ABSTRACT
Carlson AK, Bailey PE, Fincel MJ, Graeb BDS. Otoliths as elemental tracers of walleye environmental history: insights for interjurisdictional fisheries management. Lake Reserve Manage. 32:329–340.
Understanding fish natal origins and movement is important for managing interjurisdictional fisheries. We used otolith microchemistry to assess walleye (Sander vitreus) provenance, movement, and natal homing in Lake Oahe, an interjurisdictional reservoir fishery in North Dakota and South Dakota. Historical (1983–1989) water chemistry varied among 6 tributaries for strontium:calcium (Sr:Ca; μmol/mol) and barium:calcium (Ba:Ca) and between 2 main-stem sites for Ba:Ca. Current (2012) water chemistry was spatially heterogeneous for Sr:Ca and Ba:Ca. Both ratios were consistent between the historical and current periods in tributaries and main-stem sites. Sr:Ca and Ba:Ca of age-0 walleye otolith cores varied in proportion to water chemistry, resulting in high reclassification accuracies to habitat types (87%) and individual sites (78%). For adult walleye, contributions of South Dakota natal sites to the North Dakota population (48%, n = 30) and North Dakota natal sites to the South Dakota population (48%, n = 29) were highest for tributaries and embayments (e.g., Moreau and Cannonball rivers, Beaver Bay). Annual downstream movement was more prevalent than upstream movement, particularly during a flood in 2011. An average of 36% of North Dakota walleye and 33% of South Dakota walleye exhibited natal homing each year from 2009 to 2013. Otolith element:Ca ratios are effective natural tracers for evaluating walleye natal origins, movement within and between states, and natal homing. Otolith microchemistry is a tool for interjurisdictional walleye management in reservoirs, providing a methodology for assessing natal origins and movement and designing spatially informed habitat conservation programs and harvest regulations.
Ecosystem-based fisheries management is an emerging concept whereby ecological characteristics of aquatic environments are synthesized with social and economic conditions to manage fisheries as ecosystems (Pikitch et al. Citation2004). As an adaptive paradigm, ecosystem-based fisheries management is responsive to changing ecological conditions and readily accommodates scientific discoveries (Fogarty Citation2014). For instance, knowledge of fish natal origins and movement patterns in reservoirs can be used to design spatially informed management strategies. To date, implementation of ecosystem-based fisheries management has been hindered by ecosystem complexity, excessive monetary cost, and perceived ineffectiveness (Pitcher et al. Citation2009, Hilborn Citation2011, Cowan et al. Citation2012). Despite these obstacles, current governance structures, data availability, and financial and human resources render ecosystem-based fisheries management feasible (Patrick and Link Citation2015). Fisheries agencies, universities, and other institutions have established frameworks for ecosystem-based fisheries management, including long-term ecological monitoring programs for plankton and recreational and nongame fishes (Fogarty Citation2014).
Ecosystem-based fisheries management is particularly important in interjurisdictional systems. Percids (Pegg et al. Citation1997), salmonids (Keefer et al. Citation2006), acipenserids (Wishingrad et al. Citation2014), and other fishes make long-distance movements across jurisdictional boundaries (e.g., state lines) throughout their life history. Thus, it is important for fisheries managers to evaluate movement among jurisdictions to design spatially informed management strategies (McGee Citation2012). Walleye (Sander vitreus) is an ecologically and recreationally important species throughout Canada and the United States (McMahon et al. Citation1984). Walleye persist in large systems that cross state and/or federal boundaries (e.g., rivers, Great Lakes), yet natal origins and movement are often difficult to measure, hindering development of interjurisdictional management approaches. The Missouri River is the longest river in North America, subdivided into a main-stem reservoir system of 6 impoundments extending from Montana to northeastern Nebraska. Water levels are managed by the United States Army Corps of Engineers (USACE) according to a Master Manual, the primary guidance document for reservoir operation (Erickson et al. Citation2008). Lake Oahe is the second-largest impoundment in the Missouri River reservoir system, and its walleye population is jointly managed by the North Dakota Game and Fish Department (NDGF) and the South Dakota Department of Game, Fish and Parks. Mark–recapture tagging studies indicate that walleye routinely move between states (NDGF and South Dakota State University, unpubl. data), but the extent and frequency of movement are unknown. Without methods to evaluate natal origins and movement within and between states, fisheries managers have limited ability to implement spatially informed management strategies. Walleye are known to return to their natal tributaries in Lake Oahe (Riis Citation1985), but the contemporary magnitude and extent of natal homing are unknown. Moreover, site-specific natal contributions are poorly understood. Development of a reliable technique for quantifying natal origins, movement within and among jurisdictions, and natal homing would improve walleye management in Lake Oahe.
Otolith microchemistry is an effective technique for investigating fish environmental history, including natal origins (Zeigler and Whitledge Citation2010, Citation2011, Wolff et al. Citation2012) and movement (Brenkman et al. Citation2007, Allen et al. Citation2009). Otoliths accrete permanent depositions of certain trace elements (e.g., strontium [Sr], barium [Ba]) in proportion to water column concentrations, making them useful for environmental history assessment if water and otolith concentrations are spatially heterogeneous (Campana et al. Citation2000). Because the Missouri River flows through areas with diverse surficial and bedrock geology, otolith microchemistry may allow fisheries managers to evaluate natal origins, habitat- and site-specific natal contributions, and movement across jurisdictional boundaries. In turn, managers could prioritize habitat types (e.g., tributaries, embayments, main-stem locations) and specific sites for habitat protection/restoration and harvest regulations and thereby advance walleye management in Lake Oahe.
Our goal was to examine walleye natal origins, habitat- and site-specific natal contributions, and interjurisdictional movement in Lake Oahe. Our objectives were to (1) assess the utility of otolith microchemistry as an environmental history tool; (2) identify natal sites and compare the relative natal contribution of tributaries and embayments between North Dakota and South Dakota; and (3) quantify the magnitude and extent of interstate movement and natal homing under different discharge scenarios. We hypothesized that otolith microchemistry would be an effective tool for evaluating walleye environmental history in accordance with previous otolith research (Zeigler and Whitledge Citation2010, Citation2011, Martin et al. Citation2012). We expected that the natal contributions of tributaries would exceed those of embayments and main-stem locations (Riis Citation1985). Moreover, we predicted that walleye would exhibit natal homing and interstate movement, which have been documented previously in Lake Oahe (Riis Citation1985). In particular, we hypothesized that downstream movement of walleye from North Dakota to South Dakota would increase under elevated (i.e., flood) discharge.
