting that walleye in 2010 exhibited consistently larger incremental gains in length between subsequent ages compared to walleye in 2008. Similar to observations in length, mean weight of age-4 and older walleye in 2010 was greater than that in 2008 and 2009 (), although still reduced compared to 1993–1994 when rainbow smelt abundance was considerably higher.
Bioenergetics modeling
Bioenergetics estimates of gross consumption (May–Oct) for walleye age-2 to age-7 ranged from 1453 to 3663 kcal, 1578 to 4099 kcal, and 847 to 4357 kcal for walleye collected in 2008, 2009, and 2010, respectively. During this period, gross consumption was generally higher than in 2001–2002 (421–2857 kcal) but less than that reported during the high rainbow smelt period in 1993–1994 (801–6274 kcal; Graeb et al. Citation2008).
Although gizzard shad represented a significant portion of diets in 2008 and 2009, consumption of shad alone was not sufficient to solely meet maintenance energy requirements of walleye. Gizzard shad represented 28–64% and 24–44% of walleye maintenance requirements in 2008 and 2009, respectively (age-2 through age-7; ). Gizzard shad were not observed in walleye diets in 2010, but rainbow smelt were present in the diets in all years, and walleye were able to exceed maintenance energy requirements in 2010 by consuming only rainbow smelt. Depending on walleye size (age), rainbow smelt represented 1–59%, 6–45%, or 104–127% of walleye maintenance requirements in 2008, 2009, and 2010, respectively. The contribution of prey types other than gizzard shad or rainbow smelt to walleye energy requirements was similar in 2008 and 2009, but lower in 2010 when rainbow smelt were dominant (). Using the potential gizzard shad consumption model, we were able to adjust gizzard shad as a primary diet item to 1 August; however, despite the potential increase of gizzard shad consumption, walleye still failed to meet maintenance energy demands by foraging on gizzard shad alone during this extended modeling period ().
Discussion
As in previous years, walleye growth rates in Lake Oahe are considerably greater when rainbow smelt constitute a larger proportion of their diets. We believe gizzard shad would be a suitable alternative to rainbow smelt, thus producing similar growth rates; however, walleye growth was not as fast when consuming more gizzard shad and less rainbow smelt. The positive effects of rainbow smelt on walleye growth have been observed in several systems (Havey Citation1973, Kirn and Labar Citation1996, Johnson and Goettl Citation1999). Introductions of rainbow smelt or increases in their population abundance have been associated with increases in piscivore growth rates (Jones et al. Citation1994). Although positive effects of rainbow smelt on fish growth have been documented, other studies (e.g., Mercado-Silva et al. Citation2007) have shown negative effects of rainbow smelt on walleye populations via competition and predation with and on juvenile walleye, but this was not explored in the present study.
In the absence of other dominant prey types (i.e., rainbow smelt), gizzard shad are an important prey resource for walleye. In Lake Oahe, walleye derived between 40 and 60% of their maintenance energy requirements by consuming gizzard shad alone. Compared to the early-2000s, gizzard shad represent an important subsidy to Lake Oahe walleye. Other studies report similar findings where walleye growth (Santucci and Wahl Citation1993, Quist et al. Citation2002, Ward et al. Citation2007) and condition (Hartman and Margraf Citation2006) are enhanced by the availability and use of gizzard shad. Additionally, gizzard shad are particularly important when the forage base is dominated by spiny-rayed fishes (Wahl and Stein Citation1988, Einfalt and Wahl Citation1997).
Given the likelihood for increased warming in the Northern Great Plains (Poiani et al. Citation1996), gizzard shad occurrence may become more frequent in Lake Oahe and other systems throughout northern latitudes. Gizzard shad have recently been stocked in several lakes and reservoirs of the Dakotas where they are viewed as a benefit to piscivore growth and size structure (see Wuellner et al. Citation2008 review). Although gizzard shad consumption does not meet minimum maintenance energy demands, in the absence of a large rainbow smelt population, gizzard shad seem to be an important alternative forage source by acting as an energetic subsidy to other prey items in Lake Oahe.
