Abstract
Urban lakes are important assets to highly populated regions; however, extensive usage and other influences degrade their water quality, which then requires rehabilitation and maintenance. Hyper-eutrophic Swan Lake, Greater Toronto, Canada (5.5 ha, 4.4 m maximum depth) was a gravel pit that became degraded by elevated total phosphorus (TP) concentrations, mostly from internal P sources. Because Swan Lake is a terminal lake with limited flushing and small external load, a phosphate adsorbing and sediment capping agent, lanthanum-modified bentonite (Phoslock), was applied in spring 2013 to intercept the internal load. Average TP concentration decreased from 0.247 to 0.099 mg/L in the first and 0.060 mg/L in the second post-treatment year. A TP mass balance model adequately predicted post-treatment annual average TP concentration by not including the pre-treatment internal load estimate of 650 to 1100 mg/m2/yr. Phytoplankton biomass decreased only in the second post-treatment year, when Secchi transparency (highly correlated with chlorophyll concentration) increased to a growing season average of 1.4 m (range 0.7–2.7) compared to 0.5 m (0.37–0.63) before treatment. We explain the lack of response in the first treatment year with a relatively late application (29 Apr–1 May 2013), when P released from the winter bottom sediments had already been taken up by phytoplankton. Recently, a growing population of waterfowl (mostly Canada goose, Branta canadensis) were the highest contributors of nutrients (75%), as indicated by a mass balance based on literature-derived goose P export and biweekly bird census. We recommend waterfowl management or repeated treatment to further improve water quality.
Urban lakes are an important landscape feature of cities and suburbs. The high density of development and associated human population in cities creates a primarily artificial surrounding with few natural features. Traditionally, parks with ponds have assumed the role to satisfy some quest for nature in these environs. The importance of such features is obvious; many have been included in management plans and have acquired protective status in recent years (Holdren et al. Citation2001).
Urban and suburban stresses can create severely eutrophic conditions in waterbodies, however, resulting in high nutrient concentrations, low oxygen concentration and Secchi disk transparency, and high phytoplankton biomass including cyanobacterial blooms. Consequently, these lakes present a nuisance rather than a recreational asset to visitors of the park environment. Nutrient sources are not always obvious. For example, a population of geese has been spreading on and around waters that favor the typical open environment that lawns in parks provide. Increasing nuisance waterfowl abundance has been reported throughout the northern hemisphere, including the Greylag goose (Anser anser) in Europe (Carss et al. Citation2012) and Canada goose (Branta canadensis) in North America (Unckless and Makarewicz Citation2007).
Newly contributed feces combine with a legacy nutrient load accumulated in the sediment and can prevent improvement of lake water quality (Unckless and Makarewicz Citation2007). In particular, phosphorus (P) undergoes diagenesis in the lake sediments, leading to the release of biologically available phosphate into the open water. Such internal P loading is especially high under eutrophic conditions, including sediment anoxia and high organic content, and when bottom temperature is high (Søndergaard et al. Citation2001).
To determine whether this internal P load is important relative to external P inputs, both loads must be quantified and their contribution to lake P concentration established. Determination of the relative magnitude of P sources, in particular the quantification of internal loading, are most important for integrated lake management, including in-lake restoration techniques (Hickey and Gibbs Citation2009). Although small polymictic urban lakes rarely have the information readily available, models from well-studied lakes can be used to supplement any monitoring data to assess internal P load (Nürnberg Citation2009).
If such analysis indicates internal load is an important nutrient source, a proactive approach of in-lake restoration that removes or binds sediment P and prevents its release into the open water is required (Cooke et al. Citation2005, Zamparas and Zacharias Citation2014). Although many such techniques have been described, a solution for each particular lake depends on its limnology, the expected treatment effectiveness and duration, cost, and regulatory limitations.
This study evaluated the effectiveness of a treatment with a patented formulation of lanthanum-modified bentonite (Phoslock) in severely eutrophied, urban Swan Lake supporting a large population of Canada geese in the Greater Toronto Area, Ontario. Phoslock was developed by the Commonwealth Scientific and Industrial Research Organization (CSIRO) of Australia and has been used in Australia (Robb et al. Citation2003), New Zealand (Burns et al. Citation2009, Hickey and Gibbs Citation2009), China (Liu et al. Citation2012), Europe (Meis et al. Citation2012, 2013, Spears et al. Citation2013, Lürling and Van Oosterhout Citation2013), and the United States (Bishop et al. Citation2014) to treat lakes, rivers, stormwater ponds, and drinking water reservoirs.
Historic and recently observed lake characteristics and P mass balance estimates were compared before and after treatment. Modeled contributions from internal and external P sources to lake total P (TP) concentration during pre-and post-application years were used to evaluate treatment success regarding internal loading. Special consideration was given to the determination of external P input from waterfowl. This in-lake restoration by a Phoslock application is envisioned as the first step of a solution to manage water transparency and high phytoplankton biomass in Swan Lake. Remaining challenges and predictions of future water quality are discussed.
Study site
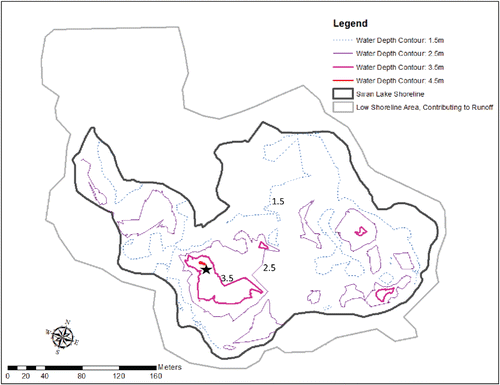
Swan Lake is located in a park-like area in the City of Markham, Greater Toronto, Ontario (43°54′, 79°15′, 208.4 m a.s.l.; mean annual precipitation = 763 mm (2012–2014); daily mean temperature = 7.4 C). This former gravel pit has turned into a highly eutrophic, hardwater lake with a history of cyanobacterial blooms after filling in 1980. Swan Lake has an approximate area of 5.5 ha in a catchment basin 8.3 times its size (, ), but much of the water is captured by 2 storm water management ponds (SWMPs) constructed in the late 1990s that drain most of the basin but have not provided inflow to Swan Lake since 2007. Variable but small runoff is available from the local catchment area, presenting the only inflow other than precipitation directly onto the lake surface. There is no surface outflow or significant groundwater flow ().
Table 1 Swan Lake morphometry, hydrology, and background chemistry.
Areas around Swan Lake have been restored to a park-like setting, and a walkway leading partially around the lake provides a semi-natural atmosphere in this otherwise developed section of the City of Markham. Despite a naturalized shoreline, there is still enough open field and lawn area in the immediate vicinity of the lake to support a sizable flock of Canada geese. The City of Markham has erected signage along the walkway and at the lake's shores to discourage feeding of wildfowl and educate visitors.
Swan Lake is polymictic but is susceptible to occasional stratification because its morphometric ratio is large (; Nürnberg Citation1995), and the lake is moderately protected from winds by a partially elevated shore line. These conditions increase the effect of any potential internal P loading on water quality, causing high phytoplankton biomass and cyanobacterial blooms throughout the growing period.
