ABSTRACT
Wildman RA, Forde NA. 2016. Effect of a moderate-size reservoir on transport of trace elements in a watershed. Lake Reserve Manage. 32:353–365.
We assessed the extent to which Grand Lake, Oklahoma (>30 m deep, >80 km long), retains Fe, Mn, P, As, Zn, Pb, and Cd. Filtered water samples and suspended sediment samples were collected upstream of, within, and downstream of the reservoir. We then estimated instantaneous, seasonal, elemental fluxes. In winter and spring, when storms brought high flows to the reservoir, Grand Lake modified flood water minimally. During these seasons, trace element distributions were determined by the passage of storm inflows through the reservoir. In summer, Fe, Mn, P, and As were enriched in anoxic bottom water and exported through the dam, which draws water from below the surface mixed layer. Concentrations of aqueous elements in the water column were lower following autumn overturn, perhaps due to precipitation of metal oxides and settling. Unlike Fe, Zn was retained in Grand Lake during all seasons. Concentrations of Cd and Pb in filtered samples were often below our detection limit. Logistic regression indicated that Zn predicted detectable Cd, and so Grand Lake probably sequesters Cd. Sequestration of Pb was unclear because detectable Pb was predicted by both Zn and Fe. This study shows that watershed hydrology determines the transport of trace elements through a reservoir during times of high flow but that vertical circulation and biogeochemistry dominate during summertime and autumn low flows. Understanding these mechanisms can aid reservoir managers who seek to reduce downstream loads of trace elements.
Reservoirs of moderate or large size influence chemical transport in their watersheds. They are often located on large rivers to maximize inflow and thus often receive large loads of dissolved and particulate chemicals relative to lakes of similar size (Kalff Citation2002). As reservoirs trap suspended sediment, they trap a particulate chemical load (Horowitz et al. Citation2001, Lee et al. Citation2001, Wildman et al. Citation2011). Trace elements have been characterized in rivers (e.g., Horowitz et al. Citation2001, Müller et al. Citation2008), but less attention has been devoted to the modification of the downstream transport of such chemicals by reservoirs.
The hydrology of sizeable reservoirs and their major tributaries can affect chemical transport downstream in 2 important ways. First, increased seasonal flow in tributaries brings larger chemical loads to a reservoir (Horowitz Citation2008). Second, at low flow, the lacustrine zones of temperate-latitude reservoirs stratify in summer with the potential for hypolimnetic anoxia and subsequent reaeration during overturn or release from the dam (Kalff Citation2002). Hypolimnetic anoxia can lead to reductive dissolution of iron (Fe)-oxide minerals, which can be released downstream (Ashby et al. Citation2004). Elements that sorb to metal-oxides, such as arsenic (As) and phosphorus (P), can behave similar to Fe in reservoirs (e.g., Kneebone and Hering Citation2000). The simultaneous influences of watershed hydrology and biogeochemistry, which vary seasonally, prevent a simple understanding of the effect of a given reservoir on elemental transport in a watershed and thus complicate management efforts to reduce contamination by trace elements.
The purpose of this study was to explore the effect of Grand Lake, a reservoir in Oklahoma, on the transport of trace elements in the watershed of the Grand River. Grand Lake is particularly interesting because it lies at the upstream end of a chain of reservoirs and downstream of multiple sources of contamination. The transport of trace elements through this reservoir is important for management of water quality in its downstream watershed. We studied metals (lead [Pb], zinc [Zn], and cadmium [Cd]), a metalloid (As), and a nutrient (P) that represent most of the primary abiotic threats to water quality in this watershed. The Tar Creek Superfund Site, a historic mining district with abundant mine tailings rich in Pb, Zn, and Cd (Schaider et al. Citation2007, Andrews et al. Citation2009), is just upstream of Grand Lake. The Grand Lake watershed contains several industrial chicken farms that might release As to the environment (Hilleman Citation2007). The US Agency for Toxic Substances and Disease Registry (ATSDR) maintains a Substance Priority List that ranks contaminants by the threat they pose to human health. On it, As, Pb, Cd, and Zn are ranked 1, 2, 7, and 75, respectively (USATSDR Citation2016); As and Pb rank high on the list because As is a carcinogen and a neurotoxin in children, and Pb is a neurotoxin in adults and is especially potent in children (USATSDR Citation2007a, Citation2007b). Cd causes bone disease and kidney damage, and, in high doses, Zn causes gastrointestinal illness, anemia, and damage to the pancreas (USATSDR Citation2012, Citation2005). Grand Lake is eutrophic because of the agricultural land in its watershed (OWRB Citation2009), and so P is a nutrient of concern. The cycling of these and many other trace elements in lakes is strongly influenced by that of Fe and manganese (Mn; e.g., Kalff Citation2002), and so Fe and Mn were included in this study as well.
