ABSTRACT
Mallin MA, McIver MR, Wambach EJ, Robuck AR. 2016. Algal blooms, circulators, waterfowl, and eutrophic Greenfield Lake, North Carolina. Lake Reserv Manage. 32:168–181.
Lake rehabilitation measures can sometimes have unintended consequences, including increasing certain eutrophication symptoms. An example involves Greenfield Lake, an urban blackwater impoundment within the City of Wilmington, North Carolina. This popular recreational impoundment has experienced noxious phytoplankton and macrophyte blooms, anoxia and hypoxia, and fish kills for many years. Chlorophyll a and biological oxygen demand are strongly correlated within the lake, indicating that decaying algal blooms are an important source of oxygen demand resulting in low dissolved oxygen. Nutrient addition bioassays demonstrated that the phytoplankton strongly responded to nitrogen (N) inputs. Spatially, ammonium, nitrate, and N/P ratios were higher in the upper lake compared with the lower lake, indicating tributary input of inorganic N, whereas phosphorus (P) was higher in the lower lake. A year-long survey indicated that waterfowl, especially cormorants, contributed somewhat to the lake's total N load but a considerable amount of P, particularly in winter. The lake sediments apparently function as P reservoirs, supplying P to the water column during summer, setting the stage for runoff-induced nitrate pulses and subsequent algal blooms. Lake restoration measures initiated in 2005 included installation of solar-powered circulators, introduction of grass carp, and herbicide treatments. These measures resulted in loss of surface macrophyte and algal mats and reduced dissolved oxygen violations but led to a significant increase in phytoplankton chlorophyll a and a tripling of chlorophyll a standard violations in comparison with pre-restoration years. The increased chlorophyll a violations have led to inclusion of the lake on the North Carolina 303(d) list of impaired waters.
Greenfield Lake, North Carolina (), is an urban lake that receives drainage from a largely commercial and residential watershed. Prior to 2005, several signs of impairment had become chronic within the lake, including low dissolved oxygen (DO), nuisance aquatic macrophyte growths, algal blooms, fish kills, and high fecal coliform bacterial counts. Most of these factors are typically related to eutrophication, a process driven by loading of excessive nutrients to a body of water (reviewed in Burkholder and Glibert Citation2013). Periodic phytoplankton blooms occurred in spring, summer, and fall. Some of the bloom-forming taxa were the cyanobacteria species Anabaena cylindrica (also referred to as Dolichospermum sp.; Wacklin et al. Citation2009) and Microcystis aeruginosa, and the filamentous chlorophytes Spirogyra and Mougeotia spp. Frequently observed free-floating macrophytes included duckweed (Lemna minor) and watermeal (Wolffia columbiana). In the water column beneath a massive duckweed bloom during summer 2004, DO concentrations were nearly anoxic, defined as having DO <0.5 mg/L. Abundant submersed macrophytes also characterized areas of the lake, including alligatorweed (Althernanthera philoxeroides), coontail (Ceratophyllum demersum), pennywort (Hydrocotyle umbellate), and water primrose (Ludwigia hexapetala; C.A. Williams, 2005, unpubl.)
Figure 1. Greenfield Lake, Wilmington, NC, showing water sampling stations, major tributaries, the road circling the lake, and locations of Solarbee mixers.
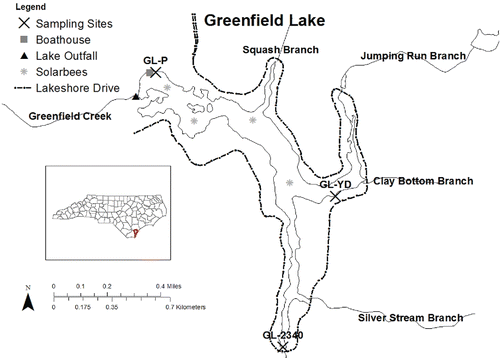
Beginning in 2005, which we refer to later as the attempted “rehabilitation period,” several steps were taken by the City of Wilmington to improve the lake (D. Mayes, Wilmington Stormwater Services, pers. comm.). In February 2005, 1000 sterile grass carp, known to consume vegetation, were introduced to the lake to control the excessive growth of aquatic macrophytes. During that same month, 4 SolarBee water circulation systems or mixers (SB10000v12 units) were installed in the main open water area of the lake with the general objectives of providing algae control, improving water quality and the fishery, reducing and/or compacting soft organics in the littoral zone, and strengthening nuisance macrophyte control. Such solar-driven circulators have a “mixed” record; they have reduced cyanobacterial abundance in some nutrient-rich lakes and reservoirs, but in other systems they have failed to control harmful algal blooms (Hudnell Citation2010). Circulators in general are easy to misapply, can cause harm to lakes if misapplied, and are not known to be effective in control of rooted nuisance aquatics (Osgood Citation2015). In addition to the grass carp and circulators, from April through June 2005 and in March and July 2006, herbicides and algicides were added to Greenfield Lake by city crews and contractors. During April 2006 and March 2007, additional grass carp (500 and 200, respectively) were added. Annually since 2007, city crews and contract firms have spot-treated areas of the lake to control macrophyte and nuisance filamentous growths with herbicide (Wilmington Stormwater Services records). Herbicide application and grass carp addition are both commonly used methods for lake nuisance vegetation control (Leslie et al. Citation1987, Ross and Lembi Citation1998); however, regeneration of inorganic nutrients and increases in phytoplankton productivity have resulted from such vegetation removal practices in some systems (e.g., Richard et al. Citation1984, Leslie et al. Citation1987, O'Dell et al. Citation1995).
