ABSTRACT
Beutel MW, Cox SE, Gebremariam S. 2016. Effects of chironomid density and dissolved oxygen on mercury efflux from profundal lake sediment. Lake Reserv Manage. 32:158–167.
Benthic macrofauna can dramatically affect the flux of dissolved compounds into and out of lake sediment. In this study, replicate experimental chambers containing profundal sediment from a relatively pristine lake were incubated under low (∼1000/m2) and high (∼3800/m2) chironomid densities and low (∼2.5 mg/L) and medium (∼5.0 mg/L) dissolved oxygen in chamber water. Dissolved methylmercury efflux rates were highest in high chironomid density/low oxygen chambers (0.35 ± 0.06 ng/m2/d, mean ± standard error, n = 4) and lowest in low density/medium oxygen chambers (0.06 ± 0.14 ng/m2/d). In contrast, dissolved total mercury efflux assessed at low dissolved oxygen (∼2.5 mg/L) was higher at low chironomid density (4.6 ± 0.7 ng/m2/d) relative to high chironomid density (2.5 ± 0.8 ng/m2/d, n = 4). Results indicate that oxygen addition, a common lake management practice, may enhance methylmercury efflux from profundal sediment as macrofauna recolonize previously anaerobic sediments. However, benthos-mediated methylmercury efflux rates are lower than hypolimnetic methylmercury accumulation rates under anaerobic conditions; therefore, relative to anaerobic conditions, oxygenation should lower net methylmercury accumulation rates in relatively pristine lakes.
Benthic macrofauna in profundal sediments, such as oligochaetes and chironomids, build burrows that they flush with overlying water to obtain food and oxygen for respiration. As a result of bioirrigation, these organisms can have a substantial effect on environmental conditions at the sediment–water interface (Aller Citation2001). Potential impacts include enhanced transport of dissolved compounds into and out of sediments, changes in redox status of surfacial sediments, and shifts in microbial ecology in sediments near burrows. Numerous studies have documented enhanced nutrient exchange and elevated oxygen uptake resulting from increased densities of chironomids in lake sediments (Pelegrí and Blackburn Citation1996, Svensson Citation1997, Hansen et al. Citation1998, Anderson et al. Citation2006, Lewandowski et al. Citation2007, Biswas et al. Citation2009). Macrofauna-mediated exchange of solutes at the sediment–water interface is a complex process affected by a range of environmental factors. Rates of bioirrigation are typically enhanced at higher dissolved oxygen (DO), higher temperature, and higher macrofauna density (Roskosch et al. Citation2012).
Macrofauna also influence the cycling of mercury (Hg) species at the sediment–water interface, including the sediment efflux of methylmercury (MeHg; Benoit et al. Citation2009), a concern because MeHg is the toxic, organic form of Hg that readily bioaccumulates in aquatic ecosystems (Selin Citation2009). Macrofauna-mediated efflux of MeHg has been implicated in a number of marine and freshwater studies (Choe et al. Citation2005, Point et al. Citation2007, Hammerschmidt and Fitzgerald Citation2008, Li et al. Citation2015) that consistently show fluxes of MeHg based on buildup rates in overlaying water exceed rates calculated using diffusive concentration gradients, with the difference being attributed to enhanced MeHg efflux due to macrofauna activity. Macrofauna density can have potentially contradictory effects on MeHg efflux from sediments (Benoit et al. Citation2009). Higher macrofauna densities could increase efflux through elevated net rates of burrow flushing that would transport MeHg from sediment pore water into overlaying water. Higher bioirrigation rates could also promote Hg methylation by advecting organic carbon and sulfate used by sulfate-reducing bacteria (SRB) into sediment and by pumping sulfide, which can inhibit SRB activity and lower Hg bioavailability for methylation, out of sediment (Hammerschmidt et al. Citation2004). Bioirrigation may also enhance sediment MeHg efflux by inhibiting anaerobic demethylating microorganisms (Nogaro and Hammerschmidt Citation2013). Alternatively, higher macrofauna densities could decrease MeHg efflux by promoting oxygen penetration into sediments, thereby repressing methylation by anaerobic organisms and/or enhancing sorption of MeHg to iron and manganese oxides in surfacial sediments (Chadwick et al. Citation2006, Matthews et al. Citation2013).
There is growing interest in the use of in-lake management strategies such as hypolimnetic oxygenation (Beutel et al. Citation2014) and thermocline deepening (Perron et al. Citation2014) to repress MeHg production and bioaccumulation in lakes and reservoirs that exhibit summertime hypolimnetic anoxia and associated MeHg buildup in bottom waters. Enhanced oxygen levels at the sediment–water interface could reduce MeHg efflux from profundal sediments via a number of mechanisms including repression of anaerobic microbial activity, such as sulfate reduction and iron reduction, responsible for methylation (Yu et al. Citation2012) and enhancement of sorption of MeHg to iron and manganese oxides in oxidized surfacial sediments (Matthews et al. Citation2013). Oxygenation of previously anaerobic sediments could open sediments to recolonization by macrofauna, however, potentially resulting in enhanced effluxes of MeHg from profundal sediments via bioirrigation (Hammerschmidt and Fitzgerald Citation2006).