Methods
Study site
This study was conducted in Lake Oahe, which spans ∼150,000 ha across a latitudinal gradient >350 km in North Dakota and South Dakota (Erickson et al. Citation2008). Cenozoic glacial sediments (e.g., Illinois and Wisconsin glacial sediments) predominate on the eastern side of Lake Oahe, whereas older Mesozoic sediments (e.g., sandstone, shale, clay) exhibit a north–south gradient west of the impoundment (SDDENR Citation2015). The study area extended from Garrison Dam (northerly boundary) to Oahe Dam (southerly boundary), a section of the Missouri River that includes 6 major tributaries with drainage areas >5000 km2 (USGS Citation2016), including the Cannonball River (drainage area: 10,619 km2), Cheyenne River (55,299 km2), Grand River (13,768 km2), Heart River (8573 km2), Knife River (5802 km2), and Moreau River (12,662 km2).
Walleye field surveys
We collected 37 age-0 walleye during their first growing season during summer 2012 in 6 tributaries (n = 19 individuals), 4 embayments (n = 12 individuals), and 1 main-stem location (n = 6 individuals) using nearshore electrofishing (pulsed DC, 60 pps, 6–8 amps; ). Total length of each individual was measured and ranged from 76 to 114 mm. Spatial separation of adult walleye sampling in upper and lower Lake Oahe (i.e., North Dakota and South Dakota) was critical to address the objectives of this study. We collected 63 adults from North Dakota (hereafter North Dakota walleye) at 5 sites in upper Lake Oahe and the riverine segment above the reservoir (both in North Dakota) during summer 2013 (). We used North American standard core gill nets (Bonar et al. Citation2009) in lentic habitats and nighttime nearshore electrofishing in riverine habitats. We collected 60 adults from South Dakota (hereafter South Dakota walleye) in 5 main-stem and embayment locations in lower Lake Oahe during summer 2013 using North American standard core gill nets (). Adults ranged from 280 to 690 mm and age-2 to age-11 with the majority (93%) age-2 to age-5.
Figure 1. Water and walleye sampling locations in Lake Oahe, North Dakota (ND) and South Dakota (SD), USA. Shapes denote locations where fish of different ages were sampled: right-angle triangles (age-0), upright triangles (adult), diamonds (age-0 and adult). Water samples were collected at all 11 sites where age-0 fish were collected (i.e., right-angle triangles and diamonds). Tributaries are represented by open shapes, and mainstem and embayment sites are represented by filled shapes. Abbreviations are BEV = Beaver Bay; BSH = Bush Landing; CAN = Cannonball River; CHY = Cheyenne River; COW = Cow Creek Bay; GAT = Garrison Tailrace; GRR = Grand River; HAZ = Hazelton; HER = Heart River; KNF = Kneifel Bay; KNR = Knife River; MIN = Minneconjou Bay; MOR = Moreau River; ODF = Oahe Dam face; OKO = Okobojo; STA = Stanton; STK = Steckel; SUT = Sutton; WEP = West Pollock Bay; WSH = Washburn Bay; WWE = West Whitlock Bay.
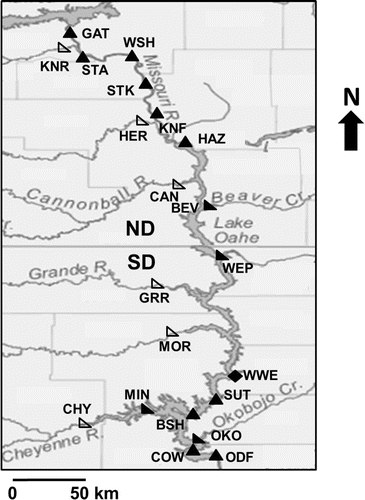
Table 1. Sampling sites for adult walleye in North Dakota (ND) and South Dakota (SD), including the number of fish (n) collected at each site and the total number collected in each state.
Otolith microchemistry
We used otolith microchemistry to assess walleye natal origins and habitat- and site-specific natal contributions, evaluate movement within and between states, and quantify natal homing. The first step in otolith microchemistry is to characterize spatial and temporal patterns in water chemistry. Water samples (n = 2–10/site) were collected from 6 tributaries, 4 embayments, and 1 main-stem locations in upper and lower Lake Oahe during summer (i.e., Jul–Sep) 2012 concurrently with walleye sampling (). Water samples were collected by researchers wearing nitrile gloves and stored in 250 mL acid-washed polyethylene bottles pre-rinsed with water from Lake Oahe. Water chemistry was measured using a syringe filtration method (Shiller Citation2003) used previously in otolith microchemistry studies (Zeigler and Whitledge Citation2010, Citation2011, Phelps et al. Citation2012). Samples were filtered with an acid-washed syringe equipped with a Whatman Puradisc PP 0.45 μm filter into acid-washed polyethylene storage vials and stored in coolers without exposure to light. Samples were shipped to the University of Southern Mississippi, acidified with a solution containing 2% nitric acid, and analyzed with high-resolution inductively coupled plasma mass spectrometry (HR-ICPMS) using 4 calibration standards prepared from National Institute of Standards and Technology (NIST) standards run after every 10 samples (Clarke et al. Citation2007). Concentrations of Sr, Ba, and calcium (Ca) were expressed in units of μg/L and subsequently converted to molar element:Ca ratios (mmol/mol).