The timing of gizzard shad availability in the Northern Great Plains plays a key role in walleye bioenergetics. Because of colder water temperatures and the subsequent delay in reproduction of gizzard shad, the timing of shad availability is delayed compared to warmer, more southern reservoirs (Wuellner et al. Citation2008). Despite late spawning, gizzard shad in the Northern Great Plains exhibit remarkable growth rates at these northern latitudes (Wuellner et al. Citation2008, Fincel et al. Citation2013). Such fast growth rates could limit foraging opportunities by walleye and other piscivores given the importance of gape-limitation and its effects on foraging efficiency (Persson et al. Citation1996, Nilsson and Brönmark Citation2000). For instance, Kocovsky and Carline (Citation2001) found that because of rapid growth, age-0 gizzard shad were absent from walleye diets as early as September as they grew too large for fish to consume. Additionally, warmer temperatures and faster growth rates could result in potential competition between age-0 gizzard shad and other prey fish or age-0 sport fish, as seen in more southerly systems (Stein et al. Citation1995).
Management implications
Fishery managers face a challenge when managing sport fisheries dependent on prey resources that vary substantially both seasonally and annually. Natural fluctuations in prey fish abundance can have dramatic effects on sport fish consumption and growth. Adding to the variability are catastrophic events that reduce prey populations. For example, a flood event in the late-1990s resulted in high entrainment of rainbow smelt on Lake Oahe and subsequent crash of the population (Graeb et al. Citation2008). Additionally, winter conditions in the late-2000s likely increased overwinter mortality of gizzard shad to a point where they were undetectable in 2010.
Although walleye seem to grow fastest on a diet consisting primarily of rainbow smelt, gizzard shad can make up a large portion of the required energy demand. Moreover, warmer, milder winters associated with climate change would likely improve recruitment and survival of gizzard shad in Lake Oahe. Interactions between gizzard shad and fish communities in systems outside their historic range are mostly unknown; however, natural or intended introductions of gizzard shad in these water bodies may increase walleye growth rates while supplementing the prey base, which could improve survival of other prey species.
Acknowledgments
G. Adams Jr., K. Edwards, R. Hanten, C. Longhenry, J. Lott, K. Potter, and J. Sorenson from South Dakota Game, Fish and Parks; and Dr. D. James, Dr. M. Brown, W. Schrek, B. Swanson, B. VanDeHey, Dr. J. VanDeHey, Dr. D. Willis, Dr. M. Wuellner, and A. Wuestewald provided technical assistance, reviews of earlier drafts, and field and laboratory assistance.
Funding
Funding for this study was provided by Federal Aid in Sport Fish Restoration, Project F-15-R, Study 1515, administered through South Dakota Department of Game, Fish, and Parks. The South Dakota Cooperative Fish and Wildlife Research Unit is jointly sponsored by the U.S. Geological Survey, South Dakota Department of Game, Fish and Parks, South Dakota State University, and the Wildlife Management Institute. Any use of trade names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- Adams G, Edwards K, Hanten R, Smith M, Potter K. 2009. Annual fish population and angler use, harvest and preference surveys on Lake Oahe, South Dakota, 2008. Pierre (SD): South Dakota Department of Game, Fish and Parks; Wildlife Division. Annual Report No. 09-12.
- Adams SM, McLean RB, Huffman MM. 1982. Structuring of a predator population through temperature mediated effects on prey availability. Can J Fish Aquat Sci. 39:1175–1184.
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46.
- Bonar SA, Hubert WA, Willis DW. 2009. Standard methods for sampling North American freshwater fishes. Bethesda (MD): American Fisheries Society.
- Bryan SD. 1995. Bioenergetics of walleye in Lake Oahe, South Dakota [master's thesis]. [Brookings (SD)]: South Dakota State University.
- Carlander KD. 1997. Handbook of Freshwater Fishery Biology, Vol. III. Ames (IA): Iowa State University Press.
- Clarke KR, Gorley RN. 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth (UK).
- Davis BA. 2004. Estimating trophic position of Lake Oahe walleye using stable isotope analysis [master's thesis]. [Brookings (SD)]: South Dakota State University.
- Einfalt LM, Wahl DH. 1997. Prey selection by juvenile walleye as influenced by prey morphology and behavior. Can J Fish Aquat Sci. 54:2616–2626.
- Faith DP, Minchin PF, Belbin L. 1987. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 69:57–68.
- Fincel MJ. 2011. Productivity and trophic interactions of the Missouri River impoundments [dissertation]. [Brookings (SD)]: South Dakota State University.
- Fincel MJ, Chipps SR, Graeb BDS, Edwards KR. 2013. Larval gizzard shad characteristics in Lake Oahe, South Dakota: A species at the northern edge of its range. J Freshwater Ecol. 28:17–26.