Although previous studies have evaluated marine systems, few if any have evaluated the effects of macrofauna density on MeHg efflux in profundal sediments from relatively pristine freshwater lakes. This study evaluated the effects of chironomid density and DO on the flux of dissolved MeHg (dMeHg) and dissolved total Hg (dTHg) from profundal lake sediments from Deer Lake, a relatively pristine lake in eastern Washington. Using replicate experimental chambers incubated in the laboratory containing undisturbed sediment–water interface samples, Hg fluxes were assessed at 2 chironomid densities (ambient and ∼4 times ambient) and 2 DO concentrations (∼2.5 and ∼5 mg/L) typical of summertime conditions in moderately productive lakes. The working hypothesis was that MeHg efflux would be highest at high chironomid density and low DO because lower DO would lead to less penetration of oxygen into the sediment, thereby moving the zone of methylation, which generally corresponds with the redox transition zone within sediment, closer to burrow walls and the sediment–water interface. This condition, combined with higher net rates of burrow flushing, would lead to higher efflux rates of MeHg. The study also included mass balance estimations that compared MeHg release rates under anaerobic conditions to those from oxygenated sediment recolonized with macrofauna.
Materials and methods
Study site
The study site was Deer Lake (surface area 445 ha, maximum depth 22.9 m), a dimictic oligo-mesotrophic lake in eastern Washington state. In the 1970s the lake exhibited characteristics of cultural eutrophication, including high algal productivity and low levels of hypolimnetic DO (Soltero et al. Citation1991). In response, a sewer system was installed to replace septic tanks, and sewage is now transported outside of the watershed to a regional wastewater treatment facility. Deer Lake thermally stratifies in the summer and exhibits bottom water hypoxia in the fall (Soltero et al. Citation1991). In late August 2011, bottom water at a depth of 20 m was 10 C and contained 4 mg/L DO (Cox Citation2011). Hg concentrations in lake water were generally <1 ng/L for THg and 0.04 ng/L for MeHg (Cox Citation2011), levels typically observed in relatively pristine lakes (Watras Citation2009). Deer Lake has low levels of THg in littoral sediments (mean of 0.55 ppm dry weight; n = 3) but somewhat elevated levels of THg in largemouth bass tissue (mean of 0.33 ppm wet weight; n = 10; WSDOE Citation2003). Bass Hg levels were near the US Environmental Protection Agency (EPA) fish tissue criterion for the protection of human health of 0.3 ppm MeHg wet weight. The high fish tissue MMHg occurred in Deer Lake despite its relatively pristine, low-Hg status, making the results presented here applicable to the vast majority of surface waters. Profundal sediments are organic and relatively iron rich. Organic content is 14.9%, iron content is 2476 mg/kg dry weight, and manganese content is 33 mg/kg dry weight (Dent Citation2012). Additional information on Deer Lake can be found in Beutel et al. (Citation2008).
Field collection of sediment and chironomids
We collected 8 chambers containing a sediment–water interface sample in July and August 2011 in the profundal zone of the lake (48°07.12′N, 117°35.32′W). The sampling station had a depth of ∼21 m. Sediment–water interface chambers were collected from Deer Lake using methods described in Beutel (Citation2006) and Beutel et al. (Citation2008), which are briefly described here. A sediment–water interface sample was first collected with an Ekman dredge and brought to the lake surface. The cohesive nature of the sediments plugged the dredge, thereby allowing the collection of a stable sediment surface with a few centimeters of overlaying water. A minimally disturbed subsampled of sediment–water interface was then carefully collected in a 9.5 m diameter, 25 cm high polycarbonate cylinder, capped on the bottom, and transferred to a secure base. The chamber was gently filled with bottom water, capped, and transported to the laboratory.
Chironomid larvae were also collected from profundal sediment to assess ambient densities and to enhance chironomid densities in experimental chambers. Sediment was collected with an Ekman dredge and placed in a sieve bucket with 541 µm stainless steel mesh. The sediment was sieved, and biota were collected by hand and stored in multiple acid-washed polyethylene bottles with bottom water. This process was repeated until at least 10 individuals were collected. The samples were chilled in coolers and transported to the laboratory. Ambient chironomids density was estimated as the number of chironomids collected divided by the area of the Ekman dredge (0.023 m2) multiplied by the number of sediment samples collected.
Chamber incubations
All chambers were incubated in the dark at 10 C, the approximate temperature of Deer Lake bottom water in the late summer. In the July experiment, 8 chambers were bubbled with a high-purity air mixture consisting of 90% nitrogen gas, 10% oxygen, and 350 ppm of carbon dioxide to maintain pH. This yielded a DO concentration in chamber water of ∼5.0 mg/L. The chambers were split into 2 treatments in quadruplicate: low ambient chironomid density (∼1100/m2) and high chironomid density (∼3900/m2). High chironomid density was achieved by gently adding 20 chironomid larvae into each high density chamber. Each chamber had a surface area of 72 cm2, and thus density was enhanced by ∼2800/m2. Water samples for dMeHg were collected at the beginning and end of a 15-day incubation period. Because the efflux of Hg from these uncontaminated sediments was anticipated to be low, a relatively long incubation period was needed to allow accumulations of measurable amounts of Hg. Fluxes of dMeHg (ng/m2/d) were calculated as the change in mass of dMeHg in chamber water (ng) divided by the area of the chamber (m2) and the incubation duration (d).
