ABSTRACT
Parks SR, McNair JN, Hausler P, Tyning P, Thum RA. 2016. Divergent responses of cryptic invasive watermilfoil to treatment with auxinic herbicides in a large Michigan lake. Lake Reserve Manage. 32:366–372.
Invasive plants are a major concern for environmental managers around the world. Cryptic invasive taxa present additional challenges because of their potential to respond differently to management efforts while being difficult to distinguish morphologically. Here, we consider the potential for different responses of Eurasian watermilfoil (Myriophyllum spicatum) and hybrid watermilfoil (Myriophyllum spicatum x Myriophyllum sibiricum), which cannot be reliably distinguished based on morphological characters. Laboratory studies show that hybrid watermilfoil genotypes can grow faster, branch more, and be less responsive to herbicide control methods designed for Eurasian watermilfoil. To date, however, there have been no statistical comparisons of the responses of hybrid versus Eurasian watermilfoil under operational management in the field. In this study, we use genetic methods to distinguish morphologically cryptic Eurasian and hybrid watermilfoil in Houghton Lake, Michigan, and test whether they exhibit divergent responses for distribution and abundance following treatment with 2,4-dichlorophenoxyacetic acid-amine and triclopyr. Our results suggest that treatment reduced the frequency of occurrence and abundance of pure Eurasian watermilfoil more than it did for hybrids in Houghton Lake. These findings highlight the importance of distinguishing these cryptic taxa during management evaluations.
Invasive aquatic plants cause billions of dollars of damage annually in the United States by impairing boating, swimming, water movement, and nutrient cycling, reducing property values, increasing sedimentation, and eliminating habitat for native species (Smith and Barko Citation1990, Lovell and Stone Citation2005, Zhang and Boyle Citation2010). Moreover, the direct cost of managing these weeds amounts to an additional $100 million annually in the United States alone (Rockwell Citation2003, Pimentel et al. Citation2005). Largely for these reasons, invasive aquatic plants have become a major concern for lake and reservoir managers.
One complication in managing invasive aquatic plants is that outcomes of specific control techniques for what appears to be a single taxon may vary markedly among sites. Cryptic invasive taxa that vary in their growth, vigor, or herbicide response are a potentially important source of variation in treatment outcomes. By “cryptic taxa,” we mean taxa that are difficult to distinguish based on traditional morphological characters. Obvious examples of cryptic taxa affecting management outcomes are the numerous cases of morphologically cryptic herbicide-resistant biotypes of agricultural weeds (Heap Citation1997). Herbicide resistant biotypes (Hydrilla verticillata; Michel et al. Citation2004, Arias et al. Citation2005) and herbicide tolerant biotypes (hybrid watermilfoil; Berger et al. Citation2012, Citation2015, Thum et al. Citation2012) have also been found in aquatic plants.
Outside of examples of herbicide resistant biotypes, cryptic taxa appear commonly in some aquatic plant species of management concern, including monoecious versus dioecious hydrilla (Hydrilla verticillata; Stewart and Van Citation1987, Michel et al. Citation2004), native versus introduced common reed (Phragmites australis; Saltonstall Citation2002), and genetically distinct biotypes of variable leaf watermilfoil (Myriophyllum heterophyllum; Thum et al. Citation2011). However, little is currently known about how common it is for such cryptic taxa to respond differently to management efforts. When such differences exist, control plans developed for one invasive taxon may be unknowingly applied to a less-sensitive cryptic taxon, resulting in variable and unpredictable control efficacy, wasted resources, and continued spread of the less sensitive taxon. Avoiding these problems requires that cryptic taxa be initially correctly identified and then monitored to determine any differences in herbicide sensitivity among the cryptic taxa and manage them individually.