Since the various treatments (artificial circulation, grass carp additions, herbicide use) were applied, lake water quality has changed, in some ways improving and in others deteriorating. Greenfield Lake provides an example of how management actions are affecting southeastern impoundments that have sustained nutrient over-enrichment, applicable to other similar systems. Here we describe the current status of the lake, provide experimental evidence of the nutrients limiting algal growth, assess sources of nutrients to the lake, examine the impacts of the lake rehabilitation treatments by comparing before-and-after water quality, and suggest future strategies for lake improvement.
Study site
Greenfield Lake is a 37 ha shallow blackwater impoundment located in the City of Wilmington, North Carolina (). It was first dammed and filled as a millpond in 1750 and was purchased for a city park in 1925. Most of the lake is 1.2–1.5 m deep, the shoreline perimeter is about 8530 m, and the watershed drains ∼1032 ha (2551 ac). Local precipitation averages about 146 cm/yr (57.5 in), and average air temperature is 17.8 C (64 F) (http://www.usclimatedata.com/climate/wilmington/north-carolina/united-states/usnc0760). The lake has one outfall but is fed by 5 perennial inflowing streams and also by several intermittent ditches. Watershed land use/land cover consists mainly of residential, office, institutional, and commercial development, with an overall impervious surface coverage of ∼37%. No sewage outfalls enter the lake. In addition to urban runoff and waterfowl guano, other potential (uncharacterized) nutrient inputs are from direct rainfall, ground water, and leaf litter from shoreline trees. Paddleboats and canoes are rented on-site, and a paved trail surrounding the lake is heavily used for walking, jogging, bicycling, and, pertinent to this study, dog walking.
Materials and methods
Field collections
Three stations within the lake were sampled every 1 to 2 months, depending on annual funding, from 1998 through 2014 (). Station GL-2340 was sampled from a bridge that crosses a bay fed by an unnamed tributary draining a subwatershed of ∼171 ha with ∼76% impervious surface coverage. That station is considered the farthest upstream site; that is, it is closest to incoming flow from a perennial stream. Station GL-YD was sampled from a dock overhanging the water (), and station GL-P in the lower lake is nearest the lake dam and outfall and was sampled from a dock at the boat house ().
Field parameters were measured at each site using a YSI 6920 Multi-parameter Water Quality Probe (sonde) linked to a YSI 650 MDS display unit. Individual probes within the instrument measured water temperature, pH, DO, turbidity, and conductivity. The YSI 6920 was calibrated prior to each sampling trip to ensure accurate measurements; our laboratory is state-certified and audited for environmental sampling. Samples were collected on-site ∼10 cm below the water surface for analysis of ammonium, nitrate + nitrite (hereafter called nitrate), total Kjeldahl nitrogen (TKN), orthophosphate, total phosphorus (TP), total suspended solids (TSS), fecal coliform bacteria, and suspended microalgal biomass as chlorophyll a. Samples were held on ice in darkness until returned to the laboratory for processing.
Laboratory methods
Samples were analyzed using State of North Carolina Division of Water Resources certified methods within acceptable holding times following American Public Health Association (APHA Citation2005) and US Environmental Protection Agency (USEPA Citation1983, Citation1997). Specific analytical methods were as follows: TSS, Method SM 2540D; ammonium + ammonia, Method SM 4500 NH3D; nitrate + nitrite, Method EPA 353.2; TKN, Method EPA 351.2; total nitrogen (TN) calculated as TKN + nitrate; orthophosphate, Method SM 4500 PE; TP, Method SM 4500 PE; biochemical oxygen demand (BOD5), Method SM 5210 B; and fecal coliform bacterial densities, Method SM 9222D MF. We also computed inorganic nitrogen to phosphorus (N/P) molar ratios.
USEPA Method 445.0 used to measure chlorophyll a was based on Welschmeyer (Citation1994) and USEPA (Citation1997). Samples were filtered through 0.7 µm glass fiber filters wrapped individually in aluminum foil, placed in an airtight container with desiccant, and stored in a freezer. Following a minimum holding time of 24 h in the freezer, each filter was ground using a Teflon grinder, followed by immersion in 10 mL of a 90% acetone solution for 3 h and analyzed for chlorophyll a concentration using a Turner AU-10 fluorometer.
Nutrient addition bioassays
This lake had sustained large visible nuisance algal blooms for years before we initiated sampling. We conducted a series of nutrient addition bioassay experiments to characterize algal responses to nutrients in this system because attempting to determine limiting nutrients from nutrient ratios alone can be uncertain (Dodds Citation2003, Lewis and Wurtsbaugh Citation2008). Nutrient addition bioassays were performed on water from stations GL-2340, GL-YD, and GL-P on 5 dates from September 1998 to August 1999. Lake water was collected in 20 L carboys cleaned by washing in dilute HCl. The water was transported to the laboratory, and 3 L were dispensed into each of thirty 4 L cubitainers. The cubitainers were then spiked with different nutrients to effect a final concentration of 100 µg/L nitrate-N (nitrate alone treatment), 50 μg/L phosphate-P (phosphate alone treatment), or N100 + P50 (combination N+P treatment). Controls were treated similarly but without nutrient additions. All treatments were in triplicate. The cubitainers were incubated outdoors in 1 m deep pools covered with 2 layers of neutral-density screens to avoid photostress (reducing surface irradiance to ∼30% of unscreened irradiance). The water in the pool was gently agitated with an aquarium pump to keep the cubitainers in motion. Samples for chlorophyll a analysis were collected daily from the cubitainers for 3 days, an incubation duration recommended elsewhere (Lewis and Wurtsbaugh Citation2008). Treatments yielding chlorophyll a significantly higher than in controls (P < 0.05) were considered to indicate the nutrient most limiting to phytoplankton growth. Note that in the late 1990s when these experiments were conducted, the Greenfield Lake watershed was already highly developed with ∼30% impervious surface coverage. In the ensuing period, no changes in nutrient inputs from point sources have been added or additional best management practice measures applied to the lake. Thus, these experimental results are pertinent to current lake conditions.