Because the limnology of a warm temperate reservoir varies seasonally, the ultimate independent variable (i.e., the presence of a reservoir in the watershed) was considered through 2 separate variables: inflow rate and state of vertical circulation. We conceptualized our dependent variable as the instantaneous mass flux of elements through the reservoir during different seasons. We compared this flux to the summed instantaneous mass loading of elements via tributaries entering the reservoir, which would be the mass flux downstream in the absence of the reservoir. Thus, this study sought to determine the effect of seasonal variation in flow and vertical circulation on the transport of a suite of trace elements through Grand Lake. Elements were studied in both aqueous and particulate form. We evaluated 4 hypotheses:
| 1. | During all seasons, the reservoir will retain particulate elements because of particle settling. | ||||
| 2. | Under vertically well-mixed conditions and base flow, transport of aqueous trace elements will be unaffected because biogeochemical conditions in Grand Lake resemble those of its tributaries. | ||||
| 3. | During stratification and base flow, the reservoir will export aqueous trace elements dissolved under anoxic conditions in the hypolimnion. | ||||
| 4. | During elevated inflows, fluxes of aqueous elements will be greater, and trends that occur at base flow will be magnified. | ||||
Study site
Grand Lake (sometimes called Grand Lake O’ the Cherokees) is a reservoir of moderate size (36 m maximum depth when not retaining floodwater, >80 km long) in northeast Oklahoma (). It is impounded by the Pensacola Dam, constructed to generate hydropower and to protect downstream areas from flooding (Hoagland Citation1986), has a volume of 1900–2000 gigaliters (GL; USACE Citation2015), and a watershed of 26,600 km2. Less than 30% falls within another flood control project well upstream (USACE Citation2015), so most of the flow into Grand Lake is unregulated and, as a mainstem reservoir, likely receives a notable sediment load (Wetzel Citation2001, Kalff Citation2002). Unregulated inflows imply considerable variation in inflow rates during a year. The median of recorded daily mean inflows in the 9 years preceding this study was 94 m3/s, although drought conditions during our study (Dec 2010 through Nov 2011) resulted in a median inflow of 35 m3/s (Wildman Citation2016). Small storms in late winter resulted in inflows from 85 to 490 m3/s, and 2 large storms in spring each increased inflows to >550 m3/s for nearly a week (Wildman Citation2016). Despite these variations, the water level of Grand Lake generally varies <1 m throughout the year because, except when it retains and later releases floodwater, outflows generally match inflows. Consequently, the reservoir water balance was near neutral in December, increasing by 40 GL/day in February during storm inflows, decreasing by 16 GL/day in May as water was released following a large storm, and decreasing slightly in August and November as part of minor drawdowns of water level (Wildman Citation2016). Accordingly, water exchange was high in February and May and low in August and November (Wildman Citation2016).
Figure 1. Location of Grand Lake in Oklahoma and the United States. Arrows indicate direction of water flow from major tributaries the Neosho River (NR), the Spring River (SR), and the Elk River (ER) and minor tributaries Tar Creek (TC), Buffalo Creek (BC), and Honey Creek (HC) through the reservoir and into the Grand River (GR). Circles indicate locations where water and suspended sediment samples were collected, although samples were not collected at all locations on all sampling excursions. Gray-black shading indicates relative depth between the shoreline and the dam face, where water is usually 36 m deep. Map adapted from OWRB (Citation2009).
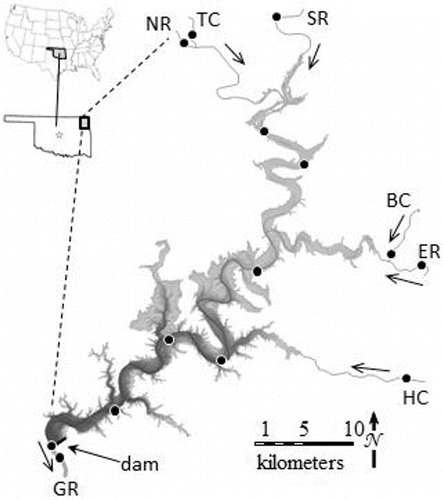
Inflows to Grand Lake come primarily from the Neosho River in the northwest, Spring River in the north, and Elk River in the east, collectively contributing 75% of the water released from the dam during this study (Wildman Citation2016). The Neosho and Spring Rivers drain predominantly agricultural land, and the Elk River drains forested uplands and receives permitted discharge from poultry plants. In the northwest, Tar Creek drains the Tar Creek Superfund Site, and in the southeast, the Honey Creek watershed drains chicken farm runoff.
The confluence of the Neosho and Spring rivers, which has been submerged by Grand Lake, was the historical source of the Grand River, which now originates from Pensacola Dam. Penstocks of the dam, used for all dam releases except during floods, are 4 m tall screened openings centered ∼16 m below the water surface and located at the southwestern end of the dam. Downstream from the dam, water from Grand Lake flows through 2 additional reservoirs on the Grand River and 2 more on the Arkansas River before meeting the Mississippi River.
Grand Lake is a warm monomictic reservoir. During our study, it turned over in October, reached an isothermal minimum temperature of 5 C in winter, and exhibited stratification by early May. In midsummer, stratification was pronounced; the surface mixed layer was 8–11 m thick (Wildman Citation2016), extending to the bottom of the reservoir at distances >52 km upstream from the dam (i.e., the vicinity of the confluence of the Elk River arm with the thalweg; Wildman Citation2016). During this study in May, dissolved oxygen (DO) was >2.5 mg/L in the deepest water by the dam and 4.0–9.5 mg/L otherwise. In summer, the metalimnion and hypolimnion were anoxic, and the entire water column readily became oxic during autumn overturn (Wildman Citation2016).
Methods
Water samples were collected in December 2010 and February–March, May, August, and November 2011. They were collected from 5 (Dec, Nov) or 6 (Feb–Aug) locations spaced at approximately equal distances across the thalweg of Grand Lake, the tributaries described earlier, and the Grand River <500 m below the dam (). Based on circulation parameters measured concurrently, 1–4 depths at each site were sampled; one sample was always collected from the surface mixed layer, and additional samples were collected from below the surface mixed layer and from water ≤1 m from the sediment–water interface. Samples were pumped through silicone tubing precleaned with dilute nitric acid and rinsed thoroughly with distilled deionized water. Before sample collection at a given site and depth, 3 tube volumes of reservoir water were passed through the tubing and discarded. Reservoir water samples were filtered through preweighed 0.45 μm polyethersulfone membranes, either within 24 h in a class-100 clean bench (Dec–May, when DO was >4.3 mg/L in all samples) or immediately inline (Aug–Nov). After filtering, samples were acidified with ultrapure nitric acid. Field blanks and occasional field duplicates verified the low background concentrations of analytes and reproducibility of field protocols.