Water chemistry in the study region is influenced primarily by surficial and bedrock geology (Gibbs Citation1970, Bickford and Hannigan Citation2005) and is likely stable over time. To confirm this, historical (1983–1989) Sr:Ca and Ba:Ca were compared to current (2012) ratios to evaluate temporal patterns in water chemistry and assess the reliability of otolith microchemistry as an environmental history tool. The US Geological Survey (USGS) National Stream Water-Quality Monitoring Network was used to collect historical Sr, Ba, and Ca concentrations (Alexander et al. Citation1996). Historical spring (Mar–May) and summer (Jul–Sep) water samples (n = 2–4/site) were collected over 5 years (1983–1986, 1989) in 6 Lake Oahe tributaries and 2 main-stem sites also sampled in this study (). Trace element concentrations were quantified using an ICPMS protocol analogous to the one employed herein. Water samples were filtered and fixed in dilute acid solution, after which flame-atomic absorption or solution-mode inductively coupled plasma mass spectrometry was used to measure elemental concentrations, depending on the element and date of sampling (Alexander et al. Citation1996).
Table 2. Mean water Sr:Ca and Ba:Ca (mmol/mol) with 95% confidence intervals for 6 Missouri River tributaries and 2 mainstem sites sampled historically (i.e., 1983–1989) and in 2012 (i.e., this study). Historical and current ranges in Sr:Ca and Ba:Ca are also provided.
All walleye were sacrificed immediately after collection and stored on ice in plastic bags (labeled by site) in sealed coolers until same-day otolith extraction in a laboratory. Plastic forceps were triple-washed in nitric acid and used to remove left and right sagittal otoliths from each individual (Campana et al. Citation2000, Brazner et al. Citation2004). The otolith with the most well-defined annuli was used for age estimation by 3 independent readers (correspondence >90%). All otoliths were triple-rinsed in ultrapure water, air-dried for a minimum of 24 h, and stored in acid-washed 2 mL polypropylene microcentrifuge tubes (Zeigler and Whitledge Citation2010). Adult otoliths were embedded in Epo-Fix epoxy and sectioned in the transverse plane (including the core) using a low-speed Isomet diamond saw (Buehler, Lake Bluff, IL) cleaned with aluminum oxide lapping film (3 µm grit) after each section to prevent contamination. Because of their small size and fragility, age-0 otoliths were placed in thermoplastic cement and ground in the sagittal plane without sectioning. All otoliths were sanded evenly until the core and annuli were at the sample surface, polished with 3M Wetordry sandpaper (400 grit) and aluminum oxide lapping film, mounted on acid-washed petrographic slides (Donohoe and Zimmerman Citation2010), triple-sonicated in ultrapure water, and dried in a Class 100 laminar flow hood for 24 h.
Trace element (i.e., 88Sr, 86Sr, 138Ba, and 137Ba) concentrations were quantified with laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) at the University of California–Davis Interdisciplinary Center for Inductively Coupled Plasma Mass Spectrometry. An Agilent Technologies 7500a quadrupole ICP-MS coupled to a New Wave Research UP-213-nm laser with helium (He) as the carrier gas was used for laser ablation analysis. Laser parameters were 70% energy, 10 Hz, 40 μ spot size, 25 s dwell time, 50 s acquisition, and 25 s background. The USGS synthetic glass standard GSE-1G was used as the calibration standard. Two reference standards (GSD-1G and MACS-3) were used as quality controls to ensure instrumental accuracy and precision. Standards were ablated in 3–5 locations after every 4 samples to adjust for possible instrument drift. Mean limits of detection for 88Sr (0.01) and 137Ba (0.07) were calculated as mean blank values plus 3 standard deviations (Wells et al. Citation2003). Concentrations of both elements were well above detection limits. Data reduction was performed using specialized computer software (Glitter 4.4; GEMOC CSIRO, Macquarie Research Ltd., Macquarie University, Sydney, Australia). Otolith trace element concentrations were background subtracted, and final ratios were obtained by matching 43Ca counts to the sample CaO concentrations obtained independently by electron microprobe. Otolith data were reported as element:Ca ratios (μmol/mol) because Ca is a pseudointernal standard (Bickford and Hannigan Citation2005, Ludsin et al. Citation2006, Whitledge et al. Citation2007).
Otoliths were ablated along a transect of spots encompassing the core, each annulus, and interannual areas as space permitted. Ablation entailed a 15 s laser warm-up time followed by a 20 s dwell time during which the otolith was ablated. The integration time for all elements (0.01 s for 43Ca, 0.05 s for 88Sr and 137Ba) was repeated throughout the 20 s dwell time. Each ablation was followed by a 95 s washout time. Otolith cores were ablated to identify natal origins of age-0 and adult walleye because their chemical composition represents that of natal environments. Along with spot ablations at annual increments, the chemistry of otolith cores was used to assess annual natal homing of adults. Recent environmental history of age-0 walleye was examined to develop site-specific elemental fingerprints for evaluating adult natal origins and homing (Ruttenberg et al. Citation2005). To temporally match otolith and water Sr:Ca and Ba:Ca, otoliths were ablated at terminal edges when water and otolith samples were collected concurrently. Hereafter, these ratios are referred to as “mean terminal.” When logistical constraints prevented same-day water and age-0 collection, age-0 fish were invariably collected after water samples, and thus otoliths were ablated at nonterminal locations synchronized with water sampling. To quantify these “adjusted mean terminal” ratios, water and otolith chemistry were temporally matched by enumerating the time span between water and age-0 sampling and ablating otoliths an equivalent number of daily rings from otolith edges. Temporal matching of water and age-0 otolith chemistry ensured otolith tracers accurately represented known capture locations and were reliable for identifying adult natal origins (Zeigler and Whitledge Citation2010, Citation2011).
Statistical analysis
Water and otolith chemistry
Normality and homoscedasticity of water and otolith element:Ca ratios were assessed using Shapiro–Wilk tests and Levene's tests, respectively. All water ratios (untransformed and log10 transformed) did not meet assumptions of normality and homoscedasticity, so a nonparametric Kruskal–Wallis test was used to compare element:Ca ratios among sites (Blair and Hicks Citation2012, Amano et al. Citation2013). Historical data were separated by habitat type due to time span differences between tributaries and main-stem locations. The Tukey–Kramer–Nemenyi test (Pohlert Citation2014) was used to perform post-hoc multiple comparisons.