Limnological characteristics in summers before treatment, including low dissolved oxygen concentration throughout the water column and elevated TP concentration at deeper depths, pointed to internal sedimentary P sources as the primary causes of the water quality problems, including cyanobacterial blooms and low Secchi disk transparency.
Materials and methods
Selection of restoration technique and Phoslock application
Dredging is not usually feasible for in-lake restoration because of high costs and environmental concerns for potential toxicity of the removed material. Techniques applicable to stratified lakes (e.g., hypolimnetic withdrawal; Nürnberg Citation2007) do not apply to shallow polymictic lakes where there is no consistent accumulation of nutrients in the bottom water. Dilution is only possible in systems with a clean water source (Welch and Jacoby Citation2009), and flushing requires a large and consistent water source. In addition, where hypolimnetic anoxia is expected to prevail, redox-sensitive chemicals do not effectively bind P unless an aeration system is installed (Zamparas and Zacharias Citation2014). Further, aeration, oxygenation, and mixing, a frequent treatment of small lakes (Welch et al. Citation2004), do not always manage to aerate the sediments sufficiently to inhibit sediment P release (Gächter and Wehrli Citation1998, Horppila et al. Citation2015) and is useless when release has become independent of the oxygen state during severe eutrophication (Katseva and Dittrich Citation2012). Oxygenation did not improve mixed-layer water quality in 4 of 5 Danish lakes (Liboriussen et al. Citation2009), and destratification has worsened eutrophication and cyanobacterial blooms (Nürnberg et al. Citation2003), although positive results have been reported in reservoirs (Wagner Citation2015). In addition to technical and limnological considerations, societal and regulatory constraints limit the applicability of restoration techniques in specific circumstances (Mackay et al. Citation2014). For example, the Canadian Provinces of Ontario and Quebec do not favor chemical applications involving aluminum (Al) because of recurring public concern about perceived Al toxicity (Gensemer and Playle Citation1999, Reitzel et al. Citation2013).
Of all possible internal load abatement techniques, only P precipitation and capping with either aluminum compounds or Phoslock were deemed limnologically feasible in Swan Lake. These treatments were judged to be especially applicable because there is neither outflow where the chemical could be lost nor large inflow with external nutrient input that could interfere. Of these chemicals, a Phoslock treatment was acceptable to regulatory agencies because of its general lack of toxicity (Ministry of Environment Citation2009, Lürling and Tolman Citation2010) and rapid disappearance of lanthanum in lakes of moderate to high alkalinity (>0.8 mEq/L; Spears et al. Citation2013). In addition, Phoslock, in combination with a low dose of flocculant (iron(III)chloride), has been used successfully for several years to treat internal P loading in a Dutch lake with a large overwintering water fowl population (Waajen et al. Citation2015). The treatment, conducted in spring 2013, was the first application to an urban Canadian lake, although Phoslock was used with limited success in Ontario on storm water management ponds and sections of a slow-moving river (Lake Simcoe Region Conservation Authority Citation2010, Moos et al. Citation2014).
Phoslock consists of bentonite clay with a high exchange capacity, in which naturally adsorbed cations have been replaced by the rare earth element lanthanum (La). In the presence of anions such as orthophosphate, La forms the highly stable mineral called rhabdophane (LaPO4 nH2O; Ross et al. Citation2008). The amount of Phoslock necessary to inactivate P is based on phosphate (soluble reactive P, SRP) in the water at the time of application and releasable P in the sediment (pore water P, iron-bound P, and labile organic P; Reitzel et al. Citation2005). Phoslock contains 5% La by weight, and P is adsorbed by La at a molar ratio of 1:1 (Ross et al. Citation2008). In dosage calculations, only the freely available P components are considered (i.e., SRP in the water and mobile P in the sediment; Meis et al. Citation2012).
We determined the effective dose for Swan Lake from rates established in laboratory experiments on eutrophic lake sediments (Egemose et al. Citation2010, Liu et al. Citation2012). This dose was also close to the combined dosage of 2 applications to a similarly shaped and enriched former gravel pit, Bärensee, in Germany (Spears et al. Citation2015). Although ideally the dose is based on measured quantities of sediment releasable P (Meis et al. Citation2012, Waajen et al. Citation2015) and, less importantly, on water SRP content of the lake to be treated, the extreme trophic state and similar characteristics to already-treated lakes permitted an effective dose estimate by using a comparably high application rate of 4.6 metric tonnes/ha. In comparison, 11 of 16 previous applications had lower application rates (Spears et al. Citation2013). A total of 25.2 metric tonnes of Phoslock was applied from 29 April to 1 May 2013 in an effort to intercept most of the internal P loading in Swan Lake at once and avoid multiple treatments. The chemical was applied by barge to the lake surface by multiple passes across Swan Lake, except for a ∼1 m zone around the shoreline. Slightly higher doses were applied preferentially to the deeper areas. The muddy consistency of the sediment and the lake's high trophic state suggested that most of the bottom sediment was involved in P release.
Sampling and analysis
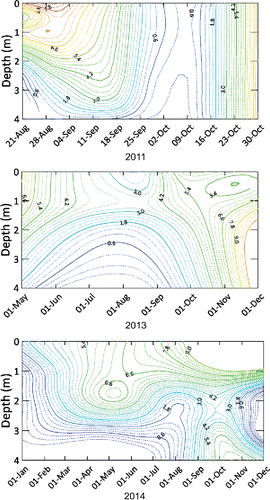
Water temperature and dissolved oxygen (DO) profiles were measured every 0.5 or 1 m from the lake surface to the bottom by submersible probes. Water was collected with a discrete layer sampler at the main deep site () twice monthly during August–October 2011 and approximately monthly May–November in both post-treatment years, 2013 and 2014. In addition, samples were taken once during winters 2012, 2013, and 2014 (on 8 Jan 2014 under ice). Secchi disk transparency was measured on each sampling event during the open water season.
In 2011, TP profiles were measured at 6 separate depths between 0.5 m and the bottom at 4–5 m, and nitrogen (N) was determined at 2 m depth. In 2013 and 2014, TP was determined at 2 (surface and 1 m above bottom) or 4 separate depths between 0.5 m and the bottom at 4–5 m, and N was determined at 2 m depth. SRP was measured at least once during midsummer of each year at 4 depths.
Water samples were analyzed for nutrients, including TP and SRP, and N, and algal biomass indicators with standard methods in the laboratories of Ontario Ministry of the Environment and Climate Change (MOECC) and AGAT Laboratories, a licensed commercial lab. Detection limits for TP and SRP were 0.004 mg/L in 2011 and 2012 and 0.02 mg/L in the post-treatment years. All units are expressed per element (e.g., P or N).
Phytoplankton biomass was determined by chlorophyll a (uncorrected for pheophytin), and Secchi disk transparency in all study years, by cell counts in 2011 (Winter et al. Citation2011), and by qualitative microscopic inspection on 4 dates in 2013. Microsystin was determined at least monthly throughout the study period (MOECC, enzyme-linked immunosorbent assay, ELISA). “True” water color (platinum units) was determined to help distinguish algae from other particles and to use multiple regression models involving Secchi disk transparency, chlorophyll, and TP concentration. La was determined on 8 August 2013 and 23 June 2014 at 1 m below surface.