Elements in the aqueous phase were quantified by inductively coupled plasma (ICP) mass spectrometry (Mn, Pb, Zn, Cd, As) and ICP optical emission spectrometry (Fe, P). Measurements were calibrated with standard solutions made by diluting commercially available stock solutions. Detection limits were 0.1 μg/L for Mn, Pb, Zn, Cd, and As and 1 μg/L for Fe and P. During a given analysis, no samples were analyzed until the instruments were tuned within operating specifications, method blanks returned concentrations below detection limits (BDL), and a linear calibration curve was produced.
Elements associated with suspended sediment were quantified after extraction from filter membranes, which were first oven-dried (at 65 C until mass was constant) and weighed to determine sediment mass. Membranes were subsequently microwave-digested in concentrated ultrapure nitric acid in Teflon digestion vessels. Digestates were diluted 1:10 with distilled, deionized water and analyzed like the water samples described earlier. Further 100- or 1000-fold dilutions with 5% ultrapure nitric acid were usually necessary to bring samples within the range of concentrations of the calibration curve. Digestion efficiency was verified by digesting NIST 2709 and 2711 certified reference materials several times along with batches of samples. Filter blanks and digestion vessel blanks verified that techniques were trace-metal clean. Detection limits of these measurements varied between samples because, although the ICP detection limits were constant, the digestate volumes, sediment masses on filters, and volumes of water filtered were not. The interquartile range (IQR) of detection limits was 0.2–1.7 mg/kg, and the median was 0.5 mg/kg.
Elemental fluxes were calculated for each sampling excursion by multiplying the average flow of the days when sampling occurred by the concentrations of elements in samples collected from tributaries and the dam tailrace and then subtracting the tailrace values from the sum of the tributary values. When concentrations were BDL, half the detection limit was used for this calculation. Volumetric flows of the Grand, Neosho, Spring, and Elk rivers and Honey and Buffalo creeks were downloaded from the US Geological Survey (USGS Citation2013). The detection limits for the elemental measurements were carried through this calculation to determine detection limits for calculated fluxes. These detection limits varied due to varying river flow rates.
Relationships between trace elements and qualitative predictor variables (e.g., depth, location, season) were explored using principal component analysis (PCA). This statistical technique expresses the variance of a many-dimensional dataset not on axes that correspond to individual variables but on new orthogonal axes called principal components (PCs) aligned with successively decreasing fractions of the variance of the dataset (e.g., Shine et al. Citation1995). This process allows visualizations of groupings of both variables and samples based on the concentrations of the trace elements measured in this study. In filtered samples, when some elements were too often BDL to permit useful inclusion in PCA, relationships with other elements were instead explored with multivariable logistic regression. These analyses treated trace elements with many concentrations available above detection limits as continuous predictor variables for low-concentration elements evaluated as bimodally distributed (i.e., above or below the detection limit) response variables.
Contour plots of elemental concentrations in Grand Lake were created from measurements at discrete depths and distances from the dam using Tecplot 360 (Tecplot, Inc.; Bellevue, WA). Vertical interpolation based on circulation parameters was required before allowing Tecplot to interpolate longitudinally between sampling locations. All available data were used to create contour plots.
Results
Concentrations of trace elements
The range of Fe in all filtered samples (Fef and similar) was BDL–220 μg/L with a median of 15 μg/L and an IQR of BDL–33 μg/L (). Concentrations in tributaries were not significantly different from those in the reservoir (t-test, P > 0.1) with one exception. In August, concentrations in anoxic summertime bottom water sometimes exceeded 100 μg/L, causing the reservoir water to be significantly higher in Fef than the tributaries (t-test, P < 0.01). Concentrations >50 μg/L also occurred in the large inflow captured in the February–March sampling, indicating that during different seasons Fe enters the reservoir in the aqueous phase from both internal loading and the watershed upstream. Downstream concentrations of Fef were similar to or lower than those in the reservoir. No other meaningful spatial trends were observed (Supplemental Fig. S1). Oxic waters were not devoid of Fef, and although concentrations were BDL in winter, they were 10–35 μg/L in February–March and May and 7–54 μg/L in November.
Figure 2. Distribution of trace element concentrations in filtered water samples. Data are depicted along several one-dimensional number lines sorted by sampling location and excursion. Circles denote samples collected upstream of Grand Lake in tributaries. Diamonds denote samples collected <8 m deep in the water column (the bottom of the summertime surface mixed layer). Triangles denote samples collected ≥8 m deep. Squares denote samples collected downstream of Pensacola Dam. Symbols appear at the median values of each subset of data, and error bars extend to the 5th and 95th percentiles of each subset of data. The numbers above the panel showing iron (Fe) concentrations indicate the number (n) of observations in each subset of samples, and these apply to the corresponding number lines for all panels other than Fe. Labels at the bottom of each panel identify sampling excursions.