Statistical analysis
Plots of bioassay data, water quality parameter concentrations among stations, and differences over time were constructed using Excel. Data were tested for normality using the Shapiro–Wilk test, with most data (except water temperature, DO, and biochemical oxygen demand [BOD5]) requiring log-transformation to achieve a normal distribution. Correlation analyses were performed among water quality parameters and meteorological data, including temperature and rainfall. The amount of rainfall used for statistical purposes was rain that fell on the day of sampling plus rainfall for the 2 days prior, collected at the Wilmington airport and accessed from the Weather Underground (www.wunderground.com). Pooled pre-rehabilitation data (Jan 2000 through Jan 2005) were tested using Student's t-tests against pooled post-rehabilitation data (Mar 2005 through Dec 2013) to assess statistically significant differences between means. Additionally, t-tests were used to compare means of pre- vs. post-rehabilitation data for each individual station. Bioassay data were analyzed using the SAS procedure of analysis of variance (ANOVA). If a difference in response means (P < 0.05) existed among the treatments, the ANOVA test was followed by treatment ranking by the least-significant-differences (LSD) procedure (Day and Quinn Citation1989). Statistical tests were performed using SAS (Schlotzhauer and Littell Citation1997) using a significance level of P < 0.05.
Waterfowl quantification and guano nutrient production estimates
Nutrient input to lakes can significantly increase as a result of guanotrophy, which is nutrient addition by bird excrement (Scherer et al. Citation1995, Moore et al. Citation1998). Thus, to effectively define nutrient sources for a given lake, waterfowl usage patterns must be considered. Past studies have indicated increases in P (Portnoy Citation1990, Marion et al. Citation1994, Moore et al. Citation1998), N (Portnoy Citation1990, Marion et al. Citation1994), and fecal coliform bacteria (Hussong et al. Citation1979, Benton et al. Citation1983, Weiskel et al. Citation1996) associated with waterfowl abundance. In other waterbodies (Scherer et al. Citation1995), nutrient loading by waterfowl was not significantly correlated with water quality; however, at Green Lake, Washington, bird droppings did influence productivity within the lake through increased P cycling (Scherer et al Citation1995). Elsewhere bird droppings have been found to be less important than human sewage inputs and agricultural runoff (Marion et al. Citation1994); however, if large numbers of waterfowl use a eutrophic lake or reservoir, guanotrophy merits consideration as a potential nutrient source.
Substantial numbers of waterfowl reside at the lake, and large numbers of seasonal migrants use the lake, particularly in winter. Thus, an objective in this study was to estimate waterfowl impact on lake nutrient and fecal bacteria abundances. From August 2000 to July 2001, waterfowl inhabiting or visiting the lake were quantified using 8 × 42 binoculars 4–6 times each month. Surveys occurred either within 2 h after sunrise or 2 h before dusk. Seven sites around the perimeter of the lake were selected for stationary counts; 4 sites were in closest proximity to station GL-P, and the remaining 3 sites were closest to GL-YD. These stations were selected for their high visibility across the lake surface and/or because waterfowl commonly roosted or foraged on land at the site. Birds on the water, in trees rooted within the water, or on the shore were included. The shore was considered to be roughly defined by a road (Lakeshore Drive; ) that surrounds the lake at a distance ranging from immediately adjacent to the lake to 250 m from the water's edge.
To calculate TN and TP input by birds on the water and shore areas, waterfowl were grouped by size and ecological niche (). For example, all dabbling ducks were summed for a daily count and then each count was averaged per month. Waterfowl use days were estimated monthly for all bird categories by multiplying the mean count by the number of days in the month. Methods used by Manny et al. (Citation1994) to estimate TN and TP defecated by ducks were used to calculate “equivalent, effective waterfowl-use days” for dabbling ducks, diving ducks, American coots (Fulica americana), and Muscovy ducks (Podilymbus podiceps). We assumed that because these birds share similar diets (including leafy vegetation, seeds, and invertebrates) and digestive processes (Ehrlich et al. Citation1988), they likely excrete equivalent nutrients relative to their body weight. Nutrients from gulls, cormorants, and herons were estimated based on percentages of nutrients found in their guano, following Marion et al. (Citation1994).
Table 1. Nutrient input factors used for various waterfowl taxon or functional groups, provided as g/dry wt of TN or TP excreted per day in feces for the individual taxon or taxa group.
Pied-billed grebes (Podilymbus podiceps) and cormorants were treated differently because these birds mostly eat a carnivorous diet of aquatic invertebrates and fish (Erhlich et al. Citation1988). Nutrients contributed to the lake by pied-bill grebe feces were derived by multiplying each monthly mean number of grebes per day by the ratio of average grebe body weight to cormorant body weight (due to the larger size of cormorants), and again by the estimated nutrients excreted daily by a cormorant. Cormorants were observed mostly on the lake prior to dusk and use the lake as a night roosting area in the winter; therefore, to calculate TN and TP excreted into the lake by cormorants, daily estimates were divided by half representing only the evening hours. Bird species other than cormorants did not show a pattern of absence from the lake and thus were assumed to utilize the lake habitat all day. The estimated monthly percentage of TN and TP contributed by each bird taxon or functional group () was calculated using the weight (in mg) of TN and TP excreted monthly by birds on the water.
Results and discussion
Greenfield Lake water quality
Water temperature in the lake ranged from 4.5 to 32.8 C during the 2006–2013 study period and did not significantly differ among sampling sites. Average turbidity and TSS were low to moderate () and likewise did not differ significantly among sites; however, average DO concentrations were significantly lower at the uppermost site GL-2340 in comparison to the mid-lake location GL-YD, which in turn was significantly lower than the farthest downstream site GL-P (). Site GL-2340 was located farthest from the solar-powered mixers () and thus received no artificial aeration; its average DO of 5.25 mg/L was near the North Carolina water quality standard of 5.0 mg/L (NCDENR Citation2003).