- Fincel MJ, Edwards K, Hanten R, Smith M, Potter K. 2012. Annual fish population and angler use, harvest and preference surveys on Lake Oahe, South Dakota, 2011. Pierre (SD): South Dakota Department of Game, Fish and Parks; Wildlife Division. . Annual Report No. 12-06.
- Forney JL. 1977. Reconstruction of yellow perch (Perca flavescens) cohorts from examination of walleye (Stizostedion vitreum vitreum) stomachs. J Fish Res Board Can. 34:925–932.
- Gallucci VF, Quinn TJ. 1979. Reparameterizing, fitting and testing a simple growth model. T Am Fish Soc. 108:14–25.
- Gigliotti LM. 1999. Statewide fish activity and harvest surveys resident and non-resident fishing. Pierre (SD): South Dakota Department of Game, Fish and Parks. . Report HD-9-00.
- Graeb BDS, Chipps SR, Willis DW, Lott JP, Hanten RP, Nelson-Stastny W, Erickson JW. 2008. Walleye response to rainbow smelt population decline and liberalized angling regulations in a Missouri River reservoir. In: Allen MS, Sammons S, Maceina MJ, editors. Balancing fisheries management and water uses for impounded river systems. 4th International Reservoir Symposium, Bethesda (MD). American Fisheries Society. 62:275–291.
- Hanson PC, Johnson TB, Schindler DE, Kitchell JF. 1997. Fish Bioenergetics 3.0. Madison (WI): Wisconsin Sea Grant, University of Wisconsin.
- Hartman KJ, Brandt SB. 1995. Estimating energy density of fish. T Am Fish Soc. 124:347–355.
- Hartman KJ, Margraf FJ. 1992. Effects of prey and predator abundances on prey consumption and growth of walleyes in western Lake Erie. T Am Fish Soc. 121:245–260.
- Hartman KJ, Margraf FJ. 2006. Relationships among condition indices, feeding and growth of walleye in Lake Erie. Fisheries Manag Ecol. 13:121–130.
- Havey KA. 1973. Effects of smelt introduction on growth of landlocked salmon at Schoodic Lake, Maine. T Am Fish Soc. 102:392–397.
- Hewett SW, Kraft CE. 1993. The relationship between growth and consumption: comparisons across fish populations. T Am Fish Soc. 122:814–821.
- Hushak LJ, Morse GW, Apraku KK. 1986. Regional impacts of fishery allocation to sport and commercial interests: a case study of Ohio's portion of Lake Erie. N Am J Fish Manage. 6:472–480.
- Jester DB, Jensen BL. 1972. Life history and ecology of the gizzard shad Dorosoma cepedianum (LeSueur) with reference to Elephant Butte Lake. New Mexico Agricultural Experiment Station Research . Report 218.
- Johnson BM, Goettl JP Jr. 1999. Food web changes over fourteen years following introduction of rainbow smelt into a Colorado Reservoir. N Am J Fish Manage. 19:629–642.
- Jones MS, Goett JP Jr, Flickinger SA. 1994. Changes in walleye food habits and growth following a rainbow smelt introduction. N Am J Fish Manag. 14:409–414.
- Kirn RA, Labar GW. 1996. Growth and survival of rainbow smelt and their role as prey for stocked salmonids in Lake Champlain. T Am Fish Soc. 125:87–96.
- Kitchell JF, Stewart DJ, Weininger D. 1977. Applications of a bioenergetics model to yellow perch (Perca Flavescens) and walleye (Stizostedion vitreum vitreum). Can J Fish Aquat Sci. 34:1910–1921.
- Kocovsky PM, Carline RF. 2001. Influence of extreme temperatures on consumption and condition of walleyes in Pymatuning Sanctuary, Pennsylvania. N Am J Fish Manage. 21:198–207.
- Lantry BF, Rudstam LG, Forney JL, VanDeValk AJ, Mills EL, Stewart DJ, Adams JV. 2008. Comparisons between consumption estimates from bioenergetics simulations and field measurements for walleyes from Oneida Lake, New York. T Am Fish Soc. 137:1406–1421.
- Madenjian CP, Wang C, O’Brien TP, Holuszko MJ, Ogilvie LM, Stickel RG. 2010. Laboratory evaluation of a walleye (Sander vitreus) bioenergetics model. Fish Physiol Biochem. 36:45–53.