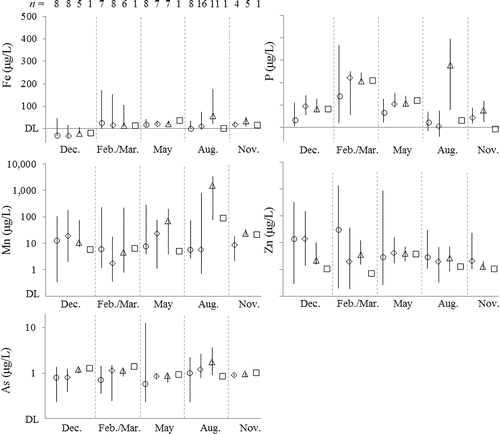
We observed similar trends but different magnitudes between Mnf, Pf, Asf, and Fef. Anoxic August bottom water contained Mn concentrations of 1–4 mg/L, and the IQR of Mn for all filtered samples was 2–57 μg/L (). Hypolimnetic anoxia in summer led to concentrations of Mnf 1–3 orders of magnitude greater than in tributaries and downstream during summer. Concentrations of Mnf in the reservoir fell between August and November to 2–35 μg/L, and downstream from the dam, releases were 93 and 22 μg/L in summer and autumn, respectively. The IQR of Pf concentrations was 22–140 μg/L (), significantly higher in the reservoir than in tributaries across all sampling excursions (t-test, P < 0.05), especially in summertime bottom water (t-test, P < 0.0005). Concentrations of Asf were <5 μg/L (), with anoxic hypolimnetic waters 4.7 μg/L and oxic water 1–2 μg/L and invariant. For each of Fef, Mnf, Asf, and Pf, concentrations of samples from anoxic summertime bottom water were significantly higher than those collected from other reservoir water (t-test, P < 0.05). With the exception of variation with depth as described here, spatial variation of Mnf, Asf, and Pf was minor (Supplemental Fig. S2–4).
Elements prevalent at the Tar Creek Superfund Site upstream behaved differently from Fef, Mnf, Pf, and Asf. Anoxic water did not show an enrichment of Znf (). In Tar Creek, Znf easily exceeded 1000 μg/L and was >100 μg/L in the main tributaries of Grand Lake and in the upper part of the reservoir. Concentrations of Znf were consistently and significantly higher (t-test, P < 0.05) in the tributaries feeding the northern end of Grand Lake than in our sampling location in the reservoir >30 km downstream from those tributary sampling location (99 vs. 24 μg/L in Dec, 157 vs. 51 μg/L in Feb, 42 vs. 8 μg/L in May, and 5 vs. 0.9 μg/L in Aug). Concentrations in the rest of the reservoir were <15 μg/L, with no clear spatial pattern (Supplemental Fig. S5). The highest concentrations of Pbf and Cdf occurred in Tar Creek in February, 1.9 μg/L and 1.7 μg/L, respectively, but were otherwise usually BDL in filtered samples.
The IQR of Fe in all suspended sediment (Fess and similar) was 7800–25,000 mg/kg, with extremes an order of magnitude lower and higher, respectively (). Maximal values occurred in bottom water during autumn and downstream in February–March; however, across all sampling excursions, deep water samples were not significantly higher in Fess than other samples from the reservoir (t-test, P > 0.1; ). Otherwise, concentrations of Fess in the reservoir and below the dam were not significantly different from those in the tributaries (t-test, P > 0.1), which did not vary appreciably throughout the year (Supplemental Fig. S6).
Figure 3. Distribution of trace element concentrations in suspended sediment samples. Because the detection limit varied between samples, only the approximate maximum detection limit is denoted where appropriate. Other details are as in .
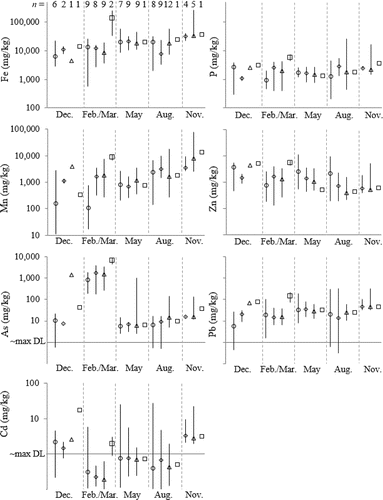
The IQRs of Mnss and Pss were 350–3300 mg/kg and 1100–2800 mg/kg, respectively (), with spatiotemporal trends closely resembling those of Fess (Supplemental Fig. S7 and S8). Concentrations of Asss were generally <50 mg/kg except for elevated values (100–8900 mg/kg) during winter (). Other than high values in February–March 1–2 orders of magnitude higher than those measured in other months, Asss in tributaries did not vary across locations or time (Supplemental Fig. S9). Like Fess, concentrations of Znss, Pbss, and Cdss were greater in some samples collected in autumn in deep water (; Supplemental Fig. S10 and S11).
Flux calculations and data analysis
Loading of trace elements via tributaries to Grand Lake varied consistently with inflow volumes (). In filtered water samples, mass fluxes of all elements were 1–3 orders of magnitude greater during high flows in February–March than in December, August, or November (). These trends were broadly similar in suspended sediment samples with one exception: for As, the February–March flux was 3–6 orders of magnitude larger than that of other months (). Fluxes of Fe, Mn, Zn, P, and As were significantly higher than those of Pb and Cd, and fluxes of Mn were significantly higher than those of As (Kruskal–Wallis test, H < 0.01).
Table 1. Water flux (GL/day) and mass flux of elements in () filtered samples (kg/d) and () suspended sediment samples (kg/d).
The aqueous and suspended sediment phases each dominated transport of trace elements through the reservoir during different seasons and for different elements (). Large positive fluxes (i.e., net loadings) of most elements measured in this study were observed in both phases in February–March, when loading via tributaries exceeded loss from the reservoir via dam releases. Net loadings to the reservoir in February were 1–2 orders of magnitude lower for Fef, Pbf, Znf, Cdf, and Asf than for these elements in suspended sediment. At other times, flux of aqueous elements was of the same order of magnitude as the flux of elements associated with suspended sediment, and sometimes it was less than an order of magnitude larger. In May, when the dam was releasing water from the downstream end of Grand Lake to accommodate large inflows at the upstream end, a net export of Fef, Fess, Mnss, Pf, and Pss was observed; however, Znf, Znss, and Mnf were loaded to the reservoir in May. In August and November, most elements were exported from the reservoir, although fluxes were smaller than in months of high flow because water flux was small. In August, flux calculations indicated a comparatively small loading of Fef to the reservoir and a much larger export of Fess.