Sediment was sampled on 25 August 2011 by taking 3 cores at the deep station and twice in each post-treatment year with a Ponar dredge. Basic sediment characteristics (TP, loss on ignition, and water content) and La concentrations were determined in 0–5 or 0–10 cm surface sediment with standard methods by AGAT Laboratories.
Trophic state was determined by Secchi disk transparency; chlorophyll, TP, and total N (TN) concentration; and observed and modeled anoxia according to Nürnberg (Citation1996) using mean growing season surface water layer (0–1 m) values.
External contributions to P budget
Obvious nutrient sources had already been reduced by hydrological bypass involving 2 SWMPs and the separation of runoff from sewage conveyance around Swan Lake.
Precipitation and runoff
The remaining main water source for Swan Lake is direct precipitation on its surface (5.5 ha; ) and runoff from the immediate shoreline area (4.7 ha, runoff coefficient 0.3; City of Markham, Jan 2015, pers. comm.). Average inflow volume into Swan Lake was estimated with a standard hydrological model for 2011–2014 resulting in an annual water load (qs) of 0.070 m/yr ().
TP input from atmospheric deposition was computed as 16.7 mg/m2/yr from long-term average wet and dry fallout for south-central Ontario (Ministry of Environment Citation2010). Most pollutants in shoreline runoff derive from the impervious walkway used by pedestrians and pets (); therefore, the median TP runoff concentration of 0.36 mg/L from residential areas in a number of studies in Ontario and northeastern United States (US EPA 1998; LaZerte and Nürnberg, 2010, unpubl. data) was used to compute export from this area.
Precipitation on the remaining watershed area (35.5 ha) is conveyed away from Swan Lake during storms with <25 mm precipitation. Only water from storms >25 mm, including 5-year event storms, enters Swan Lake. TP input from this source has not been considered in the mass balance because it has not occurred since at least 2007.
Table 2 TP contribution from waterfowl from other studies.
Waterfowl
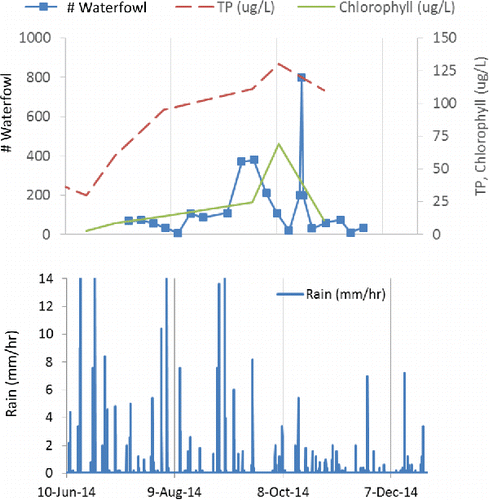
Recognizing that the number of geese is critical to water quality, waterfowl were counted by City of Markham staff almost biweekly from July to November 2014 on Swan Lake (Supplement, Appendix A). We assumed that the waterfowl counts represented an average goose number for the day of observation (i.e., the observed and counted geese would be replaced by others during the day and night) and interpolated that number between observation dates to arrive at an observed waterfowl count for 1 July to 31 December (Supplement, Appendix A).
To quantify the potential waterfowl effect on Swan Lake water quality, we investigated P contribution values for waterfowl from the scientific literature (). The value from nightly resting Canada geese on suburban northeastern US lakes (Moore et al. Citation1998) was used in the TP budget because those conditions seemed most similar to Swan Lake conditions, and waterfowl were strongly dominated by this species. Also present were 4 resident swans and, rarely, some ducks, mostly mallards (Anas platyrhynchos).
We consider the applied egestion value conservative because it is based on a 12 h day (nighttime occupancy only in this suburban Boston lake). We assumed that the waterfowl did not forage much in the lake (little or no P recycling by waterfowl) or that it may be cancelled out by some supplemental feeding by residents, despite signs discouraging this practice.
Assessment and modeling of internal load
Because of the difficulty in separating internal from external P sources in a polymictic lake (Nürnberg Citation2009), we applied a variety of independent approaches to quantify sediment-derived P in Swan Lake, similar to those applied previously (Nürnberg et al. Citation2012, 2013a). In particular we considered the following 4 approaches, depending on monitoring data obtained in 2011–2012 (additional details in Supplement, Appendix B):
in situ internal load from summer–fall water-column TP increases;
internal load determined from active sediment area release factor (AA; Nürnberg et al. Citation2013b) and areal release rates;
net and gross internal load from mass balance; and
winter internal load: P release under ice.
Approach (1) includes a potential settling of the released P and is therefore a partially net estimate. Water column average TP concentrations were available between 25 August and 26 October 2011, and thus this estimate may be low because the TP measurements do not start at the likely onset of anoxia in early summer. Approach (2) presents a gross estimate based on factors and release rates from the active sediment release area. These variables were modeled from water and sediment TP concentration collected in 2011. Approach (3) is based on TP and water budgets but was not used because it requires a more detailed mass balance than was available. For example, P retention cannot be predicted in a lake lacking an outlet and had to be calibrated (discussed later). In Approach (4) we investigated signs of internal load under ice.
Mass balance modelling and determination of lake TP concentration
Average annual TP concentration was modeled according to simple mass balance analysis (Nürnberg Citation2009). The proportion of the P loads that settle to the sediments, and therefore represent the downward flux of TP (without sediment release), is called TP retention (Rsed). Because Swan Lake lacks an outflow and its inflow is extremely small, TP retention models based on hydrology are not applicable. High retention close to one is typical of lakes with an extremely high water residence time and low outflow (Brett and Benjamin Citation2008). We estimated Swan Lake Rsed (Equationequation 1(1) ) by calibration with 2011 model input values (discussed later) as 0.983 and rounded it to
(1)
The prediction of the specific contribution (TPi, mg/L) from the individual model sources (Loadi) to lake TP concentration follows the general mass balance equation (Equationequation 2(2) ):
(2)
where Loadi (g/m2/yr). The prediction of annual average lake TP concentration (mg/L) was accomplished by adding all individual contributions from external and internal loads (Lint and Lext, g/m2/yr, Equationequation 3(3) ) corrected for annual water load (qs, discussed later) to the general mass balance equation:
(3)
We assumed that qs approximates annual water loss as groundwater seepage and evaporation in this closed lake.
Statistical and numerical analysis
Stratification was expressed as the Schmidt stability (Joules(J)/m2, computed from temperature profiles and bathymetry with the statistical program R, The R Foundation for Statistical Computing, 2014, using the “rLakeAnalyzer” subroutine), which indicates the amount of energy required to hypothetically mix the lake (Idso Citation1973). Schmidt stability values are high (>60 J/m2) during thermal stratification periods and lower during mixed conditions. It is most useful as a relative measure of stability patterns within the same lake. Nürnberg (Citation1996) regression equations were used to assess the observed response of the trophic state variables in Swan Lake relative to other lakes.
To avoid assumptions of data distribution and other constraints, nonparametric tests offered by SYSTAT v13 were applied to test for significant differences (P = 0.05) of water quality variables before treatment versus years after the treatment. The Kruskall--Wallis test was used first to compare pre- and post-treatment results. When statistically significant, multiple comparisons were conducted using Dwass--Steel--Chritchlow--Fligner and Fligner--Wolfe tests.