In the August experiment, 8 chambers were bubbled with a high-purity air mixture consisting of 95% nitrogen gas, 5% oxygen, and 350 ppm of carbon dioxide, yielding a DO concentration in chamber water of ∼2.5 mg/L. The chambers were split into 2 treatments in quadruplicate: low ambient chironomid density (∼900/m2) and high chironomid density (∼3700/m2) by adding 20 chironomid larvae into each high density experimental chamber. To simplify the experiment, in contrast to the July chambers, the August chambers were carefully drained at the start of the incubation and filled with homogenized bottom water. A single sample of the fill water was collected at the beginning of the experiment, and water samples were collected from chambers at the end of a 15-day incubation period. Because of enhanced analytical capabilities, water samples were collected for both dMeHg and dTHg, and their fluxes (ng/m2/d) were calculated as the change in Hg mass in chamber water (ng), with initial mass estimated on the single fill water concentration, divided by the area of the chamber (m2) and the incubation duration (d).
Because concentration gradients are an important driver of diffusion of dissolved substances out of sediments, there was some concern that a relatively low or high concentration of dMeHg in the bottom water added to the August chambers could enhance or repress efflux rates relative to the July experiment. An evaluation of initial dMeHg concentrations showed that values were similar for both experiments: 0.022 ± 0.01 ng/L (mean ± standard deviation; n = 8) for July chambers and 0.023 ng/L for August chambers. Because initial dMeHg concentrations were similar for both the July and August experiments, the difference in experimental methods was not expected to have impacted the mass efflux of dMeHg; thus the July and August datasets can be statistically compared without concern for the different sampling methods.
After both experiments, 2 chambers from each treatment were sieved to assess post-incubation chironomid densities. In low density chambers, chironomid densities at the end of the experiment were 141 ± 33% (mean ± standard deviation; n = 4) of densities measured at the time of sediment sampling. In high density chambers, chironomid densities at the end of the experiment were 77 ± 23% (mean ± standard deviation; n = 4) of estimated densities based on ambient densities plus added chironomids. These values indicate that initial estimates of experimental chironomid densities in chambers were reasonably accurate and there was reasonable survivorship over the course of the chamber incubations.
Mercury sampling and analysis
Hg sampling of chamber water in the laboratory followed EPA Clean Hands Dirty Hands protocol outlined in Method 1669 (USEPA Citation1996). Samples of dMeHg and dTHg were filtered through an in-line PALL AquaPrep 600 Capsule with a 0.45 µm Supor membrane. Sample bottles underwent a vigorous cleaning protocol, which included a nitric acid bath, DI rinse, and acid conditioning. Samples of dMeHg were stored in amber glass and preserved with 1% trace-metals-grade hydrochloric acid (USEPA Citation2001), and samples of dTHg were stored in clear glass bottles preserved with 0.5% bromine monochloride solution (USEPA 2002). Sample bottles were individually bagged and refrigerated for later analysis.
MeHg and THg in filtered water samples were analyzed in triplicate using the Brooks Rand MERX-M Auto Analyzer. MeHg was analyzed using distillation, aqueous ethylation, purge and trap, and CVAFS following EPA Method 1630 (USEPA Citation2001), and THg was analyzed using oxidation, purge and trap, and CVAFS according to EPA Method 1631 (USEPA 2002). Each analytical run for both compounds included quality control standards including matrix spikes (70–125% recovery criterion), ongoing precision and recovery (77–123% recovery criterion), and deionized water blanks. The relative standard deviation of triplicate analyses averaged 16.5% for MeHg (n = 16) and 9.3% for THg (n = 7). The method detection limits were 0.02 ng/L for MeHg and 0.2 ng/L for THg. Samples below detection were assumed to equal one-half of the detection limit when performing flux calculations.
Efflux rates of dMeHg from the 2 sets of experiments were evaluated for statistical significance using a 2-way ANOVA (chironomid density and DO). For the August experiment, a 2-tailed Student t-test was used to assess differences in efflux rates of dTHg and differences in the ratio of efflux rates for dMeHg and dTHg as a function of chironomid density.
Results
Efflux rates of dMeHg were highest in high chironomid/low oxygen chambers (0.35 ± 0.06 ng/m2/d, mean ± standard error, n = 4) and lowest in low chironomid/medium oxygen chambers (0.06 ± 0.14 ng/m2/d, n = 4; , ). Based on a 2-way ANOVA, the effect of chironomid density (P = 0.004) was highly significant, and the effect of DO (P = 0.054) was close to the conventional criterion for statistical significance (P ≤ 0.05). Efflux rates for dTHg, which were only measured under low DO conditions during the August experiment, showed an opposite pattern, with higher efflux in chambers with low levels of chironomids (4.6 ± 0.7 ng/m2/d, n = 4) relative to chambers with high chironomid density (2.5 ± 0.8 ng/m2/d, n = 4; , ). The P-value for the difference in means was 0.10 (2-tailed Student t-test). Because both dMeHg and dTHg effluxes were assessed in the August experiment, the ratio of dMeHg efflux to dTHg efflux could be estimated. Mean ratios were substantially lower in chambers with low chironomid density (0.04 ± 0.02, n = 4) relative to chambers with high chironomid density (0.15 ± 0.04, n = 4); however, due to the relatively high variability of efflux values in replicate chambers, the P-value for the difference in means was relatively high (2-tailed Student t-test, P = 0.22).