Table 2. Selected water quality parameters in Greenfield Lake before (Jan 2000–Jan 2005) and after (Mar 2005–Dec 2013) lake rehabilitation measures in 2005, by individual station and all data combined. Presented as mean ± standard deviation/range.
Figure 2. Water quality parameter concentrations in Greenfield Lake, 2006–2013, presented by sampling station as means ±1 standard error of the mean (n = 50 samples). Letters above the bars designate and rank significant (P < 0.05) differences among stations. Panel a = dissolved oxygen, panel b = nitrate, panel c = orthophosphate, panel d = DIN/DIP ratio, panel e = chlorophyll a, panel f = fecal coliform bacteria.
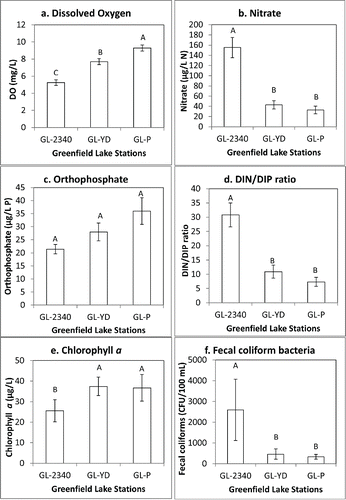
Nitrogen and P concentrations overall in Greenfield Lake from 2006 to 2013 were moderate to high (). Based on Wetzel (Citation2001, tables 13–18), average TN concentrations were in the mesotrophic range whereas average TP concentrations were indicative of eutrophic conditions in lakes and reservoirs. In addition, a statistical reanalysis of nutrient data from a large set of lakes and reservoirs placed Greenfield Lake TN in the mesotrophic range and TP concentrations in the eutrophic range (Dodds et al. Citation1998; ). TN did not significantly differ among the stations, but dissolved inorganic N (DIN) showed a defined pattern of incoming water (i.e., uppermost lake station GL-2340) having higher DIN relative to other sites. Nitrate concentrations were significantly higher in station GL-2340 (on average >4-fold higher) than concentrations at mid-lake stations GL-Y and GL-P (). Total ammonia concentrations were also significantly (P < 0.05) higher at GL-2340 than at the 2 more downstream locations. In contrast, orthophosphate did not significantly differ among sites (), and TP concentrations were significantly (P < 0.05) lower at upstream site GL-2340 (average 86.7 µg/L) compared to mid-lake stations GL-YD (97.1 µg/L) and GL-P (96.8 µg/L). Dissolved N/P molar ratios were 3- to 4-fold higher on average at GL-2340 and were significantly (P < 0.05) higher than DIN/DIP at the mid-to-lower lake stations (). These data indicate that the majority of inorganic N enters the lake via upstream tributaries, whereas P seems to have more near-lake sources. Rainfall runoff was correlated with nutrient concentrations, but results varied among sites. For all stations combined, ammonium was positively but weakly correlated with rainfall (r = 0.241, P = 0.012), as was orthophosphate (r = 0.213, P = 0.027). At GL-2340, only orthophosphate was significantly correlated with rainfall (r = 0.483, P = 0.003); at GL-YD, only nitrate was correlated with rainfall (r = 0.372, P = 0.028) whereas at GL-P none of the nutrient parameters correlated with rainfall. Based on the relationships between precipitation and nutrients as well as the watershed to lake area ratio and developed nature of the watershed, nutrient inflows and flushing are clearly important considerations for the management of Greenfield Lake.
Table 3. Monthly average total waterfowl counts and estimated waterfowl nutrient deposition to the lake and shoreline.
Average concentrations of the nutrient response variable, chlorophyll a, were representative of eutrophic conditions when compared to large numbers of lakes and reservoirs (Dodds et al. Citation1998, Wetzel Citation2001). As a further metric, North Carolina has a chlorophyll a standard for eutrophic conditions of 40 µg/L (NCDENR Citation2003). As a local comparison, Lake Waccamaw is a rare natural blackwater lake (a bay lake) with a similar depth and latitude as Greenfield Lake, located about 60 km west of Wilmington. In contrast to Greenfield Lake, its watershed is lightly developed (the Town of Lake Waccamaw population varies little, ∼1480 yearly) with many lakeside home vacation rentals. Average water column chlorophyll a in this lightly developed system is far lower than in Greenfield Lake, ranging from 1.0 to 6.0 µg/L (Cahoon and Owen Citation1996).
The within-lake distribution pattern of chlorophyll a was opposite to that of incoming nitrate and ammonium, with significantly lower chlorophyll upstream at GL-2340 compared with concentrations at mid-to-lower lake stations GL-YD and GL-P (). From 2006 to 2013, lakewide chlorophyll a was highly correlated with BOD5 (r = 0.678, P < 0.0001). In addition, chlorophyll a was strongly correlated with TSS (r = 0.584, P < 0.0001) and was also correlated with turbidity (r = 0.214, P = 0.013). Average BOD5 was 3.6 mg/L and ranged up to 16.0 mg/L (), and the more upstream station GL-2340 had significantly (P < 0.05) lower BOD5 (3.2 mg/L) than both GL-YD (4.0 mg/L) and GL-P (3.6 mg/L). BOD5 concentrations in this lake were generally high in comparison to concentrations in a wide selection of lakes and streams in the southeastern United States (Mallin et al. Citation2006).