Temporal patterns (i.e., seasonal, annual) in historical tributary and main-stem Sr:Ca and Ba:Ca were assessed by determining if interactions existed between sites and time periods (i.e., season, year) as inferred from Friedman's 2-way analysis of variance by ranks (F-ANOVA), a nonparametric alternative to 2-way ANOVA. Summer element:Ca ratios were used to determine if an interaction was present between sites and years. Moreover, long-term patterns in summer Sr:Ca and Ba:Ca were evaluated by determining if interactions existed between sites and time periods (i.e., historical, current) as inferred from F-ANOVA. Means and 95% confidence intervals for Sr:Ca and Ba:Ca were also compared between periods. Elemental fingerprints for evaluating adult environmental history were developed using age-0 terminal (i.e., mean terminal, adjusted mean terminal) otolith Sr:Ca and Ba:Ca, which are known to reflect site-specific ratios of capture locations (Zeigler and Whitledge Citation2010, Citation2011). Age-0 Sr:Ca ratios were normal with equal variances after log10 transformation; thus, concentrations were compared among sites using one-way ANOVA with a Tukey's Honestly Significant Difference post-hoc test. Untransformed and transformed terminal Ba:Ca ratios did not meet parametric assumptions; thus, a Kruskal–Wallis test with Tukey–Kramer–Nemenyi multiple comparisons was used to compare ratios among sites. Statistical significance for all analyses was set at α < 0.05.
Natal origins, movement, and natal homing
K-sample nearest neighbor discriminant analysis was used to assess the accuracy with which age-0 walleye could be reclassified to known natal habitat types (i.e., tributary, embayment, main-stem) and individual sites based on bivariate otolith chemistry (i.e., combined Sr:Ca and Ba:Ca). This nonparametric method allows reliable reclassification when otolith data do not meet assumptions of normality or homoscedasticity (Bickford and Hannigan Citation2005). It assigns age-0 individuals to natal sites to which the majority of their k nearest neighbors belong (Johnson Citation1998). The accuracy of different models (k = 2–8) was evaluated using a leave-one-out jackknife procedure, and the model with the lowest error rate (k = 2) was used to classify adults to natal sites using the known-origin dataset (Ruttenberg et al. Citation2005). Movement and natal homing of adult walleye were assessed annually using site-specific, bivariate elemental fingerprints of age-0 otoliths. Walleye were assigned to sites by matching otolith Sr:Ca and Ba:Ca with site-specific elemental fingerprints, which accurately distinguished sites (see results from K-sample nearest neighbor discriminant analysis discussed later). Movement patterns (e.g., annual downstream and upstream movement vs. residency in sites and states) and natal homing were evaluated by applying this approach across years. Adult natal contribution and homing were summarized as frequencies and percentages by site. All analyses were performed in the program R 3.1.3 (R Development Core Team Citation2015).
Results
Historical water chemistry varied among Lake Oahe tributaries for Sr:Ca (χ2 = 54.57, df = 5, P < 0.01) and Ba:Ca (χ2 = 38.24, df = 5, P < 0.01). Historical main-stem water chemistry was spatially homogenous for Sr:Ca (χ2 = 3.14, df = 1, P = 0.21) and heterogeneous for Ba:Ca (χ2 = 26.17, df = 1, P < 0.01). Spatial patterns were consistent over the historical period because Sr:Ca and Ba:Ca were stable between seasons and among years in tributaries and main-stem sites. In tributaries, interactions were not present between sites and seasons for Sr:Ca (χ2 = 1.29, df = 1, P = 0.26) or Ba:Ca (χ2 = 0.14, df = 1, P = 0.71) or between sites and years for Sr:Ca (χ2 = 2.95, df = 4, P = 0.71) or Ba:Ca (χ2 = 8.55, df = 4, P = 0.13). Similarly, in main-stem sites, interactions were not present between sites and seasons (Sr:Ca: χ2 = 2.00, df = 1, P = 0.16; Ba:Ca: χ2 = 1.00, df = 1, P = 0.32) or sites and years (Sr:Ca: χ2 = 4.21, df = 4, P = 0.38; Ba:Ca: χ2 = 4.40, df = 4, P = 0.35). Current water chemistry was also spatially variable for Sr:Ca (χ2 = 84.59, df = 9, P < 0.01) and Ba:Ca (χ2 = 84.12, df = 9, P < 0.01). Sr:Ca and Ba:Ca were consistent between historical and current time periods in tributaries (Sr:Ca: χ2 = 0.67, df = 1, P = 0.41; Ba:Ca: χ2 = 3.57, df = 1, P = 0.06) and main-stem sites (Sr:Ca: χ2 = 2.00, df = 1, P = 0.16; Ba:Ca: χ2 = 0.33, df = 1, P = 0.56). The 95% confidence intervals of all water element:Ca ratios except for Ba:Ca in the Cheyenne and Heart rivers overlapped between time periods ().
Similar to water chemistry, age-0 otolith element:Ca ratios varied among sites for Sr:Ca (ANOVA: F10,22 = 30.33, P < 0.01) and Ba:Ca (Kruskal-Wallis test: χ2 = 25.75, df = 10, P < 0.01). Linear regressions indicated a positive relationship between water and otolith Sr:Ca (Otolith Sr:Ca = 0.638 + 0.132*Water Sr:Ca, R2 = 0.60, P = 0.002; ) but not Ba:Ca (Otolith Ba:Ca = 0.0027 + 0.0152*Water Ba:Ca, R2 = 0.17, P = 0.21; ). Using bivariate element:Ca ratios, age-0 walleye were reclassified with 86.5% accuracy to habitat types and 78.4% accuracy to individual sites (). Accuracy was highest for tributaries (100.0%), followed by main-stem sites (83.3%) and embayments (66.7%).
Figure 2. Linear regression of (a) Sr:Ca and (b) Ba:Ca of the terminal otolith from age-0 walleye on equivalent ratios in water at collection sites in Lake Oahe. Error bars represent ±1 SE of the mean.