Figure 1. Dissolved methylmercury (dMeHg) flux as a function of chironomid density (low and high) and dissolved oxygen (low and high). Values are means plus one standard error (n = 4).
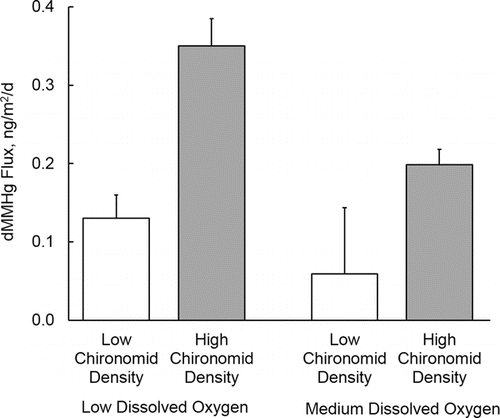
Table 1. Experimental flux results (ng/m2/d) for dissolved methylmercury.
Figure 2. Dissolved total mercury (dTHg) flux as a function of chironomid density (low and high) for August experiment under low oxygen conditions (∼2.5 mg/L). Values are means plus one standard error (n = 4).
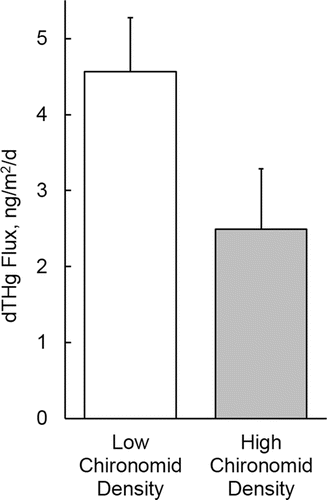
Table 2. Experimental flux results (ng/m2/d) for dissolved total mercury under low dissolved oxygen conditions (∼2.5 mg/L).
Discussion
Magnitude of methylmercury efflux
Efflux rates of dMeHg measured from sediments of Deer Lake (<0.4 ng/m2/d) were low compared to marine systems (∼10–1000 ng/m2/d; Benoit et al. Citation2009). Marine systems tend to have high levels of biological sulfate reduction that result in elevated rates of Hg methylation and enhanced dMeHg efflux from sediments (Hammerschmidt et al. Citation2004). In addition, many of these marine studies were conducted in Hg contaminated sediments with elevated MeHg efflux rates relative to non-contaminated sites like Deer Lake. Deer Lake dMeHg efflux rates were also low compared to chamber flux studies from contaminated freshwater lakes, which typically ranged from 3 to 30 ng/m2/d (Kuwabara et al. Citation2000, 2002, Citation2003, Beutel et al. Citation2013). Some studies reported diffusive fluxes of MeHg, which do not include the effect of benthic macrofauna, ranging from ∼0.2 ng/m2/d for profundal sediment of deep, oligotrophic Lake Superior (Rolfhus et al. Citation2003) to as high as 65 ng/m2/d in shallow, hypereutrophic Lake Taihu, China (Wang et al. Citation2012). Mass balance calculations from relatively pristine lakes yield rates of aerial hypolimnetic MeHg buildup ranging from 1 to 8 ng/m2/d (Watras et al. 1996, Sellers et al. Citation2001, Beutel et al. Citation2014). Aerial rates should approach values measured directly in chambers for pristine lakes because sediment efflux is usually the dominate source of MeHg to the hypolimnion in these systems (Sellers et al. Citation2001).
Effect of chironomid density and dissolved oxygen on methylmercury efflux
The most obvious explanation for elevated levels of dMeHg efflux at higher macrofauna density is enhanced exchange of water and dissolved substances between sediment and overlaying water via bioirrigation. Roskosch et al. (Citation2012), for example, showed that experimentally increasing the density of Chironomus plumosus in freshwater sediment from 360/m2 to 3600/m2 increased the bioirrigation rates from 3–6 to 15–30 mL/h. Increased C. plumosus density has also been shown to increase oxygen influx and ammonia efflux from lake sediment, presumably by enhancing the rate of water exchange between sediment and overlaying water via bioirrigation (Svensson Citation1997). A number of studies have observed enhanced dMeHg efflux with increasing macrofauna density in coastal marine sediment. Benoit et al. (Citation2009) found that higher densities of tube-building amphipods enhanced both dMeHg efflux and production in sediment. At burrow densities <500/m2, dMeHg efflux rates were <5 ng/m2/d, whereas at burrow densities of 4000–5000, efflux rates were 10–15 ng/m2/d. Point et al. (Citation2007) reported that dMeHg efflux was 19 ng/m2/d in sediment with negligible macrofauna and 60 ng/m2/d in sediment with a total macrofauna density of 510/m2 dominated by opportunistic polychaete worms. Hammerschmidt et al. (Citation2004) reported enhanced methylation rates in bioturbated sediments in coastal marine sediment. Of particular significance for Hg cycling is the potential impact of bioirrigation on sulfur cycling. Greater oxygen penetration into sediment enhances sulfide oxidation in surfacial sediment, and the resulting low sulfide concentration may enhance the bioavailability of inorganic Hg (Hg(II)) for methylation (Hsu-Kim et al. Citation2013). Sulfide oxidization may also enhance or sustain SRB activity by recycling sulfide back to sulfate for reuse as an electron acceptor, a potentially critical issue in freshwater sediment with relatively low sulfur content (Holmer and Storkholm Citation2001).
