ABSTRACT
Winston B, Scott JT, Pollock E. 2016. The synergistic effect of elevated CO2 and phosphorus on reservoir eutrophication. Lake Reserve Manage. 32:373–385.
Increased delivery of carbon (C) and phosphorus (P) as a result of climate change is predicted to significantly increase algal biomass and alter algal nutrient content. We conducted a 2-phased experiment to determine the following: (1) whether carbon dioxide partial pressure (PCO2) in the water column of reservoirs dropped low enough to limit algal biomass, (2) whether increasing the PCO2 in the water column could alleviate C limitation and increase algal biomass, and (3) whether the simultaneous increase in PCO2 and P alter algal nutrient content. In the first phase we reconstructed PCO2 in the water column of several northwest Arkansas reservoirs on timescales ranging from days to decades. During fall, winter, and spring, PCO2 was greater than atmospheric levels, suggesting the reservoirs were sources of C released to the atmosphere. During summer, PCO2 was less than atmospheric levels, suggesting that reservoir PCO2 fell low enough to potentially limit algal biomass. In the second phase, we conducted an enrichment experiment along a PCO2 gradient representative of preindustrial (250 µatm), current (400 µatm), and 2050 projected levels from the Intergovernmental Panel on Climate Change (550 µatm) along a P gradient. Our results showed that relative to preindustrial and current levels, elevated PCO2 had no significant effect on algal biomass and nutrient content. Instead, biomass increased significantly with increasing levels of P, supporting predictions that even under elevated PCO2 and global climate change scenarios, P might still be the major nutrient limiting algal growth in these reservoirs.
Before the 1970s, carbon (C) was perceived to be the primary nutrient limiting algal biomass. Numerous studies from the 1970s through the 1980s showed consistent relationships between phosphorus (P) and algal biomass (Schindler Citation1977, Hecky and Kilham Citation1988, Jones and Knowlton Citation1993) and relegated C to a minor role. To date, most studies investigating algal limitation focus on P, nitrogen (N), or combinations of both (Schindler Citation1977, Dzialowski et al. Citation2005 Scott and McCarthy Citation2010) with little to no emphasis on C.
Despite the overwhelming evidence supporting N- and P-driven limitation, C limitation is still possible under certain scenarios. For example, in softwater lakes with low or intermediate dissolved inorganic C (DIC), photosynthetic activity decreased when carbon dioxide partial pressure (PCO2) declined (Hein Citation1997). The study concluded that activity declined because the demand during photosynthesis exceeded the supply through diffusion and reaeration. Jansson et al. (Citation2012) reported phytoplankton primary production in boreal lakes 10 times greater at elevated PCO2 compared to ambient conditions. Under experimental conditions, Urabe et al. (Citation2003) reported increased algal growth with elevated PCO2 and low nutrient levels. These results suggested that C limitation might be more common than currently investigated.
If C is limiting algal biomass in northwest Arkansas (NWA) reservoirs, activities that directly or indirectly increase C may alleviate limitation and increase biomass in these reservoirs. Extreme precipitation events are predicted to occur more frequently globally (IPCC Citation2007, Citation2014), which can increase the supply of allochthonous C and provide excessive nutrients (eutrophication) to NWA reservoirs. Winston et al. (Citation2014) showed nutrient concentration at least doubled during heavy rainfall periods in a NWA reservoir.
The combined effects of increased C and nutrients are projected to alter algal nutrient content and the structure of aquatic food webs (Van de Waal et al. Citation2010) because phytoplankton with a high C-nutrient content is poor-quality food for most zooplankton species (Sterner and Elser Citation2002). An aquatic community dominated by phytoplankton with a high C-nutrient content might favor less nutrient-demanding zooplankton species, which in turn will effectively alter the structure and function of the aquatic food web. From a water management perspective, the consequences of elevated C and increased nutrients could be significant. Elevated C could result in increased algal biomass (Urabe et al. Citation2003, Jansson et al. Citation2012), which in turn could lead to increased episodes of harmful algal blooms (Paerl and Huisman Citation2009), taste and odor episodes (Winston et al. Citation2014), and the formation of disinfection byproducts (DBPs; Mash et al. Citation2014). These negative effects would likely cause massive disruption of water supplies and recreational uses of reservoirs (Paerl and Huisman Citation2009, Wagner and Adrian Citation2009).
We conducted a 2-phased experiment to determine (Equation1(1) ) whether PCO2 in the water column of reservoirs dropped low enough to limit algal biomass, (Equation2
(2) ) whether increasing the PCO2 in the water column could alleviate C limitation and increase biomass, and (3) whether the simultaneous increase in PCO2 and P would alter algal nutrient content. Algal biomass was expressed as chlorophyll a (Chl-a) and particulate carbon (PC) and algal nutrient content as seston C:P, C:N, and N:P. These proxies were chosen to compare with previous studies, some of which showed increased algal biomass and altered algal nutrient content (Urabe et al. Citation2003, Verschoor et al. Citation2013), and some of which showed contrasting results (Burkhardt et al. Citation1999, Gervais and Riebesell Citation2001).