An important example of managing cryptic invasive plant taxa in the United States is Eurasian watermilfoil sensu lato, which includes both pure parental lineages (Myriophyllum spicatum L.; EWM) and morphologically cryptic hybrid lineages (HWM) from crosses with native northern watermilfoil (Myriophyllum sibiricum Komorov; NWM). Eurasian watermilfoil has been one of the most highly managed invasive aquatic weeds in the United States since the late 1980s (Bartodziej and Ludlow Citation1998). Initial management control efforts seemed to be effective, but anecdotal accounts of lower treatment efficacy in certain populations surfaced in the late 1990s. Subsequent genetic analysis of some “abnormal” Eurasian watermilfoil populations identified them as HWM (Moody and Les Citation2002, Citation2007, Sturtevant et al. Citation2009), which led to the hypothesis that hybridization may play an important role in the evolution of invasiveness in Eurasian watermilfoil (Moody and Les Citation2002, Citation2007), including the possibility that hybrids are more difficult to control than parental EWM. Laboratory studies have demonstrated that some HWM genotypes exhibit higher vegetative growth and lower response to the commonly used herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) compared to EWM (Poovey et al. Citation2007, Glomski and Netherland Citation2010, LaRue et al. Citation2013, Berger et al. Citation2015), but these results have not been confirmed in the field.
Although managers increasingly view EWM and HWM as distinct, these 2 cryptic taxa are often confused in pre- and post-treatment vegetation surveys, where plants typically are identified using morphological characters. As a result, there is still uncertainty regarding whether, and to what extent, hybrid watermilfoils pose distinct management challenges for lake management programs (e.g., will they respond similarly or differently to a given control technique compared to pure Eurasian watermilfoil?).
In this study, we separately assessed the responses of EWM and HWM to treatment with the systemic auxinic herbicides 2,4-D and triclopyr in a large lake in central Michigan. We used molecular genetic identification methods to distinguish the 2 taxa. We tested the hypothesis that parental EWM would decrease more than HWM during the growing season following herbicide treatment.
Study site
The field study was conducted on Houghton Lake in Roscommon County, Michigan. Houghton Lake had a surface area of 8112 ha, an average depth of 2.6 m, and has been managed for watermilfoil since 2002. The first treatment in 2002 was a whole-lake fluridone treatment followed by subsequent spot treatments using a combination of 2,4-D-amine and triclopyr.
Materials and methods
Treatment
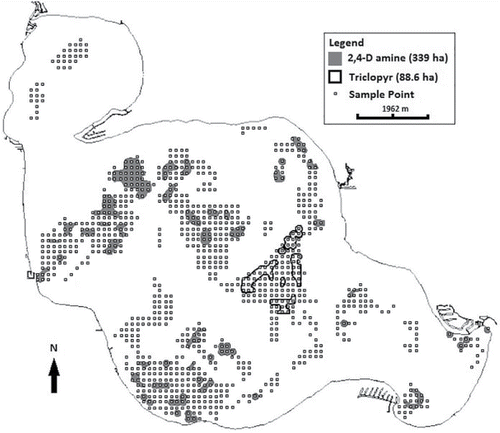
In June 2014, 339 ha (45 treatment sites containing 187 sample points) of the main portion of the lake was treated with 2,4-D-amine (Sculpin; 2,4-Dicholorophenoxyacetic acid; dimethylamine salt; granular) at 29.4 kg/ha with a target concentration of 1.145 ppm. In addition, 88.6 ha (7 treatment sites containing 49 sample points) was treated with triclopyr (Renovate OTF; 3,5,6-trichloro-2-pyridinyloxyacetic acid; trimethylamine salt; granular) at 14.9 kg/ha with a target concentration of 0.352 ppm ().
Data collection
Vegetation data were collected in June 2014 prior to treatment and at the end of the season in September. A total of 996 points were surveyed using a point-intercept grid (Mikulyuk et al. Citation2010) for both sampling events (). At each sampling point, a rake was tossed from each side of the boat to sample plants (Hauxwell et al. Citation2010, Thum et al. Citation2012). The total number of points with watermilfoil present was interpreted as the absolute frequency of occurrence. We estimated the abundance at each grid point using a semiquantitative index based on visual assessment of the amount of watermilfoil on the rake (Thum et al. Citation2012): 1 = no living watermilfoil present on the rake, 2 = <5% of the rake tines were covered with watermilfoil, 3 = 5–25% of the rake tines were covered with watermilfoil, 4 = 25–50% of the rake tines were covered with watermilfoil, and 5 = >50% of the rake tines were covered with watermilfoil. The mean of the 2 rake scores (one from each side of the boat) was interpreted as the abundance at each grid-point. Watermilfoil samples from both rake tosses were combined, and a representative plant was taken from the combined sample to conduct the genetic identification(s). In most cases, only one plant per grid point was used for genetic identification because all plants were morphologically similar. In some cases, however, the plant samples had qualitatively distinct phenotypes when examining leaf characters such as rigidity, pinnae number, and color. In these cases, one plant per putatively distinct phenotype was collected for genetic identification. Tissue samples were transported to the lab and genetically identified using restriction enzyme banding patterns for the internal transcribed spacer (ITS; Thum et al. Citation2006, Grafé et al. Citation2015).