Fecal coliform bacterial concentrations in Greenfield Lake were generally high (). Although concentrations were significantly higher at GL-2340, all 3 sites had concentrations representative of polluted waters (). For reference, in North Carolina freshwaters, geometric mean fecal coliform concentrations for 5 samples collected within 30 d that exceed 200 colony-forming units (CFU)/100 mL of water indicate impaired waters (NCDENR Citation2003). Because no point-source outfalls enter this lake, likely sources include the waterfowl inhabiting and visiting the lake (discussed later), resident urban wildlife, and the fecal contributions of pet waste and urban wildlife from stormwater draining from the urban (37% impervious surface coverage) watershed. Fecal bacterial densities for the 3 sites, considered collectively, were positively correlated with rainfall (r = 0.396, P < 0.0001). At all 3 stations, fecal coliforms were correlated with rainfall: GL-P (r = 0.344, P = 0.040), GL-YD (r = 0.540, P = 0.001), and GL-2340 (r = 0.346, P = 0.036). Thus, fecal microbes from dog, cat, urban wildlife, and waterfowl waste likely enter the lake directly from the park environs during rain events, and fecal materials are carried by runoff into the tributaries from neighborhoods in the watershed, as well. Although waterfowl can be a significant source of fecal microbial contamination to some waterways (Weiskel et al Citation1996, Hoyer et al. Citation2006), bacteria contributed by the waterfowl population were not calculated in this study because no values for excreted fecal bacteria have been determined for some of the most numerous bird groups observed on Greenfield Lake.
Nutrient bioassays
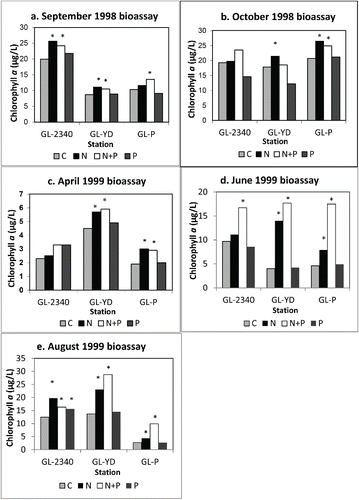
In 11 of the 15 nutrient addition bioassay experiments completed on water collected from the 3 sampling sites, chlorophyll a was significantly higher in the N-alone treatment than in controls; and in 12 of the 15 experiments, chlorophyll a was significantly higher in the N+P treatment than in controls (). In contrast, chlorophyll a was significantly higher in only 1 of 15 P-only experiments relative to control concentrations. The one exception was in water from station GL-2340, the site farthest upstream with highest N overall (); thus, experimental evidence indicates that N was clearly the principal stimulatory nutrient overall in this lake. Note that water from GL-2340 responded on fewest occasions to N (2 of 5 experiments) whereas the 2 downstream sites responded in either 4 of 5 or 5 of 5 experiments. This finding is not unexpected considering that GL-2340 yielded significantly higher nitrate, total ammonia, and DIN/DIP levels than the 2 mid-to-lower lake stations (). Phytoplankton production in inland waters most frequently responds to P inputs (Hecky and Kilham Citation1988), although some inland waters respond to N inputs (Lewis and Wurtsbaugh Citation2008). In blackwater southeastern streams and rivers, however, inputs of dissolved N rather than P seem to be most stimulatory to phytoplankton production (Mallin et al. Citation2004). Although relatively few blackwater lakes and impoundments have been tested for nutrient limitation, the response of this blackwater impoundment to N additions is similar to the responses documented for blackwater lotic systems.
Figure 3. Results of nutrient addition bioassay experiments from 1998 and 1999. Bars represent means of 3 replicates per treatment averaged over 3 days; *above bar represents significant (P < 0.05) difference from control. Panel a = September 1998, panel b = October 1998, panel c = April 1999, panel d = June 1999, panel e = August 1999.
Waterfowl nutrient contributions
Of the species and ecological groupings of waterfowl quantified in this study (), great (Phalacrocorax carbo) and double-crested (P. auritus) cormorants, Canada geese (Branta canadensis), and American widgeons (Mareca americana, a dabbling duck) were most abundant during the winter months, and among all birds considered, these species likely excreted the greatest amount of TN into the water at GL-YD. Similarly, the American coot population peaked during the winter months with a daily abundance ranging from an average of 169.3 (SE = 46.8) in December to 304.7 (SE = 32.0) in February. Calculations of nutrients defecated by American coots yielded the greatest amount of TN to the water near GL-P, an annual amount of ∼9.3 kg dry weight. At GL-P, coots frequently were observed foraging on submerged macrophytes.
Our data clearly demonstrated that the major waterfowl use of Greenfield Lake occurs November through February (). The major avian contribution of N occurred December 2000–January 2001 when populations of American coots, Canada geese, and dabbling ducks increased. The impact of waterfowl excretion on TP in the lake was much greater than that for TN. Again, the biggest impact was in winter, particularly December 2000–March 2001. Possibly as a result of P from guano, water-column orthophosphate concentrations increased throughout winter 2000–2001 over 6 months at the 2 most bird-influenced stations GL-P and GL-YD. P concentrations at GL-P peaked in April 2001 before declining again (as mg/L P; 0.005, 0.010, 0.040, 0.050, 0.030, 0.120 by month, November through April, respectively; and at GL-YD from 0.005, 0.010, NA, 0.030, 0.070, 0.110 during those same months). Significant guano contributions to lake P have been noted in other waterfowl-rich systems (Scherer et al. Citation1995, Moore et al. Citation1998). Waterfowl P load to Greenfield Lake during the study year was about 88.5 kg; for comparison, Manny et al. (Citation1994) found that during the year waterfowl contributed 88 kg of P to Wintergreen Lake, Michigan, and Marion et al. (Citation1994) found that waterfowl contributed 2000 and 2530 kg of P, respectively, to Grand-Lieu, France, during 2 different years. In Greenfield Lake, the estimated dry weight of TP excreted by waterfowl both on the water and the surrounding lake shores indicated that highest amounts were contributed by cormorants, with annual TP input estimated at ∼66.5 kg dry weight. Cormorants contributed the highest average percent of TP found in the water column per month (), followed by dabbling ducks, geese, coots, and grebes.