Results and discussion
Pre-and post-treatment limnology and trophic state
Although Phoslock binds any free phosphate (measurable as SRP) that may exist in the water column at the time of application, its main function is capping sediments to reduce or eliminate sediment P release into the open water; therefore, a decrease in lake water TP concentration due to effectively treated internal loading is expected. The ultimate goal of any decrease in internal load is decreased phytoplankton biomass and increased Secchi disk transparency, all indicating an improved trophic state.
Mixing regime
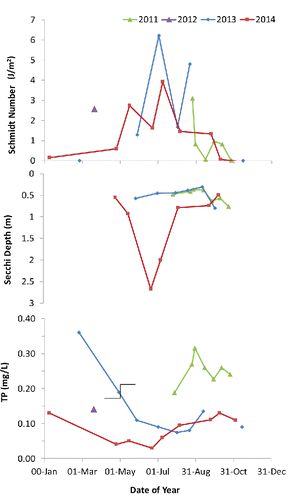
Schmidt stability was always small and fluctuated between 1 and 6 J/m2 in summers 2011 (monitoring started 25 Aug 2011), 2013, and 2014 and decreased to 0 in late summer and fall at thermal turnover (), indicating mixed conditions. Similar fluctuations in stability before treatment would have facilitated any distribution of sediment-derived substances throughout the summer by mixing, whereas short periods of stratification during warm episodes (also indicated by temperature and oxygen profiles) would have enhanced sediment oxygen demand and sediment release rates of nutrients and reduced chemicals. The observed similarity of physical stability between years before and after the treatment indicates that water quality changes were not related to changes in mixing state.
Table 3 Trophic state categories (Nürnberg Citation1996) based on water quality of the monitored pre- and post-treatment growing season surface water layer (0–1 m).
Trophic state
Pre-treatment trophic state based on historic data before 2011 indicates eutrophic–hypereutrophic conditions in Swan Lake. Blooms of the cyanobacteria Microcystis and Dolichospermum (formerly Anabaena) were reported in 1995 and 1996, which prompted a copper sulfate treatment in 1995. Swan Lake was eutrophic in 1996 (0.082 mg/L TP, growing season average Secchi depth 1.1 m) and had turned hyper-eutrophic in 2005 (0.120 mg/L TP in Dec 2005; 0.197 mg/L TP, 0.35 m Secchi depth in 2007).
Trophic state variables indicated continuing hyper-eutrophic conditions in the pre-treatment study period of 2011 (). Trophic state was not appreciably changed in the first post-treatment growing season 2013, except that nutrient concentration had declined by 60% for TP and 40% for TN. All monitored trophic state variables changed drastically from hyper-eutrophic to eutrophic in 2014 (). Hypoxia was still widespread but did not prevail throughout the water column up to the surface in 2013 and 2014, except under ice on 8 January 2014 (). Decreases in hypoxia are likely due to decreased sediment oxygen demand after the application of bentonite clay onto the sediment surfaces and the reduction of settling phytoplankton. Correspondingly, the predicted active sediment area release factor (AA) that reflects anoxia at the sediment–water interface decreased in the post-treatment years from 90 to 59 d/summer ().
TP was significantly lower in the surface layer in both post-treatment growing seasons (). The Dwass-Steel-Chritchlow-Fligner test shows significant differences, P < 0.0001, between 2011 and 2013 or 2014, but not between 2013 and 2014. The Fligner-Wolfe test shows highly significant decreases compared to the 2011 reference year. Because there was usually no elevated bottom TP concentration despite anoxia in the post-treatment years (e.g., ), differences apply to the total water column as well.
SRP was usually >0.020 mg/L in 2011 and under ice and severely elevated close to the bottom. SRP was below the detection limit (0.020 mg/L) in both summers after the treatment throughout the water column and close to the bottom (21 Aug 2013, 5 Aug 2014). TN average concentrations had already declined in the first treatment year and even further in 2014 (based on 2 observations; ). High ammonium concentration in the bottom water contributed to elevated TN ().
A bloom of Dolichospermum appeared on 21 July 2011 (Supplement, Appendix C). Two more species of cyanobacteria were identified throughout the 2011 sampling season, Anacystis sp. (probably Microcystis) and Gomphosphaeria sp. Cyanobacteria counts were elevated on 26 October 2011, mostly by Dolichospermum and Anacystis and a small amount of Oscillatoria. By comparison, cyanobacteria species were only occasionally detected in 2013, including Aphanizomenon in the August samples and Dolichospermum and Chroococcus in October (Supplement, Appendix C). Cyanobacteria abundance was low, and no blooms were visible on any monitoring dates in 2013 and 2014. Microcystin concentration was under the detection limit of 0.05 μg/L for all measured sampling occasions before and after treatment (2011–2014).
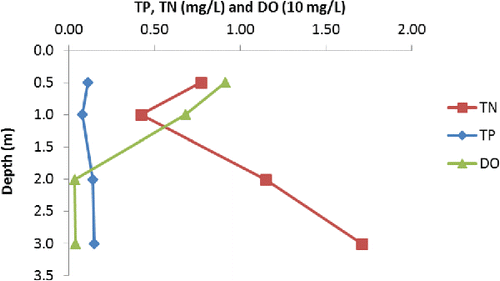
Phytoplankton abundance during summer 2013 after the treatment in May was not different from the pre-treatment summer 2011, as indicated by a lack of change in chlorophyll concentration and Secchi depth transparency (). The absence of a response can be explained by the relatively late application (postponed due to ice conditions) conducted when temperature was rising rapidly (air temperature increased from 13 C on 29 Apr 2013 before treatment, to 21 C during treatment and 26 C on 6 May 2013), and available P was already partially incorporated into phytoplankton. This finding is supported by the relatively low SRP concentration (0.040 mg/L) on the day prior to treatment, compared to 0.160 mg/L under ice on 25 February 2013 when Swan Lake was totally anoxic (0.6 mg/L DO within 1 m of surface and severely elevated ammonium concentration of 4.43 mg/L).
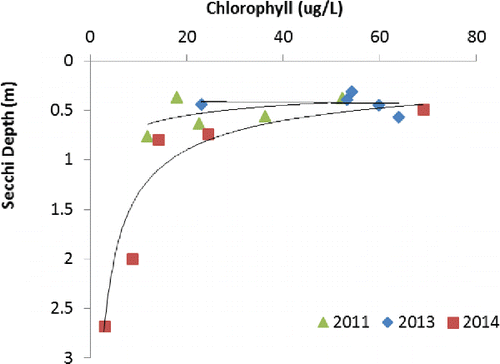
Table 4 Observed growing season surface layer averages of phytoplankton indicators (Secchi and Chlorophyll) compared to predictions from TP and color based on models of North American lakes.
Although no apparent change in biomass occurred in summer following the 2013 spring treatment, a hitherto unknown transparency of >2 m Secchi disk depth occurred in early summer of the second post-treatment year 2014 (). The significant correlation of Secchi disk transparency and chlorophyll concentration () indicates that transparency is highly correlated with algae in Swan Lake. Color also decreased and would have contributed to higher transparency in recent years (true color averaged 36 platinum units in summer 2011, 23 in 2013, and 17 in 2014).