Data analysis
Frequency of occurrence and abundance measures for each watermilfoil population were analyzed to evaluate differences between the responses of HWM and pure EWM to treatment with auxinic herbicides. NWM was excluded from all analyses because of an insufficient sample size (n = 2 pre-treatment, n = 0 post-treatment; ). Within the main lake, neither areas treated with different herbicides nor areas within versus outside of the application sites showed statistical differences in watermilfoil reduction (). Data from all areas were therefore combined for statistical analysis.
Table 1. The number of points in 996 total point that contained each taxon in the main portion of Houghton Lake before and after treatment in 2014 with auxinic herbicides in Houghton Lake, Michigan.
Because neither abundances nor probabilities of occurrence at sampling points located near each other were expected to be independent, we assessed the degree and statistical significance of spatial autocorrelation in the data using Moran's I statistic and a permutation test for significance (Cliff and Ord Citation1981). For all watermilfoil combined (EWM + HWM) and for HWM separately, we found moderate and statistically significant positive autocorrelation (I ≈ 0.3, P < 0.0001). Because positive autocorrelation would result in artificially low P values in our statistical tests for changes in frequency of occurrence and abundance, we thinned the grid-points used for these tests to increase the minimum distance between points by removing points at which no watermilfoil was ever found and used data only from alternate points in each row of the grid (like using only the black squares on a checkerboard). Additionally, all points that contained both EWM and HWM (14 points pre-treatment) were also removed because separate abundance estimates for EWM and HWM could not be obtained. These steps substantially reduced Moran's I for all components of the data (EWM + HWM, HWM only, EWM only; pre- and post-treatment) and eliminated statistical significance of spatial autocorrelation. This thinned dataset was used for all subsequent statistical analyses.
To assess the change in frequency of occurrence for each taxon individually, the corresponding data were analyzed using an exact McNemar test for dependent proportions (Hollander et al. Citation2014). The null hypothesis for a given taxon (EWM only, HWM only, or EWM + HWM) was that the proportion of grid-points changing from presence to absence following treatment was the same as the proportion changing from absence to presence; the alternative hypothesis was that the proportion of grid-points changing from presence to absence was higher.
Differences in the degree to which EWM versus HWM frequency of occurrence changed following treatment were assessed using Zelen's exact test for a common odds ratio (Hollander et al. Citation2014). The odds for population i are:
(1) where pi is the success probability defined as the proportion of grid-points at which population i was present. The odds ratio for a population is the ratio of its pre-treatment and post-treatment odds. The null hypothesis for Zelen's test was that the odds ratios are the same for EWM and HWM; the alternative hypothesis was that the odds ratios differ.
Potential reduction in the relative abundance of each taxon following treatment was assessed by calculating the post-treatment change in abundance at each grid-point and testing the null hypothesis that the mean change was zero against the alternative hypothesis that the mean change was negative. We conducted these tests using a bootstrap t-test (with 10,000 bootstrap samples) because of the numerous ties in these data and their evident non-normality.
Differences in the degree to which EWM and HWM populations abundances changed following treatment were assessed using a large-sample test for the difference between 2 success probabilities. The success probability for each taxon was defined as the proportion of grid-points at which its abundance index decreased following treatment. The null hypothesis of no difference in success probabilities between EWM and HWM was tested against the one-sided alternative that the success probability of EWM was greater, using test statistic A defined by Hollander et al. Citation(2014).
All statistical analyses were performed with the R language and statistical environment, R 3.1.0 (R Foundation for Statistical Computing 2014).