The most probable explanation for the observed increase in dMeHg efflux with lower DO was that lower oxygen penetration into sediment resulted in a methylation zone closer to the sediment–water interface in burrow walls and surfacial sediment. The resulting shorter diffusional distance combined with higher rates of burrow flushing account for the observed maximum dMeHg efflux rate in the low oxygen/high chironomid chambers. The depth of oxygen penetration in lake sediment is proportional to the square root of bulk water DO concentration (Beutel Citation2003). The difference in DO in overlaying chamber water by a factor of 2 resulted in a 2-fold increase in dMeHg efflux under both low and high chironomid density, suggesting that mechanisms in addition to enhanced diffusion may have played a role. A thinner aerobic surfacial layer may also have diminished sorption onto iron and manganese oxides, a recognized sink for MeHg in aquatic sediment (Gill et al. Citation1999).
A few studies have documented changes in MeHg efflux from aquatic sediment as a result of changes in DO in overlaying water. In general, lower DO levels correspond with higher MeHg efflux rates. Both Beutel et al. (Citation2013) and Kuwabara et al. (Citation2000) reported enhanced rates of MeHg efflux from contaminated lake sediment experimentally incubated under anaerobic vs. aerobic conditions. Using in situ benthic chambers, researchers have also documented repressed MeHg efflux from coastal marine sediments covered with benthic algae that produce DO at the sediment–water interface via photosynthesis during daylight hours (Gill et al. Citation1999, Point et al. Citation2007). A study by Hammerschmidt and Fitzgerald (Citation2008) using shipboard benthic flux chambers documented lower MeHg efflux from sediment under moderate vs. well oxygenated conditions. In-lake manipulations of oxygen dynamics in the hypolimnion via oxygen addition (Beutel et al. Citation2014) and thermocline deepening (Perron et al. Citation2014) has resulted in significant decreases in MeHg in bottom waters.
A potential negative feedback related to enhanced MeHg efflux from sediment with decreasing DO in overlaying water is changes in macrofauna activity and density. At lower DO levels, the bioirrigation rate of benthic organisms tends to slow. Roskosch et al. (Citation2012) showed that decreasing DO from >50 to 10% saturation resulted in a 2-fold decrease in bioirrigation rates by Chironomus plumosus. Lower bioirrigation rates in turn could decrease MeHg efflux. In this study, DO was ∼25% saturation in low oxygen chambers and ∼50% saturation in high oxygen chambers, levels which may have resulted in differing rates of bioirrigation by chironomids. Thus, this mechanism may have counteracted the biogeochemical processes in surfacial sediments discussed earlier that tend to enhance MeHg efflux under low DO. Higher DO levels may also support higher densities of macrofauna, which in turn could enhance net bioirrigation rates and MeHg efflux rates. This mechanism was used to explain increases in bioirrigation MeHg efflux relative to diffusional MeHg efflux with increasing DO in coastal marine sediment (Hammerschmidt and Fitzgerald Citation2006); however, the enhancement of MeHg efflux under low DO conditions outweighed increases in efflux due to enhance bioirrigation under high DO conditions.
Effects of chironomid density on total mercury efflux
As is typically observed in other studies that have assessed both THg and MeHg efflux from aquatic sediment (Gill et al. Citation1999, Kuwabara et al. Citation2000, Citation2002, Citation2003, Point et al. Citation2007, Beutel et al. Citation2013), dTHg efflux was an order of magnitude greater than dMeHg efflux for Deer Lake sediment. But in contrast to dMeHg efflux, lower dTHg efflux corresponded with higher chironomid density, suggesting different release mechanisms for inorganic and organic forms of Hg in surfacial sediment. A similar pattern of repressed THg efflux in combination with enhanced MeHg efflux with increasing macrofauna density was reported in coastal marine sediment by Point et al. (Citation2007). The relative difference in efflux capacity of inorganic and organic Hg is captured in the ratio of dMeHg efflux to dTHg efflux. The ratio of dMeHg to dTHg concentrations in sediment is sometimes used as a proxy for methylation efficiency (Mitchell and Gilmour Citation2008). The efflux ratio also captures the relative capacity of the 2 Hg species to be transported out of surficial sediment and into overlaying water.
In Deer Lake sediment, the average ratio of dMeHg efflux to dTHg efflux evaluated under low oxygen conditions (∼2.5 mg/L) was 4-fold higher in high chironomid chambers (0.15) compared to low chironomid chambers (0.04). Enhanced sorption of Hg(II) to metal oxides as a result of higher rates of bioirrigation could explain observed decreases in dTHg efflux at higher chironomid density in Deer Lake sediment. Lewandowski et al. (Citation2007) showed that oxygen influx into sediment via bioirrigation enhanced iron oxidation and phosphate co-precipitation in chironomid burrows. In their study, increasing chironomid density from 680/m2 to 4800/m2 resulted in repression of phosphate and iron efflux from burrow lining. Because iron and manganese oxides show an affinity for both inorganic and organic Hg (Hammerschmidt et al. Citation2004, Muresan et al. Citation2007), this mechanism would also repress dMeHg efflux. The fact that higher chironomid density enhanced dMeHg efflux indicates that other factors, such as enhanced SRB activity and Hg(II) bioavailability for methylation, more than compensated for any sorption-related decrease in dMeHg efflux. In a sense, Hg(II) release is a geochemical process, but dMeHg efflux is a biogeochemical process. In the case of enhanced sorption to iron and manganese oxides associated with elevated rates of bioirrigation, biological processes that favor dMeHg production, and efflux appeared to overwhelm geochemical processes repressing dMeHg efflux.