Swan Lake's Secchi depth transparency of 0.47 m was low for its 2011 growing season TP concentration and color (0.247 mg/L TP, 36 platinum units) compared to a multiple regression average of 0.66 m based on 38 eastern North American lakes (Nürnberg Citation1996; , R2 = 0.89). In 2013, Secchi transparency was similarly low, as discussed earlier, but the 2014 growing season average of 1.43 m was much higher and probably not statistically different (considering the measurement precision) from the predicted value of 1.56 m. Similar trends were apparent for chlorophyll concentration, except that Swan Lake seemed to have generally less chlorophyll per unit of TP compared to the North American model (; R2 = 0.89, n = 42), so that 65 μg/L was predicted in 2011 compared to observed 32 μg/L, and 19 μg/L in 2014 compared to 13 μg/L. Interpretation of these comparisons must consider the spatial and seasonal patchiness of chlorophyll but may indicate possible light limitation, especially for the hyper-eutrophic pre-treatment conditions. Nonetheless, individual chlorophyll concentration and Secchi depth readings were highly significantly correlated ().
Using regression equations based on average TP concentration, summer average cyanobacterial biomass (Watson et al. Citation1997) is predicted to decrease in Swan Lake from 8.0 to 3.3 mg/L. Complete anoxia occurred under ice before treatment on 25 February 2013 and after treatment on 8 January 2014 (these are the only under-ice monitoring events available because Swan Lake did not freeze over in 2012.) Pre-treatment winter TP was 0.36 mg/L at 0.3 m depth and possibly throughout the water column, and SRP was 0.16 mg/L. In comparison, the post-treatment sampling event on 8 January 2014 had a TP of 0.130 at 0.5 m and at 3 m depth. Hence, while nutrients decreased in both post-treatment years, phytoplankton measures indicate that (a) biomass decreased significantly in the second post-treatment year, (b) relationships with TP may be approaching those of other eastern North American lakes, and (c) literature-based TP–cyanobacteria relationships suggest a drastic decrease in the severity and frequency of cyanobacteria blooms.
DO concentration showed signs of improvement in the open water season; there were no water-column–wide episodes of hypoxia in the post-treatment period. Bottom sediment surfaces were still anoxic in the summer and under ice and would have released more P without treatment. Phoslock has been shown to increase P-sorption capacity of sediments, even under reducing conditions, for pH 5–9 (Ross et al. Citation2008), a typical range for Swan Lake ().
Decreases in trophic state and anoxia varied in other lakes after Phoslock applications (Dithmer et al. Citation2015, Spears et al. Citation2015). Although phytoplankton biomass had already decreased in the summer following the application in Loch Flemington, UK, decrease of cyanobacteria biomass was delayed until the second year (S. Meis, Lanaplan, Nettetal, Germany, April 2015, pers. comm.). A combined treatment by aluminum salts, which strips the water column of phytoplankton, and Phoslock, called “Flock & Lock,” avoided such a delay in a 4 ha stratified Dutch lake (Lürling and Oosterhout Citation2013).
Lanthanum concentrations in water and sediments
Although La has not been found to be toxic at most concentrations (Ministry of Environment Citation2009, Lürling and Tolman Citation2010), responsible application requires the continued monitoring of La in water and sediment after application. Because La as a rare earth element is seldom measured in routine monitoring programs, no pre-treatment data are available. Dissolved La (DLa) was 0.002 mg/L and total La (TLa) was 0.038 mg/L on 8 August 2013, just 3 months after the treatment. Both fractions were below the detection limit of 0.05 mg/L on 23 June 2014, meaning that post-treatment La was well within the tested range of 0–1.0 mg/L La, where no toxicity was determined on Daphnia magna (Lürling and Tolman Citation2010). La concentration was also well below the Daphnia magna 48-h LC50 of 245 mg/L in 4.9 g/L Phoslock and below the rainbow trout 96-h LC50 of 680 mg/L in 13.6 g/L Phoslock (Ministry of Environment Citation2009).
The La sediment (0–10 cm) concentration was 93.1 μg/g dry weight on 21 August 2013 and 300.0 on 5 August 2014. It is not clear why the concentration was so different while background characteristics (total organic carbon, moisture, total solids, loss on ignition, and calcium carbonate) were similar, except for total Kjeldahl N, which was also elevated by 5 times in 2014. Slightly different sediment depths may have been collected by the dredge and may account for the difference because La concentration was depth-dependent after Phoslock application in a Scottish lake (Meis et al. Citation2012). In that lake, mean sediment La content ranged from 28 to 35 μg/g dry weight sediment before application but increased to 99–8803 μg/g dry weight after Phoslock application, where the highest concentration was at the surficial sediment layer of 0–2 cm and the lowest at 10 cm. In comparison, Swan Lake sediment had a low La concentration throughout its surficial 10 cm layer. These sediment La concentrations are unlikely to negatively affect the macrobenthos because no toxicity was detected with standard sediment toxicity tests on Chironomus dilutus, Hexagenia spp., and Hyalella azteca (Ministry of Environment Citation2009).
Effect of Canada goose and other waterfowl
Several subspecies of Canada goose reside in the northeastern United States and southeastern Canada, at least during part of the year, and all likely frequent Swan Lake at some point. Populations of all the subspecies (B. canadensis maxima, B. canadensis canadensis, and B. canadensis interior) have increased along the flyways within the last decades because of hunting restrictions and better food sources from increased cereal grain and rice agriculture (Unckless and Makarewicz Citation2007). In addition, migration has decreased due to dryer and warmer winters with shorter ice cover periods on lakes, inducing geese to stay year-round (Moore et al. Citation1998). Similar trends were observed in Europe, where the pink-footed goose (Anser brachyrhynchus) and the greylag goose of Iceland and Greenland increased in Scotland and continental Europe due to increased feeding areas, restricted hunting, and increased protection (Carss et al. Citation2012).
P input from waterfowl has been studied in various European and North American lakes and wetlands. Geese can apparently contribute appreciably to external P sources of urban lakes. For example, estimates were reported as 70% of external TP loading in Lake Wintergreen, Michigan (Manny et al. Citation1994), 90% in Ahrend See, Germany (Rönicke et al. Citation2008), and 73% in Brown Moss, UK (Chaichana et al. Citation2010).
The single most important variable in the estimation of TP export by waterfowl is waterfowl abundance. Input of nutrients from geese (lesser snow goose [Chen caerulescens caerulescens] and Ross goose [Chen rossii]) was most sensitive to the population estimate (number of geese) but also depended on local migration patterns and food and its energy content in a New Mexico wetland (Post et al. Citation1998). For example, energy, relative content of P and N, and gut passage rates differ for different food items and affect P egestion rates.
Much of the goose feces settled to the bottom sediment as determined in a mesocosm study (Unckless and Makarewicz Citation2007), meaning it would fertilize the water and enrich the sediments of Swan Lake and possibly enter the water as anoxic sediment release and internal load unless this process is interrupted by adsorption onto La.
Comparison of TP, chlorophyll, and rain with waterfowl counts () suggests that both rain and waterfowl could have led to concentration increases in the summer of 2014, but (migrating) waterfowl may have been responsible for the increases during the relatively dry early fall.