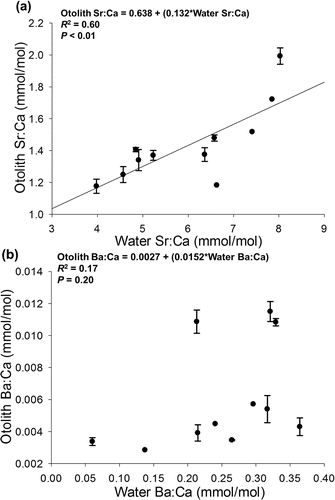
Table 3. Results of k-sample nearest neighbor discriminant analysis with leave-one-out jackknife cross-validation using Sr:Ca and Ba:Ca ratios of age-0 walleyes.
Age-0 otolith Sr:Ca and Ba:Ca were reliable fingerprints for evaluating adult environmental history. Adults from North Dakota and South Dakota hatched at sites within and outside the state where they were collected (). The contribution of South Dakota natal sites to the North Dakota population (47.6%, n = 30) was similar to that of North Dakota natal sites to the South Dakota population (48.3%, n = 29). The Moreau and Cannonball rivers were important natal sites for walleye in both states, whereas Beaver Bay contributed substantially to North Dakota but not South Dakota. Walleye from both states also hatched in tributaries (e.g., Knife, Heart, Grand rivers) and embayments (e.g., West Pollock, West Whitlock, and Minneconjou bays) with lower natal contributions.
Table 4. Percent natal contribution by site and state for adult walleye collected in North Dakota (ND, uppermost column label) and South Dakota (SD, uppermost column label).
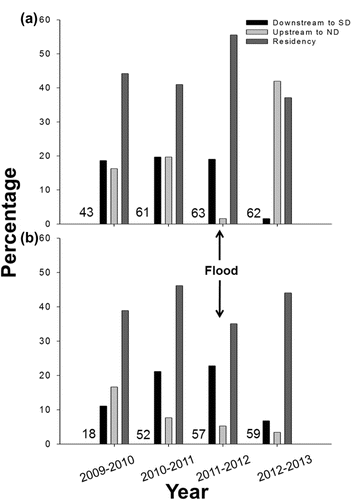
North Dakota and South Dakota walleye exhibited interjurisdictional movements between states ( and ). From 2009–2013, the cumulative percentage of North Dakota walleye that moved downstream to South Dakota (58.9%, ) was greater than the cumulative percentage of South Dakota walleye that moved upstream to North Dakota (33.0%; ). Site residency and downstream movement were prominent during the 2011 flood, whereas upstream movement was minimal (). A relatively large percentage (41.9%, n = 26) of North Dakota walleye returned to North Dakota after the flood, including 10 individuals that moved downstream during the disturbance.
Figure 3. Otolith Sr:Ca (μmol/mol) of (a) North Dakota and (b) South Dakota adult walleye demonstrating representative movement patterns. Sites are labeled, and states in which sites occur (North Dakota [ND], South Dakota [SD]) are provided in parentheses. Movement patterns include residency in one state (i.e., all Sr:Ca values from sites in that state), movement from the other state (i.e., Sr:Ca values switch states once), and movement to and from the other state (i.e., Sr:Ca values switch states twice). For example, the North Dakota walleye depicted by a triangle in plot (a) hatched in the Cannonball River (North Dakota) in 2010, moved downstream to the Moreau River (South Dakota) in 2011, and was captured in Beaver Bay (North Dakota) in 2013.
![Figure 3. Otolith Sr:Ca (μmol/mol) of (a) North Dakota and (b) South Dakota adult walleye demonstrating representative movement patterns. Sites are labeled, and states in which sites occur (North Dakota [ND], South Dakota [SD]) are provided in parentheses. Movement patterns include residency in one state (i.e., all Sr:Ca values from sites in that state), movement from the other state (i.e., Sr:Ca values switch states once), and movement to and from the other state (i.e., Sr:Ca values switch states twice). For example, the North Dakota walleye depicted by a triangle in plot (a) hatched in the Cannonball River (North Dakota) in 2010, moved downstream to the Moreau River (South Dakota) in 2011, and was captured in Beaver Bay (North Dakota) in 2013.](/cms/asset/c360d165-1486-49f8-8344-27c7427ebadb/ulrm_a_1203845_f0003_b.gif)
Figure 4. Annual movement patterns of (a) North Dakota and (b) South Dakota adult walleye from 2009 to 2013. Movement patterns are evaluated at a statewide scale and include downstream movement to South Dakota from North Dakota (Downstream to SD), upstream movement to North Dakota from South Dakota (Upstream to ND), and residency within a state (Residency). Numbers denote sample sizes for each state-time period combination.
The predominance of site residency prompted further investigation into natal homing in Lake Oahe. An average of 36.2% of North Dakota walleye and 33.2% of South Dakota individuals exhibited natal homing each year from 2009 to 2013 (). Homing percentages ranged from 30.2 to 50.0% in North Dakota and 22.2 to 50.0% in South Dakota. Homing to the Moreau River and Beaver bay (6.6–50.0%) occurred more frequently than homing to other tributaries (i.e., Heart River, Grand River; 1.6–2.5%) and embayments (i.e., West Pollock, West Whitlock, and Minneconjou bays; 1.6–3.3%; ).
Table 5. Natal homing of adult walleye in Lake Oahe. Total (ND, SD) denotes the year-specific sample size of North Dakota and South Dakota individuals.
Discussion
As ecosystem-based fisheries management becomes increasingly important for fisheries conservation (Pikitch et al. Citation2004), fisheries managers must conceptualize aquatic environments on broad scales to manage fisheries that bridge jurisdictional boundaries. Our results indicate that otolith microchemistry promotes interjurisdictional walleye management in Lake Oahe by providing novel, ecosystem-scale provenance and movement information. Tributary and embayment Sr:Ca and Ba:Ca were distinct in accordance with underlying geology, serving as accurate elemental tracers for evaluating adult natal origins, habitat- and site-specific natal contributions, and movement. These findings support previous otolith microchemistry studies on other species in different systems (Brazner Citation2004, Zeigler and Whitledge Citation2011, Martin et al. Citation2012). Our results illustrate a highly mobile, interconnected walleye population in Lake Oahe and clarify the significance, if not the necessity, of fisheries agency collaboration (e.g., cooperative otolith collection and ablation), administrative and regulatory consolidation, and long-term ecosystem monitoring for interjurisdictional fisheries management (Link Citation2010). This study indicates that otolith chemistry can promote these aspects of interjurisdictional walleye management by providing fisheries managers with science-based decision criteria for formulating management objectives (e.g., ecological, social, economic; Fogarty Citation2014).