Variance in the PCA performed on filtered samples (PCAf) was broadly distributed, with just 38% described by the first PC (PC1f and similar), 21% by PC2f, and 16% by each of PC3f and PC4f. A biplot showing the relationship of variables and samples to these axes (termed “loadings” and “scores,” respectively; see Shine et al. Citation1995) is characterized by high values on PC1f for all elements included in this analysis and a range of values on PC2f with Fef and Asf together and opposite from Znf, and also with Mnf and Pf near each other (). This finding indicates general covariance of Fef and Asf across all the samples of the dataset, covariance of Mnf and Pf across all samples in the dataset, and noncovariance of Znf with Fef and Asf. Because of numerous concentrations BDL, Cdf and Pbf were excluded from the PCAf.
Figure 4. Biplot of principal component analysis of elemental concentrations in filtered samples. Loadings are depicted as open double circles and correspond to upper and right-hand axes. Scores are other symbols and correspond to lower and left-hand axes. Scores are represented as: (filled diamonds) upstream samples from February-March and May (i.e., months of high flow); (open diamonds) other upstream samples; (filled squares) summertime surface mixed layer; (open squares) other lake samples from <8 m deep; (filled triangles) summertime metalimnion and bottom water; (open triangles) other lake samples from ≥8 m deep; and (filled circles) downstream samples. PC1 and PC2 described 38% and 21% of the variance in the dataset, respectively.
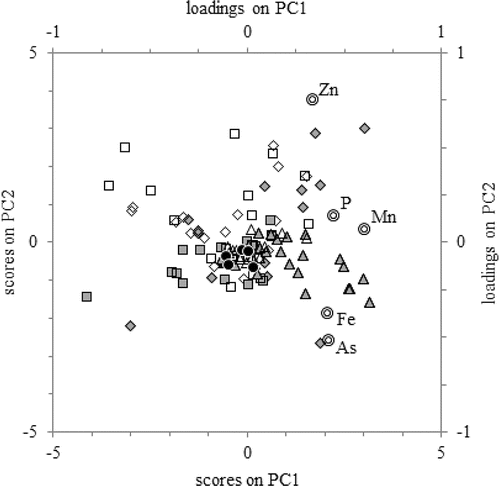
The plot of PCf scores showed that most samples collected in river inflows during months of high flow plotted with high values on PC1f and PC2f, whereas many samples collected from depth in summer plotted with high values on PC1f and comparatively low values on PC2f (). These 2 groupings, the most pronounced of the dataset, corroborate other observations in this study by showing that, across 5 variables, the highest concentrations in this dataset occurred during large inflows or anoxic bottom water. Comparing these PC scores to the PC loadings of the variables indicates that the river inflows were comparatively low in Fef and Asf and high in Znf, whereas the opposite was true for anoxic bottom water.
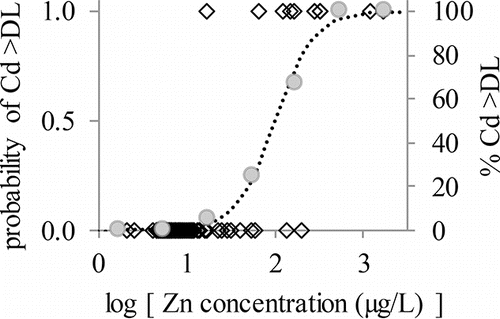
Multivariate logistic regression identified the logarithm of concentrations of Znf as the only significant predictor for Cdf (); the relationship was robust, with a McFadden's pseudo R2 of 0.69. Conversely, Pbf was predicted by the logarithms of concentrations of Fef and Znf in a multivariate logistic regression that resulted in a McFadden's pseudo R2 of only 0.39.
Figure 5. Plot of logistic function relating occurrence of detectable Cdf to logarithm of Znf concentration by showing observed values (open diamonds, left vertical axis), the percentage of Cd observations that exceeded the detection limit for a given 0.5-log-unit window on the horizontal axis (gray circles, right vertical axis), and the logistic function fitting the observed data (dotted line, left vertical axis).
In suspended sediment samples, PC1–3 explained 42, 25, and 15% of the variance of the dataset, respectively. No clear trends could be discerned from the biplots resulting from this PCA (data not shown).
Discussion
The concentrations of Fess, Mnss, Znss, Pbss, and Cdss measured in this study were generally comparable to previous measurements of streambed sediment from the Neosho River, the Spring River, and Tar Creek (Andrews et al. Citation2009). However, concentrations of Fess in all downstream samples and in bottom water samples in November exceeded the median of 12,000 mg/kg measured by Andrews et al. (Citation2009) in tributaries. One sample in particular, 1 of 2 collected downstream of the dam in February–March, exceeded 250,000 mg/kg, a concentration suggesting that some particles exported downstream during elevated dam releases can be substantially enriched in Fess. A previous study reported fluvial sediment samples collected from many river and stream sites in the United States (Horowitz and Stevens Citation2008). Median values of samples from rivers that drained either >50% agricultural land or >50% rangeland (Mnss, Pss, and Asss near 800, 100, and 7 mg/kg, respectively) fell between the 25th and 50th percentiles of concentrations of samples measured in our study. In our study, both Znss and Cdss were consistently 10–100 times higher and Pbss was ∼10 times higher than median values of 90, 0.4, and 20 mg/kg, respectively, found by Horowitz and Stevens (Citation2008). These comparisons indicate that the suspended sediment of Grand Lake is enriched in metals derived from upstream mining and can also be enriched in other elements under certain limnologic conditions, described later. The elevated concentrations of Asss across Grand Lake in February–March matched high concentrations in tributaries during that time and occurred after the water column had been well-mixed and oxygenated for several weeks. Therefore, they were probably caused by variations in watershed hydrology (perhaps related to storm runoff and high river flows), not lacustrine biogeochemistry.