TP mass balance and concentration model
To determine which TP sources were most likely to create the high concentration leading to hyper-eutrophy before treatment, external and internal loads were assessed and contributions to the lake water TP concentration determined.
External load
Atmospheric deposition of TP onto the lake surface amounted to 0.9 kg/yr, and runoff from the immediate shoreline averaged 3.9 kg/yr (range: 3.86–3.99) in the study years. Because there were no major storm events (larger than a 5-year event storm), there has been no flow or input from the SWMPs into Swan Lake since at least 2007.
The number of counted geese in 2014 was 17,672 from July to December 2014 and 5480 estimated geese from January to June (Supplement, Appendix A), yielding a TP input of 14.1 kg/yr. It is unknown whether goose abundance has changed within recent years, but a 2013 estimate (without detailed counting) yielded similar loads (15.8 kg/yr). However, an earlier estimate (without any census data) for the pre-treatment year 2011 assumed a lower goose impact of 36 geese per day from March to November, yielding a TP input of only 6 kg/yr. This estimate was used in the retention model calibration (Equationequation 1(1) ).
To even out the “goose effect,” which cannot be estimated for the pre-treatment year with certainty, we applied the 2014 census-based estimate to all 3 study years; however, it is possible that waterfowl number was smaller before the restoration treatment. Fluctuating waterfowl abundance is likely; other studies noted that waterfowl abundance was not constant between years in European lake environments, and goose numbers increased (Carss et al. Citation2012). A sensitivity analysis on variable goose P export determined that its effect on predicted pre-treatment TP concentration was small compared to internal load (Supplement, Appendix D).
In summary, we estimated the combined external TP input into Swan Lake as 18.9 kg/yr for all 3 study years 2011, 2013, and 2014, except that a possibly lower geese abundance in 2011 would yield only 10.8 kg/yr in that year.
Internal load
Details of internal load estimates are presented in Supplement, Appendix B. Based on monitoring data, areal 2011 internal load estimates ranged almost 2-fold, from 650 to 1100 mg/m2/yr, or 34–59 kg/yr, including the internal load of 90 mg/m2, or 5 kg in the winter. Literature P release rates suggest a considerably higher load. Although this estimate represents a large range and an associated uncertainty, even the smallest estimate is large compared to the combined external load for 2011. In contrast, there was no evidence of summer internal loading in 2013 and only minor evidence in 2014 (one elevated TP sample at 3 m depth, 8 Jul 2014, 0.31 mg/L) that may be explicable by waterfowl abundance (discussed earlier).
Internal loading into the open water would be expected to end if La was sufficient to adsorb all phosphate released from any iron-hydroxides or organic compounds within the sediments, as attempted in the Swan Lake application. In this case, the release rate would be “0” even though anoxia was still wide-spread and the modeled active area was still 70 and 59 d/yr in the post-treatment years. Laboratory-based studies revealed that Phoslock intercepts P release even under anoxic conditions (Ross et al. Citation2008), probably because it can shift P from the potentially releasable fraction to apatite and other residual fractions generally considered not involved in P release (Bishop et al. Citation2014). Nonetheless, it is possible that some P may have been released from the sediments despite the treatment. P fractionation studies of P from saturated Phoslock under laboratory conditions indicated that ∼21% of P bound by Phoslock was still in a form that could be released under reducing conditions (Meis et al. Citation2012).
TP concentration contribution from individual loads
Assuming a constant waterfowl abundance, as described earlier, predicted pre- and post-treatment years differed mainly and substantially in the contribution from internal load to Swan Lake average concentration (). Of all loads, the largest contribution is from this internal load in 2011, next are contributions from waterfowl, and less important is the load from shoreline runoff and from atmospheric contributions.
Table 5 Modeled total phosphorus (TP) concentration (annual, whole water column; for retention, R = 0.98, not the calibrated R = 0.983) attributable to specific P sources compared to monitored TP.
Predicted TP concentrations compare reasonably well to monitored concentrations, especially for the post-treatment years (). Using the census-based goose number of 2014 rather than the assumed smaller number in 2011 explains the slightly higher predicted pre-treatment concentration (using the assumed smaller 2011-based goose load would predict about 12% lower TP concentration.) External loading alone explains the observed 2014 whole water column TP average of 0.091 mg/L (; modeled 0.099 mg/L), meaning there is no obvious indication of an unaccounted-for load, such as the sediments. It also indicates that most likely the treatment effectively intercepted internal P loading from the sediments, as already observed in 2013, the first year of application.
The similarity between observed and predicted values lends support to the internal load estimate and the chosen approach including active release area predictions and areal release rate (discussed in previous section; Supplement, Appendix B). This general agreement also indicates that mass balance modeling can be applied to evaluate restoration treatment success, even though the lake cannot be expected to be in steady state. We assume that the small and closed features of Swan Lake, its low hydrological connectivity with only small inputs, and the lack of an outlet contribute to the almost instant response predicted by the model.
To obtain further confidence in the predictions, we determined the sensitivity of the results to various model inputs. The 3 largest loads expectedly create the largest errors if wrongly estimated. The chosen input compares well with monitoring results whereas hypothetical deviations, including prevailing internal loading, do not predict observed concentrations well (Supplement, Appendix D). Further, P mass balance models are most sensitive to retention, and only small deviations create large differences in the concentrations. The value of 0.98 as calibrated for pre-treatment conditions also best predicted TP concentration in post-treatment years; but the model is not very sensitive to annual water load, which is another input into the mass balance model (discussed earlier; Supplement, Appendix D).
Prognosis and conclusions
Depending on its abundance, waterfowl alone substantially contributes to the water TP concentration in Swan Lake (). Although the Phoslock treatment effectively reduced P return from sediments in 2 monitored post-treatment years, it is unclear how long it will be able to adsorb P from future settled loads that may accumulate as feces from the waterfowl that frequently visit Swan Lake. Considering the goose density in 2014 and the high P retention of 0.98, it is likely that the feces accumulating on the sediment surfaces above the La-modified clay layer will release P in due time. This process may be delayed because the sediment surfaces were still anoxic, which delays conversion of organic P of the feces to bioavailable inorganic phosphate. The decrease in internal load should last several years at least, although the exact longevity of the Phoslock treatment in Swan Lake cannot be predicted with certainty. In this context we recommend the continuation of routine monitoring. At the least, future monitoring of transparency determined as Secchi disk depth is recommended if economic constraints do not permit more detailed studies. The plausible and significant relationship between Secchi depth transparency and chlorophyll for Swan Lake 2014 () indicates that that Secchi depth may closely track phytoplankton biomass.
Two main options seem to insure that the current water quality prevails or even further improves: (1) aggressive goose management, including culling, nest and egg destruction, sterilization for resident geese, and chasing migrating geese, which may be legally protected from harsher measures, with dogs; (2) if goose management is not possible, further treatment could be used to intercept P release from the newly accumulated P-rich sediment. In that case, the areal distribution of La within the sediments and calculated La:P ratios could be used to determine where the applied dosage was or has become insufficient, so that a more specific and economic areal application of Phoslock can be accomplished in the future, similar to a repeat application in a hyper-eutrophic German lake (Yasseri and Epe Citation2015). Ideally, an in-lake treatment as described here would be used when external load is low, so that subsequent improved water conditions would cause settling of material lower in nutrients, and the newly accumulated sediment would be less prone to release P as internal loading in the future.