Results from this study have important applications for interjurisdictional walleye management in Lake Oahe. Natal homing was most common for adults, supporting the notion that philopatry is an adult-learned behavior whereby first-time spawners reproduce in areas used by older individuals (Olson et al. Citation1978). Walleye are known to exhibit this behavior in Lake Oahe (Riis Citation1985), but this study expanded previous knowledge by revealing spatiotemporal variability in natal homing. For instance, annual natal homing from 2009–2013 (≤50%) was considerably lower than a historical estimate (>85%; Riis Citation1985), suggesting temporal dampening in homing and greater intrastate and interstate movement. Although the walleye collected in Lake Oahe for this study could have originated in the upstream reservoir (i.e., Lake Sakakawea) where walleye fingerlings are routinely stocked (2.49 × 106 to 4.28 × 106 fingerlings/yr from 2009 to 2013; NDGF Citation2015), movement rates between Lake Sakakawea and Lake Oahe are largely unknown and did not represent an objective of this study because Lake Sakakawea, located entirely in North Dakota, is not an interjurisdictional walleye fishery like Lake Oahe. A useful follow-up investigation would be to use otolith microchemistry to quantify movement rates between Lake Sakakawea and Lake Oahe, enabling fisheries managers to estimate the relative natal contributions of these 2 reservoirs to the Lake Oahe walleye population. In the present study, walleye tended to exhibit a lake resident-embayment spawning or a lake resident-river spawning life history typology (Bozek et al. Citation2011) characteristic of populations in Lake Nipigon (Dymond Citation1926), Oneida Lake (Adams and Hankinson Citation1928), and Lake Erie (Schneider and Leach Citation1977, Schram et al. Citation2010, Fielder et al. Citation2010). Compared to main-stem environments, Lake Oahe tributaries and embayments tend to be warm and shallow, conditions favorable for reproductive success (Kaemingk et al. Citation2007, Graeb et al. Citation2009, Bozek et al. Citation2011). Although spawning was not the focus of this research, future studies that compare habitat features among tributaries, embayments, and main-stem sites may reveal mechanisms driving differential natal contribution and inform management strategies to protect and restore natal environments.
Overall, this study indicates that ecosystem-based approaches are needed to manage the interjurisdictional walleye population in Lake Oahe. Interstate partnerships are necessary to protect natal environments from habitat degradation (e.g., sedimentation, desiccation caused by tributary damming). Moreover, public outreach activities and stakeholder surveys (e.g., mail surveys, personal interviews, internet surveys; Marshall et al. Citation2011, Seidl and Klepeis Citation2011, Varble and Secchi Citation2013) need to be conducted at an interjurisdictional scale. Fisheries managers should consider interstate movement and natal homing of walleye in developing harvest regulations to ensure they are compatible between North Dakota and South Dakota. Current regulations are similar but not identical (i.e., North Dakota: 5 fish daily limit, 10 in possession; South Dakota: 4 fish daily limit, 8 in possession, 1 > 20 inches). It will be particularly important for fisheries managers to monitor factors that affect regulatory congruence between states (e.g., disturbances, discharge, prey availability) to ensure interjurisdictional management strategies are effective. Although this study demonstrates the significance of interjurisdictional walleye management, fisheries managers must recognize that fisheries management is only one component of a complex Missouri River management framework. The USACE manages reservoir water levels for flood damage reduction, water supply and irrigation, navigation, hydropower, fish and wildlife, and recreation (Erickson et al. Citation2008). Interjurisdictional walleye management will therefore require fisheries managers to collaborate with reservoir managers to design strategies that align with the multiple federally mandated uses of Missouri River reservoirs. For example, although otolith microchemistry enables fisheries managers to prioritize walleye natal habitats for protection and restoration, they must ensure that walleye management strategies do not interfere with navigation, hydropower, recreation, and other reservoir uses.
Otolith microchemistry is a reliable technique for evaluating walleye environmental history and clarifies the importance of ecosystem-based approaches for walleye management in Lake Oahe, an interjurisdictional fishery. This novel information would be difficult to obtain using traditional techniques (i.e., mark–recapture, radiotelemetry) and advances knowledge of Missouri River walleye ecology. Moreover, it adds to a sparse literature on walleye otolith microchemistry (Bickford and Hannigan Citation2005, Pflugeisen and Calder Citation2013) and informs future environmental history research. With spatially variable but temporally stable water chemistry, Lake Oahe is a suitable reservoir for otolith microchemistry research to assess natal origins (Zeigler and Whitledge Citation2010, Citation2011, Wolff et al. Citation2012), movement (Brenkman et al. Citation2007, Allen et al. Citation2009), and entrainment of diverse fish species (Galat et al. Citation2005). Other Missouri River impoundments may be suitable for similar studies. Moreover, isotopic markers (e.g., δ13C, δ18O, 87Sr/86Sr) also warrant further investigation because they may improve the spatial and/or temporal resolution of environmental history research. Overall, otolith microchemistry represents an instrument for interjurisdictional walleye management in Lake Oahe, providing a methodology for evaluating natal origins and movement and designing spatially informed habitat conservation programs and harvest regulations.
Acknowledgments
We thank W. Radigan, R. Johnston, C. Hayer, T. Rapp, and J. Mecham of South Dakota State University for field assistance. We thank C. Longhenry, B. Hanten, H. Meyer, K. Potter, G. Knecht, J. Sorensen, R. Trible, and B. Larson of the South Dakota Department of Game, Fish and Parks; J. Barstad of the North Dakota Game and Fish Department; and Q. Phelps of the Missouri Department of Conservation and Southeast Missouri State University for technical advice and assistance. We thank A. Shiller and lab members at the University of Southern Mississippi for assistance with water sampling and trace element measurement. We thank G. Barford, J. Commisso, and J. Glessner at the University of California–Davis for assistance with otolith microchemistry analyses.