Compared to previous measurements of water samples collected in the Neosho River, the Spring River, and Tar Creek (Andrews et al. Citation2009), Fef, Mnf, Znf, Pbf, and Cdf data from this study fall in the same ranges with 2 exceptions. First, redox-active metals (i.e., Fef and Mnf) were higher in floodwater and in the anoxic bottom water sampled in this study (median Fef measured by Andrews et al. [Citation2009] in the Neosho and Spring Rivers was <20 μg/L; median Mn ranged between 10 and 80 μg/L). Second, Andrews et al. (Citation2009) measured much higher concentrations of Znf, Pbf, and Cdf in Tar Creek (1000–10,000, 0.1–1, and 3–90 μg/L, respectively) than we measured in Grand Lake. We are aware of no previous measurements of these elements in the Elk River or Honey Creek or of profiles of aqueous metals in Grand Lake.
In this study, flux of Fe occurred mostly in particulate form, whereas in most seasons >50% of the flux of P and As occurred in filtered samples. In February and August, the flux of Mnf was greater than that of Mnss, and the reverse was true otherwise. Flux of Zn species were similarly inconsistent across sampling excursions. Although the majority of mass flux can frequently be attributed to the aqueous phase in some elements in this study, this does not imply that the solid phase does not dominate the yearly mass balance of all elements, as has been observed in some major rivers (Horowitz et al. Citation2001), because at least 12 hydrologically spaced measurements are needed to accurately determine a yearly flux (Horowitz Citation2008). Despite this limitation, our flux calculations provide insight into the effect of hydrology on the transport of elements through Grand Lake because they describe a range of inflow rates that can be taken as representative of a range of hydrologic events.
The rising limb of a storm inflow (observed in February–March) loaded trace elements into Grand Lake, which can be explained by the imbalance of inflows rich in elements and outflows of lower concentration from a long reservoir that is not longitudinally mixed. During the falling limb of a storm inflow (observed in May), several elements in both the aqueous phase and suspended sediment were exported out of the reservoir along with the excess water retained during the flood. Both of these periods of high inflow were also characterized by little difference between the trace element chemistry of the reservoir and its tributaries, which we attribute to low water residence time, low primary production, weak or nonexistent stratification, and an oxic water column that made biogeochemical modification of trace elements unlikely. These observations resemble those from another well-flushed reservoir basin in which water chemistry matched that of the inflowing river (Bellanger et al. Citation2004). Our data from Grand Lake suggest that, during stormy seasons, managers of Grand Lake can expect minimal modification of floodwater by the reservoir and spatial distributions of trace elements determined mostly by the passage of floodwater through the system. During this study, the volume of inflows during winter and spring was >230% of the volume of the reservoir, and each of 2 large storms may have flushed the thalweg completely in as little as 1 week (Wildman Citation2016).
When elevated inflows from storms subsided, summer stratification led to hypolimnetic anoxia (Wildman Citation2016). Subsequent elevated concentrations of Fef and Mnf in bottom water samples suggest reductive dissolution of metal oxides in sediment and settling particles. Elevated concentrations of Pf and Asf suggest desorption of these elements from metal oxides. Grand Lake exported Fe, Mn, and P from its anoxic summertime hypolimnion, with Mn and Fe measured downstream predominantly in filtered and particulate samples, respectively. However, Fe may be leaving the dam in the aqueous phase and precipitating immediately upon entering oxic tailwater due to the rapid oxidation kinetics of Fe2+ (Morgan Citation2005). This event likely explains the low concentrations of Fef below the dam that led to an apparent net loading of Fef to the reservoir in August. The export of Fe, Mn, P, and As in August, despite the low volume of water exchanged (Wildman Citation2016), indicates that reductive dissolution of particles in anoxic bottom water represents a source of dissolved trace elements to the river below the dam. The shift of control of trace element cycling from inflow hydrology to lacustrine biogeochemistry when inflow rates were low is consistent with research elsewhere (Bellanger et al. Citation2004).
Much lower concentrations of Fef, Mnf, Pf, and Asf in the water column shortly after autumn overturn suggest oxidative precipitation of Fe and Mn. The concentrations of Fef and Mnf in November that were higher than those of the preceding December suggest that, in this natural setting, oxidation kinetics might have been slower than in laboratory settings (e.g., Morgan Citation2005) and that oxidative precipitation may not have been complete at the time of our sampling. This interpretation is supported by the low flow through the reservoir during and between our summer and autumn sampling excursions, indicating that water exchange was too low to explain the decrease in the concentrations of trace elements in filtered samples (Wildman Citation2016). Scavenging of Pf and Asf onto newly precipitated particles within the reservoir may explain the decrease in these elements in filtered samples from August to November. These interpretations are consistent with our observation of some elevated concentrations of Fess, Mnss, Pss, and Asss in bottom waters during this season. During a vertically mixed period of low flow (observed in our December excursion), the reservoir closely resembled its tributaries for all elements in the aqueous phase and in suspended sediment, except it passed P downstream in both phases.
The broad distribution of variance in both PCAs suggests that elements in this study respond to a range of relatively balanced influences. Although these PCAs alone do not indicate clear trends, the loadings of variables on PC2f combine with spatial distributions and mass flux calculations to indicate that, although Asf and Fef covary in this system, Fef has minimal influence over Znf. This observation resembles those made in the anoxic hypolimnion of a eutrophic lake where many previous studies have occurred (Achterberg et al. Citation1997). In Grand Lake, Znf decreased as water entered the reservoir in all seasons and did not respond to biogeochemical depletion of DO. Verification of this sequestration through analysis of sediment samples was beyond the scope of this study, but sediment cores collected by Andrews et al. (Citation2009) from the upper region of Grand Lake contained Zn concentrations an order of magnitude higher than in the Neosho River upstream of its confluence with Tar Creek.