Results
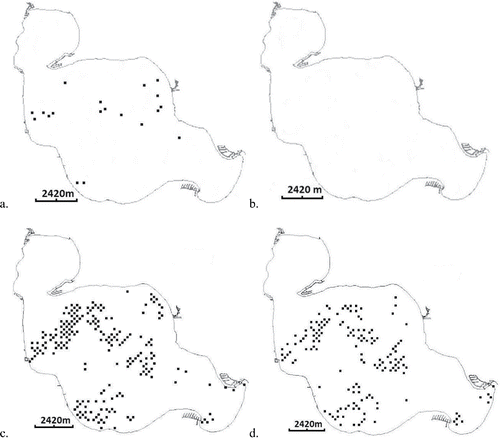
EWM and HWM both exhibited a statistically significant decrease in frequency of occurrence following treatment (EWM: P < 0.0001, percent change = −100%; HWM: P < 0.0001, percent change = −26.2%; ). Zelen's test on the odds ratio confirmed that the proportions of grid-points occupied by EWM and HWM in the main lake changed by different amounts following treatment (P < 0.0001).
Figure 2. The number of points (frequency of occurrence) with plants present for Eurasian watermilfoil only (a and b) and hybrid watermilfoil only (c and d) in Houghton Lake, Michigan, before (left) and after (right) treatment in 2014 with auxinic herbicides.
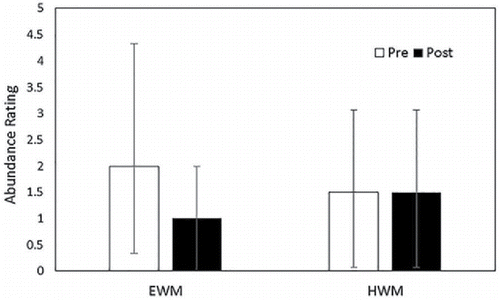
The bootstrap test supported the alternative hypothesis that mean abundance decreased following treatment for both taxa in the main lake (P < 0.0001 for both EWM and HWM; ). Also, a greater proportion of points with EWM exhibited a decrease in abundance following treatment compared to HWM (A = 3.727; P < 0.0001; percent decreasing = 100% for EWM and 57.8% for HWM).
Figure 3. Abundance distributions for points with Eurasian (EWM) and hybrid (HWM) watermilfoil present in the main portion of Houghton Lake before and after treatment with auxinic herbicides in 2014. Bars indicate the median abundance scores and the error bars indicate the interquartile range of abundance scores.
Discussion
Here, we have presented results from the first study to compare the responses of EWM and HWM to treatment with auxinic herbicides in the field. We found that the within-season reduction in watermilfoil abundance following treatment was greater for EWM than for HWM in Houghton Lake. Our study demonstrates that HWM poses management challenges distinct from pure EWM in Houghton Lake and provides a quantitative empirical example (as opposed to anecdotal report) that some HWM can be more difficult to control than EWM and may therefore require a different management strategy to achieve equally effective control.
If studies on other lakes similarly document divergent responses of EWM and HWM to specific control strategies, the most important question then becomes: what management strategy will be most effective for achieving similar (or acceptable) control of HWM compared to EWM? Increasing interest in and concern about HWM has led to numerous proposed alternative management strategies (e.g., increased concentration, longer exposure, early- versus late-season timing of applications, whole-lake versus spot treatment applications, herbicide mixtures, etc.). Further assessment of these alternative strategies is needed, particularly in lakes with quantitatively documented reduced HWM responses compared to those for EWM.
Three limitations of our study warrant further and careful investigation. First, the temporal scale of our June pre-treatment and September post-treatment sampling does not allow us to distinguish the cause(s) of reduced seasonal control efficacy of HWM compared to pure EWM. One possibility is that the lower reduction of HWM compared to EWM results from lower mortality on the standing crop of plants to which the auxinic herbicides were applied (i.e., higher survivorship of HWM). Another is that the lower reduction of HWM results from faster recolonization of treated areas. For example, HWM from untreated areas may have spread more quickly to recolonize treated areas, or plants observed in the post-treatment sampling may have colonized from the seed bank. The alternative explanations are not mutually exclusive, given that laboratory studies have demonstrated lesser effects of 2,4-D on HWM, faster vegetative growth of HWM, and greater germination of HWM compared to EWM (LaRue et al. Citation2013; D. Grimm, Montana State University, August 2014, unpubl. data). Future studies should use repeated temporal sampling and methods that can more definitively quantify shorter versus longer term effects of herbicide exposure and source(s) of regrowth.