Guanotrophy contributes to a reservoir of P in the sediments during the winter months, some of which becomes available to phytoplankton and free-floating macrophytes (such as duckweed) in warmer months when DO is lower (Scherer et al. Citation1995). Water-column TP was positively correlated with water temperature (r = 0.296, P = 0.0002), and P from shallow lake and reservoir sediments is known to enter the water column in summer (Wetzel Citation2001). In other shallow eutrophic lakes, sediment release of labile P can account for a substantial portion of, or even exceed, P loading from outside of the lake (Fisher et al. Citation2005, Kelton and Chow-Fraser Citation2005). This P loading to Greenfield Lake would support the formation of the blooms of N-fixing (i.e., heterocyte-containing) Anabaena sp., which occurred in the past and continue today. Whereas guanotrophy can supply large amounts of P to Greenfield Lake in winter, avifauna seem to contribute little N to this N-limited system overall.
This study indicates that cormorants add considerably more TP compared to other bird groups. Cormorants were observed flying early in the morning west from the lake toward the direction of the Cape Fear River (<1 km away), then in the evening they congregated in several cypress trees located in the lake's center. Cormorants feed mostly on small fish during the day and therefore likely added “new” nutrients sourced from the river into the lake system, whereas other bird species were essentially recycling nutrients from the water and surrounding shoreline. As noted elsewhere (Scherer et al. Citation1995), this nutrient recycling by waterfowl changes the form of the nutrient from an organic prey item to a mostly more labile remineralized form that can be assimilated by algae, macrophytes, and bacteria. The cormorant behavior is an example of translocation of nutrients (i.e., when an animal feeds in one location and goes elsewhere to excrete; Vanni Citation2002). The long-term impact of cormorants and other waterfowl utilizing Greenfield Lake as a roosting habitat is likely enriching the sediments with P, stimulating subsequent algal blooms and growth of free-floating macrophytes such as duckweeds and ultimately exacerbating the eutrophication of this urban lake.
Impact of rehabilitation measures
We considered 2 questions. First, what were the impacts to water quality of the set of lake rehabilitation measures performed, and second, why did rehabilitation measures cause a significant increase in algal bloom frequency and biomass as chlorophyll a? First, visually, based on decreased public complaints and city maintenance staff observations, excessive surface macrophyte and algal growth has declined. Based on the overall means between the pre- and post-rehabilitation periods, no significant lake-wide differences were observed in concentrations of DO, turbidity, TSS, or fecal coliform bacteria () or significant differences among the individual stations for the physical parameters (). Incidences of DO violations <5.0 mg/L decreased from 27 to 20% of total samples (). Thus, aeration improvement from the solar mixers seem to have had a positive impact from a regulatory standpoint (i.e., fewer DO violations) and aided in preventing the occasional near-anoxic conditions that occurred pre-rehabilitation.
Table 4. Number and percent of dates when water samples exceeded North Carolina water quality standards before (Jan 2000–Jan 2005) and after (Mar 2005–Dec 2013) lake aeration, grass carp additions, and herbicide treatments.
There were no statistically significant changes in TN, TP, or orthophosphate concentrations based on overall means during the 2 periods, but there was a significant overall 50% decrease in ammonium (). There were highly significant ammonium reductions at stations GL-P and GL-YD and a significant nitrate reduction at GL-P (). This observation was noteworthy because we originally assumed there would be notable inorganic nutrient release from macrophyte decomposition (Dierberg et al. Citation1993), especially following herbicide treatments (O'Dell et al. Citation1995), and also from excretion of nutrients via grass carp feeding (see review in Leslie et al. Citation1987). The ammonium (and to a degree nitrate) decrease was also likely responsible for a decrease in the DIN/DIP ratio (). Whereas aeration due to the artificial mixing might be expected to enhance microbial nitrification, overall mean nitrate did not increase pre- to post-rehabilitation effort. Average orthophosphate and TP concentrations qualitatively suggested an apparent downward trend post-rehabilitation effort (), but there was no significant difference in concentrations during the 2 periods for the pooled data or at individual stations (P > 0.05); additionally, median orthophosphate concentrations stayed the same pre- and post-rehabilitation, at 20 µg/L P, and median TP increased from 60 to 80 µg/L P following rehabilitation. Evidently, TP inputs to the water column vary from year to year depending on the waterfowl community composition and abundance, a factor not affected by the present lake management efforts.
A noteworthy and statistically significant (P < 0.0001) finding was the 74% increase in overall mean chlorophyll a concentrations following the attempted rehabilitation measures (); additionally, median chlorophyll a increased from 9.5 to 22.0 µg/L. Chlorophyll increases were significant at both GL-P and GL-YD (). Pertinent to a regulatory standpoint, the percent of chlorophyll a water quality standard violations tripled following installation of mixers, introduction of grass carp, and herbicide treatments (). Average BOD5 (correlated with chlorophyll a) increased by 20% following attempted rehabilitation measures (), but this change was not statistically significant. The increase in chlorophyll violations led the North Carolina Department of Environment and Natural Resources (NCDENR) in 2014 to add Greenfield Lake to the North Carolina 303(d) list for chlorophyll violations. Regarding cyanobacteria, surface scums of Microcystis aeruginosa have not been noted following rehabilitation measures, but water-column blooms of N-fixing Anabaena sp. continue to occur, with lake-wide blooms recorded by our laboratory and NCDENR in 2011 and 2014. Evidently, the ammonium, and likely also the bioavailable P (Dierberg Citation1993), remineralized from the various restoration measures promoted increased algal blooms, as reported elsewhere following herbicide and grass carp treatments (Richard et al. Citation1984, O'Dell et al. Citation1995). Nitrate entering from the tributaries continues to supply the principal stimulatory nutrient, N (as “new” N), to the phytoplankton. In other blackwater systems, nitrate, total ammonia, and urea have all been shown to stimulate phytoplankton growth (Mallin et al. Citation2004). Moreover, in other southeastern ecosystems, ammonium has been demonstrated to preferentially enhance cyanobacterial production in summer (Siegel et al. Citation2011). Additionally, Greenfield Lake is a shallow lake, and the circulators are designed to bring bottom-associated waters to the surface; thus, nutrients associated with the bottom are likely entrained in this water and brought into the upper euphotic zone where they become available for use by phytoplankton.