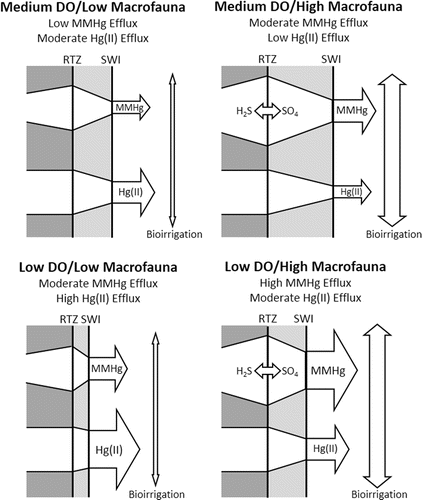
A conceptual model () encapsulates the finding from this study, as well as results from the literature, on how DO and macrofauna density interact to control MeHg and Hg(II) efflux from profundal lake sediment. In the model, MeHg is produced at the redox transition zone (RTZ) and diffuses through aerobic surficial sediment to the sediment–water interface (SWI). MeHg production is enhanced at high macrofauna density as a result of high bioirrigation rates that promote Hg methylation by SRB at the RTZ. Potential factors that promote methylation include the influx of labile organic carbon, enhanced recycling of sulfur that sustains SRB activity, and low sulfide levels that promote bioavailability of Hg(II) for methylation. A key potential sink for MeHg as it diffuses to the SWI is sorption to iron and manganese oxides. Thus, efflux is impeded by a larger aerobic zone (distance between RTZ and SWI) under high oxygen conditions and/or high macrofauna density. The resulting conceptual model yields high MeHg efflux under low DO/high macrofauna conditions and low MeHg efflux under moderate DO/low macrofauna conditions. Hg(II) efflux is mainly controlled by the thickness of the aerobic zone, which is a sink via sorption to iron and manganese oxides. High Hg(II) efflux is associated with low DO penetration into sediment under low DO/low macrofauna conditions, and low efflux is associated with elevated DO penetration into sediment under moderate DO/high macrofauna conditions.
Figure 3. Conceptual model of methylmercury (MeHg) and inorganic mercury (Hg(II)) efflux from freshwater sediment as a function of dissolved oxygen (DO) in overlaying water and macrofauna density in sediment. MeHg is produced at the redox transition zone (RTZ) and diffuses through aerobic surficial sediment to the sediment–water interface (SWI) of a burrow wall. Macrofauna density enhances bioirrigation rates and enlarges the thickness of the aerobic zone. MeHg efflux is mainly controlled by macrofauna bioirrigation, which enhances efflux, and sorption to metal oxides in the aerobic zone, which represses efflux. Bioirrigation enhances MeHg efflux partly by promoting the recycling of sulfide (H2S) and sulfate (SO4), which promotes the methylation activity of SRB and the bioavailability of Hg(II) for methylation.
Implications for lake and reservoir management
Results from this study support the contention that increased macrofauna densities correspond with higher sediment efflux of MeHg, a potent bioaccumulatory toxin in aquatic environments. An associated concern related to lake oxygen addition is that previously “dead” sediments will be recolonized by respiring macrofauna that promote the efflux of Hg. This rationale was used, in part, to support the use of nitrate rather than oxygen to repress MeHg efflux from contaminated lake sediments in Onondaga Lake, New York (Matthews et al. Citation2013). By using nitrate, sediments were oxidized and MeHg efflux was repressed, but sediments were not opened to recolonization by respiring aerobic macrofauna.
In the case of relatively pristine lakes like Deer Lake, enhanced MeHg efflux caused by colonizing macrofauna does not seem to be a valid concern. The peak level of dMeHg efflux from Deer Lake sediment measured under low DO/high chironomid conditions was ∼0.4 mg/m2/d (). Comparable rates of anaerobic MeHg accumulation in South Twin Lake, a relatively pristine mesotrophic lake in eastern Washington that experiences summertime anoxia in bottom waters, were ∼12 ng/m2/d (Beutel et al. Citation2014). This finding indicates that anaerobic dMeHg efflux rates are 1–2 orders of magnitude greater than efflux rates from aerobic, macrofauna-rich sediment and further suggests that oxygen addition, while enhancing bioirrigation-mediated MeHg efflux, will result in lower net efflux rates of MeHg. A potentially important implication of the Deer Lake study is that even with enhanced macrofauna density, higher DO levels in overlaying water decreased dMeHg efflux. At high chironomid density, a 2-fold increase in DO led to a 2-fold decrease in dMeHg efflux, indicating that enhanced MeHg efflux resulting from macrofauna colonization can be partly mitigated by maintaining moderate DO levels (>5 mg/L) at the sediment–water interface. While promoting MeHg efflux, a diverse and functioning macrofauna resulting from bottom water oxygen addition can have a number of additional positive ecological benefits. A critical benefit is enhanced influx of oxygen into sediment, which is indicative of faster rates of oxidation of organic matter is sediment. Over the long term, bioirrigation in combination with oxygen addition at the sediment–water interface should promote oxygen penetration into surfacial sediment, a key to repressing the release of redox-sensitive pollutants from sediment to overlaying water.