A second limitation of our study is that we cannot rule out effects of environmental factors on the reduction of each taxon. It will be difficult in practice to have true control versus treated lakes to precisely quantify the effects of herbicide treatment versus environmental factors, but sampling events over the span of multiple years may further solidify patterns of response to herbicide treatments. As such, monitoring EWM and HWM on multiple lakes during operational management is necessary. In addition, where possible, laboratory herbicide screening would provide complementary data for differences in herbicide susceptibility.
A third limitation is whether our observations of EWM versus HWM in Houghton Lake in 2014 will be representative of quantitative observations conducted on other lakes. Laboratory studies demonstrate that HWM can exhibit faster vegetative growth and reduced 2,4-D response compared to EWM (LaRue et al. Citation2013). However, other laboratory studies demonstrate that hybrid accessions may differ in their growth and response to herbicides, including some hybrid accessions that respond similarly to EWM accessions used for comparison (Poovey et al. Citation2007, Slade et al. Citation2007, Glomski and Netherland Citation2010, Berger et al. Citation2012, Citation2015). Thus, EWM and HWM populations would possibly exhibit similar responses to each other on other lakes, depending on the specific genotypes present. Continued assessment of EWM and HWM on other lakes, in addition to herbicide screens comparing different genotypes, will begin to determine if the responses observed on Houghton Lake are representative of general patterns for how each taxon will respond to treatment versus if responses of HWM populations vary from one lake to another.
Our study shows for the first time that cryptic hybrid and Eurasian watermilfoil can exhibit substantially different responses to control under operational management conditions. A clear management implication is that correctly distinguishing between these cryptic taxa is important to plan, implement, and assess programs aimed at controlling them. Although traditional morphological characters are not sufficient for this purpose, we found that genetic identification worked well and can be recommended as a practical and reliable method.
Acknowledgments
We are grateful to Lindsey Schulte, Rick Buteyn, Ryan Scott, and Evan Thomas at Progressive AE for their aid in the collection of the data for this study. We would like to thank 3 anonymous reviewers and Andrew Pyman, Ryan Sheik, Greg Chorak, Jeremy Newton, Fatouma Abdoulaye, Danielle Grimm, and Jeff Pashnick for helpful comments that improved the manuscript.
Funding
We are grateful to the Houghton Lake Improvement Board and the Michigan Economic Development Corporation for funding.
References
- Arias RS, Netherland MD, Scheffler BE, Atul P, Dayan FE. 2005. Molecular evolution of herbicide resistance to phytoene desaturase inhibitors in Hydrilla verticillata and its potential use to generate herbicide-resistant crops. Pest Manage. 61:258–268.
- Bartodziej W, Ludlow J. 1998. Aquatic vegetation monitoring by natural resources agencies in the United States. Lake Reserv Manage. 13:109–117.
- Berger ST, Netherland MD, MacDonald GE. 2012. Evaluating fluridone sensitivity of multiple hybrid and Eurasian watermilfoil accessions under mesocosm conditions. J Aquat Plant Manage. 50:135–144.
- Berger ST, Netherland MD, MacDonald GE. 2015. Development of a rapid assay to detect reduced fluridone sensitivity in invasive watermilfoils. Weed Technol. 29:605–610.
- Cliff AD, Ord JK. 1981. Spatial processes: models and applications. London (UK): Pion.
- Glomski LM, Netherland MD. 2010. Response of Eurasian watermilfoil and hybrid watermilfoil to low use rates and extended exposures of 2,4-D and triclopyr. J Aquat Plant Manage. 48:12–14.
- Grafé SF, Boutin C, Pick FR. 2015. A PCR-RFLP method to detect hybridization between the invasive Eurasian watermilfoil (Myriophyllum spicatum) and the native northern watermilfoil (Myriophyllum sibiricum), and its application in Ontario lakes. Botany. 93:117–121.
- Hauxwell J, Knight S, Wagner K, Mikulyuk A, Nault M, Porzky M, Chase S. 2010. Recommended baseline monitoring of aquatic plants in Wisconsin: sampling design, field and laboratory procedures, data entry and analysis, and applications. Madison (WI): Wisconsin Department of Natural Resources Bureau of Science Services. PUB-SS-1068 2010.