The second question considered is why the rehabilitation measures caused a significant increase in algal bloom frequency and biomass as chlorophyll a. We hypothesized that none of the attempted improvement measures addressed the underlying, fundamental cause of eutrophication in Greenfield Lake, which is excessive nutrient inputs, especially N. The mixers do nothing to reduce nutrient inputs; to the contrary, they likely make bottom-associated nutrients more available to the phytoplankton in the water column. Grass carp consume macrophyte vegetation but also excrete substantial dissolved nutrients (such as ammonium) into the water column. Herbicides kill macrophytes and filamentous algae, which decompose and release N and P into the water column for further stimulation of the phytoplankton.
Conclusions and the way forward
Rehabilitation measures, applied with the best of intentions, can clearly have unintended consequences. The measures performed on Greenfield Lake have improved the appearance of the lake to the public and have improved DO concentrations by eliminating near-anoxia incidents (); thus, DO standard violations have decreased by 26%. These measures also have led to a tripling of chlorophyll a violations, however, which have placed this lake on the North Carolina 303(d) list of state impaired waters, which will require a total maximum daily load (TMDL). Chlorophyll a is strongly correlated with BOD5 in this lake, indicating that the algal blooms can lead to hypoxia. At present, the solar-powered mixers bring hypolimnetic water to the surface for aeration, and if they are removed, DO standard violations likely will increase.
Greenfield Lake has clearly received long-term excessive nutrient loading and will continue to display symptoms of eutrophication (algal blooms and elevated BOD) until nutrient inputs are decreased. Nutrient reduction is considered the key to eutrophication control (Smith et al. Citation1999). We note here that control of both N, which tends to principally enter the lake from tributaries, and P, which seems to have more localized sources, is important. Controlling only N runs the risk of increasing the number of blooms of N-fixing cyanobacteria (Lewis and Wurtsbaugh Citation2008, Schindler et al. Citation2008), and Greenfield Lake already has the flora (Anabaena) with the potential to do so. A positive step would be to initiate an investigation of nutrient loading from the tributaries vs. lake outflow nutrient discharge to determine which tributaries should be most effectively targeted for best management practices (such as buffers, rain gardens, constructed wetlands) designed to reduce nutrient inputs, as well as how precipitation events impact spatial loading and flushing. Some of the best management practices are likely to reduce fecal bacteria pollution as well.
A complementary approach, if dredging nutrient-rich sediments becomes an option, would be to perform comprehensive lake-wide sediment sampling to determine where sediment P concentrations (as well as N) are highest. Decreasing P deposition from resident waterfowl and seasonal waterfowl defecation can be accomplished by nonlethal means (use of border collies and Shetland sheepdogs to harass waterfowl into moving elsewhere is effective); however, removal of waterfowl may not prove politically feasible (i.e., popular). In addition, pet waste manure is abundant, has low N/P ratios, and contains high fecal bacterial loads and should therefore be vigorously targeted. The city has a pet waste ordinance, although law enforcement does not aggressively target owners who do not collect their pet's waste. In addition to the internal release of P from sediments, ammonium, and orthophosphate will continue to be remineralized from grass carp feces and decaying macrophytes treated by herbicides and rapidly cycled into new phytoplankton. Thus, an additional option that might prove useful in nutrient removal from the main lake as well as targeted tributaries is use of floating macrophyte islands to enhance nutrient removal by vegetation uptake, repackaging, and especially denitrification (Borne et al. Citation2013). The measures taken to date to rehabilitate this lake do not include an aggressive nutrient-removal component, and algal blooms will continue to flourish, including nuisance cyanobacterial blooms.
Acknowledgments
For facilitation, we thank Dr. J.F. Merritt of UNCW, and D.B. Mayes and M. Hayes of Wilmington Stormwater Services. For field and laboratory assistance, we thank L. Blaylock, L. Bohrer, J. Cook, D. Elks, Dr. S.H. Ensign, J. Harding, V. Johnson, T. MacPherson, D. Parsons, A. Roberts, M. Turner and H. Wells. We thank Drs. J.M. Burkholder and L.B. Cahoon for manuscript comments, as well the manuscript reviewers.
Funding
We thank the City of Wilmington Stormwater Services Department and the University of North Carolina Wilmington for funding support.
References
- [APHA] American Public Health Association. 2005. Standard methods for the examination of water and wastewater, 21st ed. Washington (DC).
- Benton C, Khan F, Monoghan P, Richards WN, Shedden CB. 1983. The contamination of a major water supply by gulls (Larus sp.). Water Res. 17:789–798.
- Borne KE, Tanner CC, Fassman-Beck EA. 2013. Stormwater nitrogen removal performance of a floating treatment wetland. Water Sci Technol. 68:1657–1664.
- Burkholder JM, Glibert PM. 2013. Eutrophication and oligotrophication. In: Encyclopedia of biodiversity, vol. 3. Elsevier. p. 347–371.
- Cahoon LB, Owen, DA. 1996. Can suspension feeding by bivalves regulate phytoplankton biomass in Lake Waccamaw, North Carolina? Hydrobiologia. 325:193–200.
- Day RW, Quinn GP. 1989. Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr. 59:433–463.
- Dierberg FE. 1993. Decomposition of desiccated submersed aquatic vegetation and bioavailability of released phosphorus. Lake Reserv Manage. 8:31–36.