Acknowledgments
We thank graduate students Stephen Dent, Lanka DeSilva, and Piper Marshall for assistance during this project. Thanks also to the anonymous reviewers for their constructive comments on the manuscript. The views expressed herein are solely those of the authors and do not represent the official policies or positions of any supporting agencies.
Funding
This project was funded in part by the National Science Foundation though CAREER Grant #0846446.
References
- Aller RC. 2001. Transport and reaction in the bioirrigated zone: In: Boudreau BP, Jorgensen BB, editors. The benthic boundary layer. Oxford University Press. p. 269–301.
- Anderson FØ, Jørgensen M, Jensen HS. 2006. The influence of Chironomus plumosus larvae on nutrient fluxes and phosphorus fractions in aluminum treated lake sediment. Water Air Soil Pollut: Focus. 6:101–110.
- Benoit JM, Shull DH, Harvey RM, Beal SA. 2009. Effect of bioirrigation on sediment–water exchange of methylmercury in Boston Harbor, Massachusetts. Environ Sci Technol. 43:3669–3674.
- Beutel MW. 2003. Hypolimnetic anoxia and sediment oxygen demand in California drinking water reservoirs. Lake Reserv Manage. 19:208–221.
- Beutel MW. 2006. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol Eng. 28:271–279.
- Beutel MW, Dent SR, Reed B, Marshall P, Gebremariam S, Moore BC, Cross B, Shallenberger E. 2014. Effects of hypolimnetic oxygen addition on mercury bioaccumulation in Twin Lakes, Washington, USA. Sci Tot Environ. 496:688–700.
- Beutel MW, Duvil R, Drury D. 2013. Effect of oxygen, nitrate and aluminum addition on methylmercury efflux from mine-impacted reservoir sediments. SETAC Europe 2013.
- Beutel MW, Leonard TM, Dent SR, Moore BC. 2008. Effects aerobic and anaerobic conditions on P, N, Fe, Mn and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Res. 42:1953–1962.
- Biswas JK, Rana S, Bhakta JN, Jana BB. 2009. Bioturbation potential of chironomid larvae for the sediment-water phosphorus exchange in simulated pond systems of varied nutrient enrichment. Ecol Eng. 35:1444–1453.
- Chadwick S, Babiarz C, Hurley J, Armstrong D. 2006. Influences of iron, manganese, and dissolved organic carbon on the hypolimnetic cycling of amended mercury. Sci Total Environ. 368:177–188.
- Choe K-Y, Gill GA, Lehman RD, Han S, Heim WA, Coale KH. 2005. Sediment–water exchange of total mercury and monomethylmercury in the San Francisco Bay-Delta. Limnol Oceanogr. 49:1512–1527.
- Cox SE. 2011. Effects of chironomid density and dissolved oxygen on mercury efflux from profundal lake sediment from Deer Lake, Washington [master's thesis]. [Pullman (WA)]: Washington State University.
- Dent SR. 2012. Effects of oxygenation on metal cycling in lakes [dissertation]. [Pullman (WA)]: Washington State University.
- Gill GA, Bloom NS, Cappellino S, Driscoll C, Dobbs C, McShea L, Mason RP, Rudd J. 1999. Sediment-water fluxes of mercury in Lavaca Bay, Texas. Environ Sci Technol. 33:663–669.
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Visscher PT. 2004. Biogeochemistry of methylmercury in sediments of Long Island Sound. Mar Chem. 90:31–52.
- Hammerschmidt CR, Fitzgerald WF. 2006. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch Environ Contam Toxicol. 51:416–424.
- Hammerschmidt CR, Fitzgerald WF. 2008. Sediment–water exchange of methylmercury determined from shipboard benthic flux chambers. Marine Chem. 109:86–97.
- Hansen K, Mouridsen S, Kristensen E. 1998. The impact of Chironomus plumosus larvae on organic matter decay and nutrient (N, P) exchange in a shallow eutrophic lake sediment following a phytoplankton sedimentation. Hydrobiologia. 364:65–74.
- Holmer M, Storkholm P. 2001. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshwater Biol. 46:431–451.
- Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses A. 2013. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Technol. 47:2441–2456.
- Kuwabara JS, Alpers CN, Marvin-DiPasquale MM, Topping BR, Carter JL, Stewart AR, Fend SV, Parchaso F, Moon GE, Krabbenhoft DP. 2003. Sediment–water interactions affecting dissolved-mercury distributions in Camp Far West Reservoir, California. US Geological Survey Water Resources Investigations Report 03-4140. 66 p.
- Kuwabara JS, Berelson WM, Balistrieri LS, Woods PF, Topping BR, Steding DJ, Krabbenhoft DP. 2000. Benthic flux of metals and nutrients into the water column of lake Coeur d'Alene, Idaho. US Geological Survey Water-Resources Investigations Report 00-4132. 74 p.
- Kuwabara JS, Marvin-DiPasquale MM, Praskins W, Byron E, Topping BR, Carter JL, Fend SV, Parchaso F, Krabbenhoft DP. 2002. Flux of dissolved forms of mercury across the sediment–water interface in Lahontan Reservoir, Nevada. US Geological Survey Water Resources Investigations Report 02-4138. 48 p.