Previous research at the Tar Creek Superfund Site can provide insight into a possible mechanism of Znf sequestration in the upper region of Grand Lake. In Tar Creek, some Zn(aq) precipitates as Zn-carbonate or sorbs to Fe-oxides (Bostick et al. Citation2001, Schaider et al. Citation2014) while the remaining Zn(aq) serves as the probable source of the high concentrations of Znf observed in this study. The lack of association between Znf and Fef in Grand Lake implies that sorption of excess Znf to Fe-oxide particles from the Neosho and Spring rivers is an unlikely mechanism for Znf sequestration. However, attenuation of Znf by precipitation as carbonate minerals is plausible because the Spring River is likely rich in carbonate (suggested by abundant limestone in its watershed and elevated pH relative to the Neosho River; Andrews et al. Citation2009) and because precipitation of Zn-carbonate particles is thermodynamically likely in Tar Creek waters (Schaider et al. Citation2014). Further research would be required to investigate this potential mechanism of Znf sequestration but would be relevant for reservoir management by demonstrating the transferability of observations at Grand Lake to other reservoirs downstream of mining areas.
Sequestration of Zn by Grand Lake implies that reservoir managers in the Grand River Basin can rely on Grand Lake to limit the downstream transport of mine waste across a range of hydrologic conditions. The logistic regression model showing that Znf predicts the presence of detectable Cdf indicates that Grand Lake probably also sequesters Cdf. This prediction is particularly advantageous for reservoir managers because it implies that broad trends of Cdf can be understood indirectly by measuring Znf, which requires less sensitive instrumentation to characterize in this system because of its higher concentrations. The behavior of Pbf is less clear, in part because the best model that predicted Pbf used both Fef and Znf as predictors and because that model was not robust.
Future work pertaining to trace element flux through Grand Lake might focus on the spatial distribution of elements in the reservoir sediment. Between 55 and 65 km from the dam (i.e., the vicinity of the confluence of the Elk River arm with the thalweg), the summertime surface mixed layer reached the reservoir bed and most sedimentation occurred (Wildman Citation2016), indicating that this region is probably the transition zone of the reservoir (Wetzel Citation2001). Because fine sediment contains elevated concentrations of trace elements (Horowitz and Elrick Citation1987, Wildman et al. Citation2011), future work could test the hypothesis that the sediment of the transition zone is enriched in trace elements. During floods, however, sedimentation can occur at Pensacola dam (Wildman Citation2016), and, consequently, future research could also explore the hypothesis that much of the thalweg sediment is enriched in trace elements relative to background.
Conclusions
During our study period, the flux of Fe, Mn, P, and As through Grand Lake was controlled by hydrology when inflows were low and high (i.e., winter and spring, respectively) and the water column was vertically mixed. During these times, spatial distribution of trace elements in the reservoir was without meaningful trends, the reservoir chemically resembled its tributaries, and it did little to alter the transport of aqueous trace elements downstream. These findings support the second and fourth hypotheses of this study. When flows were minimal and the water column was stratified, elements that entered the aqueous phase in the anoxic hypolimnion were exported from the reservoir, which supports the third hypothesis of this study. Additionally, these trends were not observed for Zn, which was loaded to the reservoir in all seasons. Concentrations of Pb and Cd were consistently low, but Cdf concentrations above the detection limit were predicted by Znf concentrations. This finding implies that the frequency of large inflows and the volume of summertime hypolimnetic water released from Pensacola Dam will be primary drivers of the yearly flux of several metals through Grand Lake, and that Grand Lake sequesters Zn, preventing its transport downstream. The presence of Grand Lake in the watershed of the Grand River does not always imply that all elements associated with suspended sediment are trapped in this reservoir, refuting the first hypothesis of this study. Instead, some elements are passed downstream under some conditions, indicating that Grand Lake is not large enough for all particles entering from upstream to settle.
ULRM_A_1204397_Supplement.docx
Download MS Word (1.4 MB)Acknowledgments
This study was enabled by the generous logistical, laboratory, and personnel support of the Grand River Dam Authority, specifically Darrell Townsend, Sam Ziara, Jacklyn Jaggars, Scott Cox, and Sean Allred. Steve Nikolai, Andy Dzialowski (Oklahoma State University), and Lance Phillips (Oklahoma Water Resources Board) shared important ancillary data. Mollie Thurman and Emily Estes (Harvard) provided essential sampling support, and Nick Lupoli, Chitra Amarasiriwardena, and Zhao Dong (Harvard) provided analytical support. Rebecca Jim, Earl Hatley (L.E.A.D. Agency), Laurel Schaider (Harvard), and Marjorie Wonham (Quest) provided key background information and helpful discussion. Jim Shine (Harvard) provided essential support in all aspects of this project. The final manuscript benefitted from 3 anonymous reviews and the work of Associate Editor R. Thomas James.
Funding
This project was funded by a French Environmental Fellowship granted to Wildman through the Harvard University Center for the Environment and by a gift from the Akatsuka Orchid Company, Ltd.
References
- Achterberg EP, van den Berg CMG, Boussemart M, Davison W. 1997. Speciation and cycling of trace metals in Esthwaite Water: a productive English lake with seasonal deep-water anoxia. Geochim Cosmochim Acta. 61:5233–5253.
- Andrews WJ, Becker MF, Mashburn SL, Smith SJ. 2009. Selected metals in sediments and streams in the Oklahoma part of the Tri-State mining district. Washington (DC): US Geological Survey, Scientific Investigations Report 2009–5032.