- Dodds WK. 2003. Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J N Am Benth Soc. 22:171–181.
- Dodds WK, Jones JR, Welch EB. 1998. Suggested classification of stream trophic state: distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res. 32:1455–1462.
- Ehrlich PR, Dobkin DS, Wheye D. 1988. The birder's handbook: a field guide to the natural history of North American birds. New York (NY): Simon & Schuster Inc.
- Fisher MM, Reddy KR, James RT. 2005. Internal nutrient loads from sediments in a shallow, subtropical lake. Lake Reserv Manage. 21:338–349.
- Hecky RE, Kilham P. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr. 33:796–822.
- Hoyer MV, Donze JL, Schulz EJ, Willis DJ, Canfield DE Jr.. 2006. Total coliform and Escherichia coli counts in 99 Florida lakes with relation to some common limnological factors. Lake Reserv Manage. 22:141–150.
- Hudnell HK. 2010. Freshwater harmful algal blooms (FHAB) suppression with solar powered circulation (SPC). Harmful Algae. 9:208–217.
- Hussong D, Damare JM, Limpert RJ, Sladen WJL, Weiner RM, Colwell RR. 1979. Microbial impact of Canada Geese (Branta canadensis) and Whistling Swans (Cygnus columbianus columbianus) on aquatic ecosystems. Appl Environ Microbiol. 37:14–20.
- Kelton N, Chow-Fraser P. 2005. A simplified assessment of factors controlling phosphorus loading from oxygenated sediments in a very shallow eutrophic lake. Lake Reserv Manage. 21:223–230.
- Leslie AJ Jr, Van Dyke JM, Hestand III RS, Thompson BZ. 1987. Management of aquatic plants in multi-use lakes with grass carp (Ctenopharyngodon idella). Lake Reserv Manage. 3:266–276.
- Lewis WM Jr, Wurtsbaugh WA. 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. Int Rev Hydrobiol. 93:446–465.
- Mallin MA, Johnson VL, Ensign SH, MacPherson TA. 2006. Factors contributing to hypoxia in rivers, lakes and streams. Limnol Oceanogr. 51:690–701.
- Mallin MA, McIver MR, Ensign SH, Cahoon LB. 2004. Photosynthetic and heterotrophic impacts of nutrient loading to blackwater streams. Ecol Appl. 14:823–838.
- Manny BA, Johnson WC, Wetzel RG. 1994. Nutrient additions by waterfowl to lakes and reservoirs: predicting their effects on productivity and water quality. Hydrobiologia. 279/280:121–132.
- Marion L, Clergeau P, Brient L, Bertru G. 1994. The importance of avian contributed nitrogen (N) and phosphorus (P) to Lake Grand-Lieu, France. Hydrobiologia. 279/280:133–147.
- Moore MV, Zakova P, Shaeffer KA, Burton RP. 1998. Potential effects of Canada geese and climate change on phosphorus inputs to suburban lakes of the northeastern U.S.A. Lake Reserv Manage. 14:52–59.
- [NCDENR] North Carolina Department of Environment and Natural Resources. 2003. Amended effective 1 April 2003. “Redbook” - Surface waters and wetlands standards (NC Administrative Code 15A NCAC 02B .0100 & .0200). Raleigh (NC).
- O'Dell KM, Van Arman J, Welch BH, Hill SD. 1995. Changes in water chemistry in a macrophyte dominated lake before and after herbicide treatment. Lake Reserv Manage. 11:311–316.
- Osgood D. 2015. Do you want something that works? NALMS Lakeline 35:8–16.
- Portnoy JW. 1990. Gull contributions of phosphorus and nitrogen to a Cape Cod kettle pond. Hydrobiologia. 202:61–69.
- Richard DI, Small JW Jr, Osborne JA. 1984. Phytoplankton responses to reduction and elimination of submerged vegetation by herbicides and grass carp in four Florida lakes. Aquat Bot. 20:307–319.
- Ross MA, Lembi CA. 1998. Applied weed science, 2nd ed. Upper Saddle River (NJ): Prentice Hall.
- Scherer NM, Gibbons HL, Stoops KB, Muller M. 1995. Phosphorus loading of an urban lake by bird droppings. Lake Reserv Manage. 11:317–327.
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-comment whole-ecosystem experiment. P Natl Acad Sci. 105:11254–11258.
- Schlotzhauer SD, Littell RC. 1997. SAS system for elementary statistical analysis, 2nd ed. Cary (NC): SAS Institute, Inc.
- Siegel A, Cotti-Rausch B, Greenfield DI, Pinckney JL. 2011. Nutrient controls of planktonic cyanobacteria biomass in coastal stormwater detention ponds. Mar Ecol Prog Ser. 434:15–27.
- Smith VH, Tilman GD, Nekola JC. 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine and terrestrial ecosystems. Environ Pollut. 100:179–196.
- [USEPA] United States Environmental Protection Agency. 1983. Methods for chemical analysis of water and wastes. Washington (DC): EPA-600/4-79-020.
- [USEPA] United States Environmental Protection Agency. 1997. Methods for the determination of chemical substances in marine and estuarine environmental matrices, 2nd ed. Cincinnati (OH): EPA/600/R-97/072.
- Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Ann Rev Ecol Syst. 33:341–370.
- Wacklin P, Hoffman L, Komarek J. 2009. Nomenclature validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet et Flahault) comb. Nova. Fottea. 9:59–64.
- Weiskel PK, Howes BL, Heufelder GR. 1996. Coliform contamination of a coastal embayment: sources and transport pathways. Environ Sci Technol. 30:1872–1881.
- Welschmeyer NA. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and phaeopigments. Limnol Oceanogr. 39:1985–1993.
- Wetzel RG. 2001. Limnology: lake and river ecosystems, 3rd ed. San Diego (CA): Academic Press.