Funding
Funding for this project was provided by the Federal Aid in Sport Fish Restoration program, Project 3M3328, Study 1526, administered by the South Dakota Department of Game, Fish and Parks, United States Fish & Wildlife Service, South Dakota Agricultural Experiment Station, and by South Dakota State University.
References
- Adams CC, Hankinson TL. 1928. The ecology and economics of Oneida Lake fish. Roosevelt Wild Life Annals. 1:235–548.
- Alexander RB, Slack JR, Ludtke AS, Fitzgerald KK, Schertz TL. 1996. Data from selected U.S. Geological Survey national stream water-quality monitoring networks (WQN). USGS Digital Data Series DDS-37; [cited 9 Nov 2015]. Available from: http://pubs.usgs.gov/dds/wqn96cd/
- Allen PJ, Hobbs JA, Cech JJ Jr, Van Eenennaam JP, Doroshov SI. 2009. Using trace elements in pectoral fin rays to assess life history movements in sturgeon: estimating age at initial seawater entry in Klamath River green sturgeon. T Am Fish Soc. 138:240–250.
- Amano Y, Kuwahara M, Takahashi T, Shirai K, Yamane K, Amakawa H, Otake T. 2013. Otolith elemental and Sr isotopic composition as a natal tag for Biwa salmon Oncorhynchus masou subsp in Lake Biwa, Japan. Aquat Biol. 19:85–95.
- Bickford N, Hannigan R. 2005. Stock identification of walleye via otolith chemistry in the Eleven Point River, Arkansas. N Am J Fish Manage. 25:1542–1549.
- Blair JM, Hicks BJ. 2012. Otolith microchemistry of koi carp in the Waikato region, New Zealand: a tool for identifying recruitment locations? Inland Waters. 2:109–118.
- Bonar SA, Hubert WA, Willis DW. 2009. Standard methods for sampling North American freshwater fishes. Bethesda (MD): American Fisheries Society. 325 p.
- Bozek MA, Haxton TJ, Raabe JK. 2011. Walleye and sauger habitat. In: Barton BA, editor. Biology, management, and culture of walleye and sauger. Bethesda (MD): American Fisheries Society. p. 133–197.
- Brazner JC, Campana SE, Tanner DK. 2004. Habitat fingerprints for Lake Superior coastal wetlands derived from elemental analysis of yellow perch otoliths. T Am Fish Soc. 133:692–704.
- Brenkman SJ, Corbett SC, Volk EC. 2007. Use of otolith chemistry and radiotelemetry to determine age-specific migratory patterns of anadromous bull trout in the Hoh River, Washington. T Am Fish Soc. 136:1–11.
- Campana SE, Chouinard GA, Hanson JM, Frechet A, Brattey J. 2000. Otolith elemental fingerprints as biological tracers of fish stocks. Fish Res. 46:343–357.
- Clarke AD, Telmer KH, Shrimpton JM. 2007. Elemental analysis of otoliths, fin rays and scales: a comparison of bony structures to provide population and life-history information for the Arctic grayling (Thymallus arcticus). Ecol Freshw Fish 16:354–361.
- Cowan JH Jr, Rice JC, Walters CJ, Hilborn R, Essington TE, Day JW Jr, Boswell KM. 2012. Challenges for implementing an ecosystem approach to fisheries management. Mar Coast Fish. 4:496–510.
- Donohoe CJ, Zimmerman CE. 2010. A method of mounting multiple otoliths for beam-based microchemical analyses. Environ Biol Fish. 89:473–477.
- Dymond JR. 1926. The fishes of Lake Nipigon. University of Toronto Studies in Biology Series 27, Publications of the Ontario Research Laboratory. 27:1–108.
- Erickson JW, Rath MD, Best D. 2008. Operation of the Missouri River reservoir system and its effects on fisheries management. In: Allen MS, Sammons S, Maceina MJ, editors. Balancing fisheries management and water uses for impounded river systems. Bethesda (MD): American Fisheries Society. p. 117–134.
- Fielder DG, Liskauskas AP, Gonder DJA, Mohr LC, Thomas MV. 2010. Status of walleye in Lake Huron. Great Lakes Fishery Commission Technical Report. 69:71–90.
- Fogarty MJ. 2014. The art of ecosystem-based fishery management. Can J Fish Aquat Sci. 71:479–490.
- Galat DL, Berry CR, Gardner WM, Hendrickson JC, Mestl GE, Power GJ, Stone C, Winston MR. 2005. Spatiotemporal patterns and changes in Missouri River fishes. In: Rinne JN, Hughes RM, Calamusso B, editors. Historical changes in large river fish assemblages of the Americas. Bethesda (MD): American Fisheries Society. p. 249–291.
- Gibbs RJ. 1970. Mechanisms controlling world water chemistry. Science. 170:1088–1090.
- Graeb BDS, Willis DW, Spindler BD. 2009. Shifts in sauger spawning locations after 40 years of reservoir aging: influence of a novel delta ecosystem in the Missouri River, USA. River Res Appl. 25:153–159.
- Hilborn R. 2011. Future directions in ecosystem based fisheries management: a personal perspective. Fish Res. 108:235–239.
- Johnson DE. 1998. Applied multivariate methods for data analysis. Pacific Grove (CA): Brooks/Cole Publishing. 425 p.
- Kaemingk MA, Graeb BDS, Hoagstrom CW, Willis DW. 2007. Patterns of fish diversity in a mainstem Missouri River reservoir and associated delta in South Dakota and Nebraska, USA. River Res Appl. 23:786–791.
- Keefer ML, Peery CA, Caudill CC. 2006. Long-distance downstream movements by homing adult Chinook salmon. J Fish Biol. 68:944–950.
- Link JS. 2010. Ecosystem-based fisheries management: confronting trade-offs. Cambridge (UK): Cambridge University Press. 224 p.
- Ludsin SA, Fryer BJ, Gagnon JE. 2006. Comparison of solution-based versus laser ablation inductively coupled plasma mass spectrometry for analysis of larval fish otolith microelemental composition. T Am Fish Soc. 135:218–231.