In conclusion, small lakes are an important feature in urban and suburban landscapes, but past use and deteriorated water quality, including the accumulation of potentially toxic cyanobacteria and scums during warm months, have caused problems for traditional engineering and park departments of municipalities and cities. This study presents a collaboration where a small monetary budget and frequent exchange among the authors and city employees (Swan Lake is owned by the City of Markham), the field sampling organization (Toronto Region Conservation Authority), and experienced treatment consultants (Institut Dr. Novak, Ottersberg, Germany) provided the first step of a solution to the management of low water transparency and high phytoplankton biomass at Swan Lake. Comprehensive monitoring, ideally for several pre- and post-treatment years, is advisable (Hickey and Gibbs Citation2009) to determine further treatment pressure (e.g., waterfowl management). Highlights of this study are summarized as follows:
The Swan Lake Phoslock application was the first to be conducted on a Canadian lake.
Specific features of shallow, polymictic Swan Lake made it a perfect candidate for in-lake restoration that addresses internal P loads from sediments. Swan Lake
had limited inflow and hence only a small external P loading,
lacks an outflow,
exhibited an obvious and large internal P load, and
was occasionally completely anoxic throughout the water in the summer, fall, and under ice.
The time of treatment determines the onset of treatment success. Despite decreases in lake TP concentration indicating the successful cessation of internal loading from the bottom sediments, phytoplankton (chlorophyll and Secchi depth) did not improve until the second post-treatment growing season, possibly because of the relatively late application when phosphate was already incorporated into phytoplankton biomass.
In the second post-treatment year, water transparency was much improved in early summer, and elevated phytoplankton measured as chlorophyll concentration was detected only once in the fall. Neither cyanobacterial blooms nor cyanotoxins were identified.
Because Phoslock successfully intercepted internal P load, trophic state has improved significantly from hyper-eutrophy to eutrophy; therefore Phoslock proves to be an acceptable treatment for small, closed, polymictic and shallow lakes.
Unconventional nutrient input (waterfowl) must be considered in a mass balance as well as in a lake management plan. It will be interesting to see whether this trend of improved water quality lasts despite continuing visits by waterfowl (Canada goose), which currently is estimated as the single most important P source for Swan Lake.
Quantitative information from other lakes, including regressions and models, are useful to corroborate and explain water quality changes after a lake treatment.
Acknowledgments
We thank the City of Markham staff (Rob Muir, Denisa Necula, Soran Sito, and Cynthia Tam) for their steady support and enthusiasm. C. Tam graciously provided Figure 1. Friendly exchange with Said Yasseri of Institute Dr. Nowak, Ottersberg, Germany, and Nigel Traill of Phoslock Water Solutions Ltd. helped in the product and dosage selection process and application. The numerous helpful comments by Ken Wagner and other reviewers are appreciated.
Funding
Funding for all monitoring, data analysis, and 3 reports on Swan Lake by the first author was provided by the City of Markham. MOECC funded initial lab analyses and all phytoplankton biomass and microcystin related analysis.
ULRM_A_1133739_Supplement.pdf
Download PDF (942.3 KB)References
- Ayers CR, DePerno CS, Moorman CE, Yelverton FH. 2010. Canada goose weed dispersal and nutrient loading in turfgrass systems. Appl Turfgrass Sci. 7:0–0.
- Bishop WM, McNabb T, Cormican I, Willis BE, Hyde S. 2014. Operational evaluation of Phoslock phosphorus locking technology in Laguna Niguel Lake, California. Water Air Soil Pollut. 225:2018–2029.
- Brett MT, Benjamin MM. 2008. A review and reassessment of lake phosphorus retention and the nutrient loading concept. Freshwater Biol. 53:194–211.
- Burns N, McIntosh J, Scholes P. 2009. Managing the lakes of the Rotorua District, New Zealand. Lake Reserv Manage. 25:284–296.
- Carss D, Spears BM, Quinn L, Cooper R. 2012. Long-term variations in waterfowl populations in Loch Leven: identifying discontinuities between local and national trends. Hydrobiologia. 681:85–104.
- Chaichana R, Leah R, Moss B. 2010. Birds as eutrophicating agents: a nutrient budget for a small lake in a protected area. Hydrobiologia. 646:111–121.
- Cooke GD, Welch EB, Peterson SA, Nichols SA. 2005. Restoration and management of lakes and reservoirs. 3rd ed. Boca Raton (FL): CRC.
- Dithmer L, Nielsen UG, Lundberg D, Reitzel K. 2015. Influence of dissolved organic carbon on the efficiency of P sequestration by a lanthanum modified clay. Water Research. doi: 10.1016/j.watres.2015.07.003
- Egemose S, Reitzel K, Andersen FØ, Flindt MR. 2010. Chemical lake restoration products: sediment stability and phosphorus dynamics. Environ Sci Technol. 44:985–991.
- Gächter R, Wehrli B. 1998. Ten years of artificial mixing and oxygenation: no effect on the internal P loading of two eutrophic lakes. Environ Sci Technol. 32:3659–3665.
- Gensemer RW, Playle RC. 1999. The bioavailability and toxicity of aluminum in aquatic environments. CRC Cr Rev Environ Sci Technol. 29:315–450.
- Hahn S, Bauer S, Klaassen M. 2007. Estimating the contribution of carnivorous waterbirds to nutrient loading in freshwater habitats. Freshwater Biol. 52:2421–2433.
- Hahn S, Bauer S, Klaassen M. 2008. Quantification of allochthonous nutrient input into freshwater bodies by herbivorous waterbirds. Freshwater Biol. 53:181–193.
- Hickey CW, Gibbs MM. 2009. Lake sediment phosphorus release management–Decision support and risk assessment framework. New Zeal J Mar Freshw Res. 43:819–856.
- Holdren C, Jones W, Taggart J. 2001. Managing lakes and reservoirs. 3rd ed. Madison (WI): North American Lake Management Society, Terrene Institute in cooperation with Office Water Assessment Watershed Protection Division US Environ, Protection Agency.
- Horppila J, Köngäs P, Niemistö J, Hietanen S. 2015. Oxygen flux and penetration depth in the sediments of aerated and non-aerated lake basins. Int Rev Hydrobiol. doi:10.1002/iroh.201401781
- Idso SB. 1973. On the concept of lake stability. Limnol Oceanogr. 18:681–683.
- Katseva S, Dittrich M. 2012. Modeling of decadal scale phosphorus retention in lake sediment under varying redox conditions. Ecol Model. 251:246–259.
- Lake Simcoe Region Conservation Authority. 2010. PhoslockTM Report.
- Liboriussen L, Søndergaard M, Jeppesen E, Thorsgaard I, Grünfeld S, Jakobsen T, Hansen K. 2009. Effects of hypolimnetic oxygenation on water quality: results from five Danish lakes. Hydrobiologia. 625:157–172.
- Liu B, Liu XG, Yang J, Garman D, Zhang K, Zhang HG. 2012. Research and application of in-situ control technology for sediment rehabilitation in eutrophic water bodies. Wat Sci Technol. 65:1190–1199.