- Meerbeek JR, Isermann DA, Willis DW. 2002. Influence of age-0 yellow perch abundance on walleye populations in two eastern South Dakota lakes. Proc SD Acad Sci. 81:93–99.
- Mercado-Silva N, Sass GG, Roth BM, Gilbert S, Vander Zanden MJ. 2007. Impact of rainbow smelt (Osmerus mordax) invasion on walleye (Sander vitreus) recruitment in Wisconsin lakes. Can J Fish Aquat Sci. 64:1543–1550.
- Michaletz PH. 1998. Population characteristics of gizzard shad in Missouri Reservoirs and their relation to reservoir productivity, mean depth and sportfish growth. N Am J Fish Manage. 18:114–123.
- Nelson-Stastny W. 2001. Estimates of abundance, biomass and distribution of rainbow smelt and other pelagic fish in Lake Oahe using hydroacoustic techniques, 1996-1999. Pierre (SD): South Dakota Department of Game, Fish and Parks; Wildlife Division. Annual Report.
- Ney JJ. 1993. Bioenergetics modeling today: Growing pains on the cutting edge. Applications of bioenergetics models to fish ecology and management: Where do I go from here? T Am Fish Soc. 122:1019–1030.
- Ney JJ, Orth DJ, Stroud RH, editors. 1986. Coping with future shock: The challenge of matching predator stocking programs to prey abundance. Fish culture in fisheries management. Bethesda (MD): American Fisheries Society. p. 81–92.
- Nilsson PA, Brönmark C. 2000. Prey vulnerability to a gape-size limited predator: Behavioural and morphological impacts on northern pike piscivory. Oikos. 88:539–546.
- Persson L, Andersson J, Wahlström E, Eklöv P. 1996. Size-specific interactions in lake systems: Predator gape limitation and prey growth rate and mortality. Ecology. 77:900–911.
- Poiani KA, Johnson WC, Swanson GA, Winter TC. 1996. Climate change and northern prairie wetlands: Simulations of long-term dynamics. Limnol Oceanogr. 41:871–881.
- Porath MT, Peters EJ. 1997. Use of walleye relative weights (Wr) to asses prey availability. N Am J Fish Manage. 17:628–637.
- Quist MC, Guy CS, Bernot RJ, Stephen JL. 2002. Seasonal variation in condition, growth and food habits of walleye in a Great Plains reservoir and simulated effects of an altered thermal regime. J Fish Biol. 61:1329–1344.
- Santucci VJ, Wahl DH. 1993. Factors influencing survival and growth of stocked walleye (Stizostedion vitreum) in a centrarchid-dominated impoundment. Can J Fish Aquat Sci. 50:1548–1558.
- SAS Institute. 2010. SAS/STAT user's guide. Cary (NC): SAS Institute.
- Slipke JW, Duffy WG. 1997. Food habits of walleye in Shadehill Reservoir, South Dakota. J Freshwater Ecol. 12:11–17.
- Stein RA, DeVries DR, Dettmers JM. 1995. Food-web regulation by a planktivore: Exploring the generality of the trophic cascade hypothesis. Can J Fish Aquat Sci. 52:2518–2526.
- Wahl DH, Stein RA. 1988. Selective predation by three esocids: The role of prey behavior and morphology. T Am Fish Soc. 117:142–151.
- Ward MJ, Willis DW, Miller BH, Chipp SR. 2007. Walleye consumption and long-term population trends following gizzard shad introduction into a western South Dakota reservoir. J Freshwater Ecol. 22:339–345.
- Whitledge GW, Bajer PG, Hayward RS. 2006. Improvement of bioenergetics model predications for fish undergoing compensatory growth. T Am Fish Soc. 135:49–54.
- Wuellner MR, Chipps SR, Willis DW, Adams WE Jr. 2010. Interactions between walleyes and smallmouth bass in a Missouri River reservoir with consideration of the influence of temperature and prey. N Am J Fish Manage. 30:445–463.
- Wuellner MR, Graeb BDS, Ward MJ, Willis DW. 2008. Review of gizzard shad population dynamics at the northwestern edge of its range. In: Allen MS, Sammons S, Maceina MJ, editors. Balancing fisheries management and water uses for impounded river systems. 4th International Reservoir Symposium, Bethesda (MD). American Fisheries Society. 62:637–653.