- Marshall NA, Friedel M, van Klinken RD, Grice AC. 2011. Considering the social dimension of invasive species: the case of buffel grass. Environ Sci Policy. 14:327–338.
- Martin J, Bareille G, Berail S, Pécheyran C, Gueraud F, Lange F, Daverat F, Bru N, Beall E, Barracou D, Donard O. 2012. Persistence of a southern Atlantic salmon population: diversity of natal origins from otolith elemental and Sr isotopic signatures. Can J Fish Aquat Sci. 70:182–197.
- McGee S. 2012. We really can get there from here: important steps toward ecosystem-based fishery management. In Stephenson RL, Annala JH, Runge JA, HallArber M, editors. Advancing an ecosystem approach in the Gulf of Marine. Bethesda (MD): American Fisheries Society. p. 35–40.
- McMahon TE, Terrell JW, Nelson PC. 1984. Habitat suitability information: walleye. U.S. Fish and Wildlife Service Biological Report No. 82(10.56).
- [NDGF] North Dakota Game and Fish Department. 2015. ND Game & Fish Department fish stocking list: major waters by lake name. [cited 4 May 2016]. Available from: https://gfapps.nd.gov/reports/fisheries/FishStockin-gMajorWaters.pdf
- Olson DE, Schupp DH, Macins V. 1978. An hypothesis of homing behavior of walleyes as related to observed patterns of passive and active movement. In: Kendall RL, editor. Selected coldwater fishes of North America. Bethesda (MD): American Fisheries Society. p. 52–57.
- Patrick WS, Link JS. 2015. Myths that continue to impede progress in ecosystem-based fisheries management. Fisheries. 40:155–160.
- Pegg MA, Bettoli PW, Layzer JB. 1997. Movement of saugers in the Lower Tennessee River determined by radio telemetry, and implications for management. N Am J Fish Manage. 17:763–768.
- Pflugeisen BM, Calder CA. 2013. Bayesian hierarchical mixture models for otolith microchemistry analysis. Environ Ecol Stat. 20:179–190.
- Phelps QE, Whitledge GW, Tripp SJ, Smith KT, Garvey JE, Herzog DP, Ostendorf DE, Ridings JW, Crites JW, Hrabik RA, et al. 2012. Identifying river of origin for age-0 Scaphirhynchus sturgeons in the Missouri and Mississippi rivers using fin ray microchemistry. Can J Fish Aquat Sci. 69:930–941.
- Pikitch EK, Santora C, Babcock EA, Bakun A, Bonfil R, Conover DO, Dayton P, Doukakis P, Fluharty D, Heneman B, et al. 2004. Ecosystem-based fishery management. Science. 305:346–347.
- Pitcher TJ, Kalikoski D, Short K, Varkey D, Pramod G. 2009. An evaluation of progress in implementing ecosystem-based management of fisheries in 33 Countries. Mar Policy. 33:223–232.
- Pohlert T. 2014. The pairwise multiple comparison of mean ranks package (PMCMR). R package. R: a language and environment for statistical computing. Vienna (Austria).
- R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Riis JC. 1985. Walleye movement, harvest and angler use on Lake Oahe, South Dakota, 1981–1984. Pierre (SD): South Dakota Game, Fish and Parks, Completion Report 84–4.
- Ruttenberg BI, Hamilton SL, Hickford MJH, Paradis GL, Sheehy MS, Standish JD, Ben-Tzvi O, Warner RR. 2005. Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Mar Ecol Prog Ser. 297:273–281.
- Schneider JC, Leach JH. 1977. Walleye (Stizostedion vitreum vitreum) fluctuations in the Great Lakes and possible causes, 1800–1975. J Fish Res Board Can. 34:1878–1889.
- Schram ST, Seider MJ, Furlong PD, Friday MJ. 2010. Status of walleye in Lake Superior. Great Lakes Fishery Commission Technical Report. 69:1–13.
- [SDDENR] South Dakota Department of Environment & Natural Resources. 2015. South Dakota geology. [cited 9 Nov 2015]. Available from: http://www.sdgs.usd.edu/geologyofsd/geosd.html
- Seidl DE, Klepeis P. 2011. Human dimensions of earthworm invasion in the Adirondack State Park. Hum Ecol. 39:641–655.
- Shiller AM. 2003. Syringe filtration methods for examining dissolved and colloidal trace element distributions in remote field locations. Environ Sci Technol. 37:3953–3957.
- [USGS] United States Geological Survey. 2016. WaterWatch. cited [4 May 2016]. Available from: http://waterwatch.usgs.gov/
- Varble S, Secchi S. 2013. Human consumption as an invasive species management strategy. A preliminary assessment of the marketing potential of invasive Asian carp in the US. Appetite. 65:58–67.
- Wells BK, Rieman BE, Clayton JL, Horan DL, Jones CM. 2003. Relationships between water, otolith, and scale chemistries of westslope cutthroat trout from the Coeur d'Alene River, Idaho: the potential application of hard-part chemistry to describe movements in freshwater. T Am Fish Soc. 132:409–424.
- Whitledge GW, Johnson BM, Martinez PJ, Martinez AM. 2007. Sources of nonnative centrarchids in the upper Colorado River related to stable isotope and microchemical analyses of otoliths. T Am Fish Soc.136:1263–1275.
- Wishingrad V, Carr MK, Pollock MS, Ferrari MCO, Chivers DP. 2014. Lake sturgeon geographic range, distribution, and migration patterns in the Saskatchewan River. T Am Fish Soc. 143:1555–1561.
- Wolff BA, Johnson BM, Breton AR, Martinez PJ, Winkelman DL. 2012. Origins of invasive piscivores determined from the strontium isotope ratio (Sr-87/Sr-86) of otoliths. Can J Fish Aquat Sci. 69:724–739.
- Zeigler JM, Whitledge GW. 2010. Assessment of otolith chemistry for identifying source environment of fishes in the lower Illinois River, Illinois. Hydrobiologia. 638:109–119.
- Zeigler JM, Whitledge GW. 2011. Otolith trace element and stable isotopic compositions differentiate fishes from the Middle Mississippi River, its tributaries, and floodplain lakes. Hydrobiologia. 661:289–302.