- Lürling M, Tolman Y. 2010. Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna. Water Res. 44:309.
- Lürling M, Van Oosterhout F. 2013. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 47:6527–6537.
- Mackay EB, Maberly SC, Pan G, Reitzel K, Bruere A, Corker N, Douglas G, Egemose S, Hamilton D, Hatton-Ellis T, et al. 2014. Geoengineering in lakes: welcome attraction or fatal distraction?Inland Waters. 4:349–356.
- Manny BA, Johnson WC, Wetzel RG. 1994. Nutrient additions by waterfowl to lakes and reservoirs: predicting their effects on productivity and water quality. Hydrobiologia. 279/280:121–132.
- Meis S, Spears BM, Maberly SC, O’Malley MB, Perkins RG. 2012. Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): investigating changes in sediment elemental composition and phosphorus fractionation. J Environ Manage. 93:185–193.
- Meis S, Spears BM, Maberly SC, Perkins RG. 2013. Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Res. 47:4460–4473.
- Ministry of Environment. 2009. Phoslock toxicity testing with three sediment dwelling organisms (Hyalella azteca, Hexagenia spp. and Chironomus dilutus) and two water column dwelling organisms (Rainbow Trout and Daphnia magna). Toronto (Ontario): Ministry of the Environment, Technical Memorandum.
- Ministry of Environment. 2010. Lakeshore capacity assessment handbook: protecting water quality in inland lakes on Ontario's Precambrian Shield. Toronto (Ontario). www.ontario.ca/environment
- Moore MV, Zakova P, Shaeffer KA, Burton RP. 1998. Potential effects of Canada Geese and climate change on phosphorus inputs to suburban lakes of the Northeastern U.S.A. Lake Reserv Manage. 14:52–59.
- Moos MT, Taffs KH, Longstaff BJ, Ginn BK. 2014. Establishing ecological reference conditions and tracking post-application effectiveness of lanthanum-saturated bentonite clay (Phoslock®) for reducing phosphorus in aquatic systems: an applied paleolimnological approach. J Environ Manage. 141:77–85.
- Nürnberg GK. 1995. Quantifying anoxia in lakes. Limnol Oceanogr. 40:1100–1111.
- Nürnberg GK. 1996. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake Reserv Manage. 12:432–447.
- Nürnberg GK. 2007. Lake responses to long-term hypolimnetic withdrawal treatments. Lake Reserv Manage. 23:388–409.
- Nürnberg GK. 2009. Assessing internal phosphorus load – problems to be solved. Lake Reserv Manage. 25:419–432.
- Nürnberg GK, LaZerte BD, Olding DD. 2003. An artificially induced Planktothrix rubescens surface bloom in a small kettle lake in southern Ontario compared to blooms world-wide. Lake Reserv Manage. 19:307–322.
- Nürnberg GK, LaZerte BD, Loh PS, Molot LA. 2013a. Quantification of internal phosphorus load in large, partially polymictic and mesotrophic Lake Simcoe, Ontario. J Great Lakes Res. 39:271–279.
- Nürnberg GK, Molot LA, O’Connor E, Jarjanazi H, Winter JG, Young JD. 2013b. Evidence for internal phosphorus loading, hypoxia and effects on phytoplankton in partially polymictic Lake Simcoe, Ontario. J Great Lakes Res. 39:259–270.
- Nürnberg GK, Tarvainen M, Ventelä A-M, Sarvala J. 2012. Internal phosphorus load estimation during biomanipulation in a large polymictic and mesotrophic lake. Inland Waters. 2:147–162.
- Post DM, Taylor JP, Kitchell JF, Olson MH, Schindler DE, Herwig BR. 1998. The role of migratory waterfowl as nutrient vectors in a managed wetland. Conserv Biol. 12:910–920.
- Reitzel K, Hansen J, Andersen FØ, Jensen HS. 2005. Lake restoration by dosing aluminum relative to mobile phosphorus in the sediment. Environ Sci Technol. 39:4134–4140.
- Reitzel K, Jensen HS, Egemose S. 2013. pH dependent dissolution of sediment aluminum in six Danish lakes treated with aluminum. Water Res. 47:1409–1420.
- Robb M, Greenop B, Goss Z, Douglas G, Adeney J. 2003. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: preliminary findings. Hydrobiologia. 494:237–243.
- Rönicke H, Doerffer R, Siewers H, Buttner O, Lindenschmidt KE, Herzsprung P, Beyer M, Rupp H. 2008. Phosphorus input by nordic geese to the eutrophic Lake Arendsee, Germany. Fund Appl Limnol/Arch Hydrobiol. 172:111–119.
- Ross G, Haghseresht F, Cloete TE. 2008. The effect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae. 7:545–550.
- Søndergaard M, Jensen JP, Jeppesen E. 2001. Retention and internal loading of phosphorus in shallow, eutrophic lakes. ScientificWorld. 1:427–442.
- Spears BM, Lürling M, Yasseri S, Castro-Castellon AT, Gibbs M, Meis S, McDonald C, McIntosh J, Sleep D, Van Oosterhout F. 2013. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: an analysis of water column lanthanum data from 16 case study lakes. Water Res. 47:5930–5942.
- Spears BM, Mackay EB, Yasseri S, Gunn ID, Waters KE, Andrews C, Cole S, De Ville M, Kelly A, Meis S, et al. 2015. A meta-analysis of water quality and aquatic macrophyte responses in 18 lakes treated with lanthanum modified bentonite (Phoslock®). Water Res. doi: 10.1016/j.watres.2015.08.020
- Unckless RL, Makarewicz JC. 2007. The impact of nutrient loading from Canada Geese (Branta canadensis) on water quality, a mesocosm approach. Hydrobiologia. 586:393–401.
- [US EPA] US Environmental Protection Agency. 1998. National strategy for the development of regional nutrient criteria, US-EPA No. 822-R-98-002.
- Waajen G, Van Oosterhout F, Douglas G, Lürling M. 2015. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant – Lanthanum modified bentonite treatment. Water Res. doi: 10.1016/j.watres.2015.11.034
- Wagner K. 2015. Oxygenation and circulation to aid water supply reservoir management. Water Research Foundation. No. 4222c.
- Watson SB, McCauley E, Downing JA. 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr. 42:487–495.
- Welch EB, Jacoby G, Lindell T. 2004. Pollutant effects in fresh waters: applied limnology. 2nd ed. London (UK): Taylor & Francis.
- Welch EB, Jacoby JM. 2009. Phosphorus reduction by dilution and shift in fish species in Moses Lake, WA. Lake Reserv Manage. 25:276–283.
- Winter JG, Young JD, Landre A, Stainsby E, Jarjanazi H. 2011. Changes in phytoplankton community composition of Lake Simcoe from 1980 to 2007 and relationships with multiple stressors. J Great Lakes Res. 37:63–71.
- Yasseri S, Epe TS. 2015. Analysis of the La:P ratio in lake sediments–vertical and spatial distribution assessed by a multiple-core survey. Water Res. doi: 10.1016/j.watres.2015.07.037
- Zamparas M, Zacharias I. 2014. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci Total Environ. 496:551–562.