- Heap IM. 1997. The occurrence of herbicide-resistant weeds worldwide. Pestic Sci. 51:235–243.
- Hollander M, Wolfe DA, Chicken E. 2014. Nonparametric statistical methods, 3rd ed. Hoboken (NJ): John Wiley and Sons. p. 497, 506–508, and 527–530.
- LaRue EA, Zuellig MP, Netherland MD, Heilman MA, Thum RA. 2013. Hybrid watermilfoil lineages are more invasive and less sensitive to a commonly used herbicide than their exotic parent (Eurasian watermilfoil). Evol Appl. 6:462–471.
- Lovell SJ, Stone SF. 2005. The economic impacts of aquatic invasive species: a review of the literature. National Center for Environmental Economics. Working Paper #05–02.
- Michel A, Arias RS, Scheffler BE, Duke SO, Netherland M, Dayan FE. 2004. Somatic mutation-mediated evolution of herbicide resistance in the nonindigenous invasive plant hydrilla (Hydrilla verticillata). Mol Ecol. 13:3229–3237.
- Mikulyuk A, Hauxwell J, Rasmussen P, Knight S, Wagner KL, Nault ME, Ridgley D. 2010. Testing a methodology for assessing plant communities in temperate inland lakes. Lake Reserv Manage. 26:54–62.
- Moody ML, Les DH. 2002. Evidence of hybridity in invasive watermilfoil (Myriophyllum) populations. P Nat Acad Sci. 19:14867–14871.
- Moody ML, Les DH. 2007. Geographic distribution and genotypic composition of invasive hybrid watermilfoil (Myriophyllum spicatum x M. sibiricum) populations in North America. Biol Invasions. 9:559–570.
- Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental costs associated with alien-invasive species in the United States. Ecol Econ. 52:273–288.
- Poovey AG, Slade JG, Netherland MD. 2007. Susceptibility of Eurasian watermilfoil (Myriophyllum spicatum) and a milfoil hybrid (M. spicatum x M. sibiricum) to triclopyr and 2,4-D amine. J Aquat Plant Manage. 45:111–115.
- R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
- Rockwell HW. 2003. Summary of a survey of the literature on the economic impact of aquatic weeds. In: The economic impact of aquatic weeds. p. 1–18.
- Saltonstall K. 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. P Nat Acad Sci. 99:2445–2449.
- Slade JG, Poovey AG, Netherland MD. 2007. Efficacy of fluridone on Eurasian and hybrid watermilfoil. J Aquat Plant Manage. 45:116–118.
- Smith CS, Barko JW. 1990. Ecology of Eurasian watermilfoil. J Aquat Plant Manage. 28:55–64.
- Stewart KK, Van TK. 1987. Comparative studies of monoecious and dioecious hydrilla (Hydrilla verticillata) biotypes. Weed Sci. 35:204–210.
- Sturtevant AP, Hatley N, Pullman GD, Sheick R, Shorez D, Bordine A, Mausolf R, Lewis A, Sutter R, Mortimer A. 2009. Molecular characterization of Eurasian watermilfoil, northern watermilfoil, and the invasive interspecific hybrid in Michigan lakes. J Aquat Plant Manage. 47:128–135.
- Thum RA, Lennon JT, Connor J, Smagula AP. 2006. A DNA fingerprinting approach for distinguishing among native and non-native milfoils. Lake Reserv Manage. 22:1–6.
- Thum RA, Wcisel DJ, Zuellig MP, Heilman M, Hausler P, Tyning P, Huberty L, Netherland MD. 2012. Field documentation of decreased herbicide response by a hybrid watermilfoil population. J Aquat Plant Manage. 50:141–146.
- Thum RA, Zuellig MP, Johnson RL, Moody ML, Vossbrinck C. 2011. Molecular markers reconstruct the invasion history of variable leaf watermilfoil (Myriophyllum heterphyllum) and distinguish it from closely related species. Biol Invasions. 13:1687–1709.
- Zhang C, Boyle KJ. 2010. The effect of aquatic invasive species (Eurasian watermilfoil) on lakefront property values. Ecol Econ. 70:394–404.