- Ashby SL, Faulkner SP, Gambrell RP, Smith BA. 2004. Assessing iron dynamics in the release from a stratified reservoir. Lake Reserv Manage. 20:65–75.
- Bellanger B, Huon S, Steinmann P, Chabaux F, Velasquez F, Vallès V, Arn K, Clauer N, Mariotti A. 2004. Oxic-anoxic conditions in the water column of a tropical freshwater reservoir (Peña-Larga dam, NW Venezuela). Appl Geochem. 19:1295–1314.
- Bostick BC, Hansel CM, LaForce MJ, Fendorf S. 2001. Seasonal fluctuations in zinc speciation within a contaminated wetland. Environ Sci Technol. 35:3823–3829.
- Hilleman B. 2007. Arsenic in chicken production. Chem Eng News. 85:34–35.
- Hoagland BW. 1986. Rivers, lakes, and reservoirs. In: Goins CR, Goble D, editors. Historical atlas of Oklahoma. Norman (OK): University of Oklahoma Press. p. 12.
- Horowitz AJ. 2008. Determining annual suspended sediment and sediment-associated trace element and nutrient fluxes. Sci Tot Environ. 400:335–343.
- Horowitz AJ, Elrick KA. 1987. The relation of stream sediment surface area, grain size and composition to trace element chemistry. Appl. Geochem. 2:437–451.
- Horowitz AJ, Elrick KA, Smith JJ. 2001. Annual suspended sediment and trace element fluxes in the Mississippi, Columbia, Colorado, and Rio Grande drainage basins. Hydrolog Process. 15:1169–1207.
- Horowitz AJ, Stevens VC. 2008. The effects of land use on fluvial sediment chemistry for the conterminous U.S. – Results from the first cycle of the NAWQA Program: trace and major elements, phosphorus, carbon, and sulfur. Sci Total Environ. 400:290–314.
- Kalff J. 2002. Limnology. Upper Saddle River (NJ): Prentice Hall.
- Kneebone PE, Hering JG. 2000. Behavior of arsenic and other redox-sensitive elements in Crowley Lake, CA: a reservoir in the Los Angeles Aqueduct system. Environ Sci Technol. 34:4307–4312.
- Lee G, Faure G, Bigham JM, Williams DJ. 2001. Metal release from bottom sediments of Ocoee Lake No. 3, a primary catchment area for the Ducktown mining district. J Environ Qual. 37:344–352.
- Morgan JJ. 2005. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim Cosmochim Acta. 69:35–48.
- Müller B, Berg M, Yao ZP, Zhang XF, Wang D, Pfluger A. 2008. How polluted is the Yangtze river? Water quality downstream from the Three Gorges Dam. Sci Total Environ. 402:232–247.
- [OWRB] Oklahoma Water Resources Board. 2009. Hydrographic survey of Grand Lake. Oklahoma City (OK).
- Schaider LA, Senn DB, Brabander DJ, McCarthy KD, Shine JP. 2007. Characterization of zinc, lead, and cadmium in mine waste: implications for transport, exposure, and bioavailability. Environ Sci Technol. 41:4164–4171.
- Schaider LA, Senn DB, Estes ER, Brabander DJ, Shine JP. 2014. Sources and fates of heavy metals in a mining-impacted stream: temporal variation and the role of iron oxides. Sci Total Environ. 490:456–466.
- Shine JP, Ika RV, Ford TE. 1995. Multivariate statistical examination of spatial and temporal patterns of heavy metal contamination in New Bedford Harbor marine sediments. Environ Sci Technol. 29:1781–1788.
- [USATSDR] US Agency for Toxic Substances and Disease Registry. 2005. Public Health Statement: Zinc; [cited 23 Apr 2016]. Available from: http://www.atsdr.cdc.gov/phs/phs.asp?id=300&tid=54
- [USATSDR] US Agency for Toxic Substances and Disease Registry. 2007a. Public Health Statement: Arsenic; [cited 23 Apr 2016]. Available from: http://www.atsdr.cdc.gov/phs/phs.asp?id=18&tid=3
- [USATSDR] US Agency for Toxic Substances and Disease Registry. 2007b. Public Health Statement: Lead; [cited 23 Apr 2016]. Available from: http://www.atsdr.cdc.gov/phs/phs.asp?id=92&tid=22
- [USATSDR] US Agency for Toxic Substances and Disease Registry. 2012. Public Health Statement: Cadmium; [cited 23 Apr 2016]. Available from: http://www.atsdr.cdc.gov/phs/phs.asp?id=46&tid=15
- [USATSDR] US Agency for Toxic Substances and Disease Registry. 2016. Priority List of Hazardous Substances; [cited 23 Apr 2016]. Available from: http://www.atsdr.cdc.gov/spl/
- [USACE] US Army Corps of Engineers. 2015. PENO2: Grand Lake O’ the Cherokees, Pensacola Dam. Available from: http://www.swt-wc.usace.army.mil/pens.lakepage.html
- [USGS] US Geological Survey. 2013. USGS current water data for Oklahoma. Available from: http://waterdata.usgs.gov/ok/nwis/rt
- Wetzel RG. 2001. Limnology: lake and river ecosystems, 3rd ed. San Diego (CA): Elsevier.
- Wildman RA. 2016. Mercury and methylmercury in a reservoir during seasonal variation in hydrology and circulation. Lake Reserv Manage. 32:89–100.
- Wildman RA, Pratson LF, DeLeon M, Hering JG. 2011. Physical, chemical, and mineralogical characteristics of a reservoir sediment delta (Lake Powell, USA) and implications for water quality during low water level. J Environ Qual. 40:575–586.