- Lewandowski J, Laskov C, Hupfer M. 2007. The relationship between Chironomus plumosus burrows and the spatial distribution of pore‐water phosphate, iron and ammonium in lake sediments. Freshwater Bio. 52:331–343.
- Li Q, Jiang L, Wang D, Luo X. 2015. Sediment profile and fluxes of mercury and methyl mercury in Weihe Watershed in Henan, China. Bull Environ Contam Toxicol. 95:51–55.
- Matthews DA, Babcock DB, Nolan JG, Prestigiacomo AR, Effler SW, Driscoll CT, Todorova SG, Kuhr KM. 2013. Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake, NY. Environ Res. 125:52–60.
- Mitchell CP, Gilmour CC. 2008. Methylmercury production in a Chesapeake Bay salt marsh. J Geophy Res. 113:G00C04. doi:10.1029/2008JG000765
- Muresan B, Cossa D, Jézéquel D, Prévot F, Kerbellec S. 2007. The biogeochemistry of mercury at the sediment–water interface in the Thau Lagoon. 1. Partition and speciation. Estuar Coast Shelf Sci. 72:472–484.
- Nogaro G, Hammerschmidt CR. 2013. Influence of benthic macrofauna on microbial production of methylmercury in sediments on the New England continental shelf. Hydrobiologia. 701:289–299.
- Pelegrí SP, Blackburn TH. 1996. Nitrogen cycling in lake sediments bioturbated by Chironomus plumosus larvae, under different degrees of oxygenation. Hydrobiologia. 325:231–238.
- Perron T, Chételat J, Gunn J, Beisner BE, Amyot M. 2014. Effects of experimental thermocline and oxycline deepening on methylmercury bioaccumulation in a Canadian Shield lake. Environ Sci Technol. 48:2626–2634.
- Point D, Montperrus M, Tessier E, Amouroux D, Donard OFX, Chauvaud L, Thouzeau G, Jean F, Amice E, Grall J, et al. 2007. Biological control of trace metal and organometal benthicfluxes in a eutrophic lagoon (Thau Lagoon), Mediterranean Sea, France. Estuar Coast Shelf Sci. 72:457–471.
- Rolfhus KR, Sakamoto HE, Cleckner LB, Stoor RW, Babiarz CL, Back RC, Manolopoulos H, Hurley JP. 2003. Distribution and fluxes of total and methylmercury in Lake Superior. Environ Sci Technol. 37:865–872.
- Roskosch A, Hette N, Hupfer M, Lewandowski J. 2012. Alteration of Chironomus plumosus ventilation activity and bioirrigation-mediated benthic fluxes by changes in temperature, oxygen concentration, and seasonal variations. Freshwater Sci. 31:269–281.
- Selin NE. 2009. Global biogeochemical cycling of mercury: a review. Ann Rev Environ Resour. 34:43–63.
- Sellers P, Kelly CA, Rudd JW. 2001. Fluxes of methylmercury to the water column of a drainage lake: the relative importance of internal and external sources. Limnol Oceanog. 46:623–631.
- Soltero RA, Wainwright ML, Sexton LM, Humphreys LM, Buchanan JP, Seigmund BL, Anderson DE, Oppenheimer JO, Morency DA. 1991. Water quality assessment of Deer Lake, Washington. Cheney (WA): Eastern Washington University, Biology Department. 415 p.
- Svensson JM. 1997. Influence of Chironomus plumosus larvae on ammonia flux and denitrification (measured by the acetylene blockage- and the isotope pairing-technique) in eutrophic lake sediment. Hydrobiologia. 346:157–168.
- [USEPA] United States Environmental Protection Agency. 1996. Method 1669: Sampling ambient water for trace metals at EPA water quality criteria levels. Washington (DC): EPA-821 R-96-008.
- [USEPA] United States Environmental Protection Agency. 2001. Method 1630: Methylmercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. Washington (DC): EPA-821 R-01-020.
- [USEPA] United States Environmental Protection Agency. 2002. Method 1631, revision E: Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Washington (DC): EPA-821 R-02-019.
- Wang SF, Xing DH, Jia YF, Biao L, Wang KL. 2012. The distribution of total mercury and methyl mercury in a shallow hypereutrophic lake (Lake Taihu) in two seasons. Appl Geochem. 27:343–351.
- [WSDOE] Washington State Department of Ecology. 2003. Mercury in edible fish tissue and sediments from selected lakes and rivers of Washington State. Olympia (WA): Publication No. 03-03-026. 104 p.
- Watras CJ. 2009. Mercury pollution in remote freshwater lakes. In: Likens G, editor. Encyclopedia of inland waters. New York: Elsevier. p. 100–109.
- Watras CJ, Morrison KA, Back, RC. 1996. Mass balance studies of mercury and methyl mercury in small temperate/boreal lakes of the northern hemisphere. In: Global and regional mercury cycles: Sources, fluxes and mass balances. Netherlands: Springer. p. 329–358.
- Yu RQ, Flanders JR, Mack EE, Turner R, Mirza MB, Barkay T. 2012. Contribution of coexisting sulfate and iron reducing bacteria to methylmercury production in freshwater river sediments. Environ Sci Technol. 46:2684–2691.