Site description
The research was conducted in 5 lakes in the NWA area. Beaver Lake, Lake Brittany, Lake Rayburn, and Lake Norwood were used to reconstruct PCO2, and Lake Fayetteville was used to validate the model used in the reconstruction. All lakes were located in the Springfield Plateau, composed of karstified limestone and dolostone mantled by soil and insoluble regolith. The plateau is deeply dissected with well-developed dendritic drainage underlain by Paleozoic strata from the Ordovician through Mississippian periods (Croneis Citation1930, Haley et al. Citation1993).
Beaver Lake (94°12′N, 36°18″W) is a warm monomictic lake with a surface area of 113 km2 and an average depth of 18 m. The reservoir was built between 1960 and 1966 on the upper portions of the White River for flood control, hydropower generation, and water supply. The watershed drainage area is 3087 km2, with 57% forest, 32% agriculture, and 5% urban (Galloway and Green Citation2007). The trophic status of Beaver Lake is variable and ranges from eutrophic in the riverine portions to oligotrophic near the dam. Average annual total N (TN), total P (TP), Chl-a, and pH are 800 µg/L, 5 µg/L, 5 µg/L, and 8.53, respectively ().
Table 1. Select reservoir characteristics for Beaver Lake and Lakes Brittany, Norwood, Rayburn, and Fayetteville. Data from Ben Thompson's master's thesis and Bryant Baker's master's thesis, and from the Arkansas Department of Environmental Quality.
Lakes Brittany (36°28′08″N, 94°12′04″W), Norwood (36°28′43.01″N, 94°14′48.62″W), and Rayburn (36°27′43″N, 94°14′21″W) are warm monomictic water bodies, similar in size (<1 km2), depth (24 m), watershed dimensions, and land-use characteristics. The reservoirs were built in the 1970s, predominantly for recreational purposes. Forests account for the majority of the land use (64–77%) followed by urban (14–25%), which is mostly single-family homes and roadways. Lake Brittany had the lowest average annual TN, TP, Chl-a, and pH of all the smaller lakes (), with annual averages of 400 µg/L, 15 µg/L, 6 µg/L, and 7.88, respectively.
Lake Fayetteville (36°13′56″N, 94°13′9″E) was built in 1949 as a reservoir for the city of Fayetteville, Arkansas, but now is used for recreational purposes. The reservoir is also warm and monomictic, with a surface area <1 km2 (Scott and Grantz Citation2013) and elevated levels of TN, TP (ADEQ Citation2004), and seston Chl-a ().
Materials and methods
To assess C limitation of algal biomass, we reconstructed water-column PCO2 over long-term, short-term, and daily scales for 4 lakes in NWA. Carbon limitation was defined as water-column PCO2 less than atmospheric PCO2, which averaged 397 µatm in 2013 measured at the Mauna Loa Observatory.
Long-term and short term PCO2 reconstruction
All PCO2 reconstructions are based on C chemistry equations from Stumm and Morgan (Citation1996) and Henry's Law.
Stumm and Morgan (Citation1996):
(1) where [CO2] is the concentration of carbon dioxide in moles per liter, [HCO3−] is the concentration of bicarbonate in moles per liter, Ka is the acid dissociation constant, and pH is the negative log of the hydrogen ion concentration.
Henry's Law:
(2) where KH is Henry's constant (29.41 L atm/mol) at standard temperature and pressure. Our longest PCO2 reconstruction was for Beaver Lake based on a historical dataset from 1986 to 2010. Long-term pH, temperature, and bicarbonate concentration data were obtained from Beaver Water District, the regional water management authority.
Short-term PCO2 reconstructions were from data compiled over a 2-year period from in situ monitoring of physical water quality parameters at Lakes Brittany, Norwood, and Rayburn. A HACH datasonde recorded pH, temperature, and dissolved oxygen (DO) at 15-min intervals. Every 2 weeks, the probe was retrieved and data downloaded. The probe was also recalibrated and DO sensors were checked and replaced as necessary. There was no significant fouling of the sensors; therefore, DO corrections were not required.
Daily reconstructions were based on data retrieved from Beaver Lake on the 22 June, 22 July, and 22 August (JJA) 2011 and 2012. These months were chosen because they generally coincided with warm temperatures, stable water column, and increased algal biomass in the reservoir. To calculate daily fluctuations in PCO2, we first developed a linear relationship between pH and DO as follows. First, pH, temperature, and DO were measured at 15-min intervals using a HACH datasonde for 1 week at a depth of 0.5 m from the surface. The probe was returned to the lab, data downloaded, and the linear relationship between pH and DO assessed. Second, we obtained 5-min interval DO and temperature data measured by the US Geological Survey Lake Diagnostic System (LDS, http://waterdata.usgs.gov/ar/nwis) for 2011 and 2012. Briefly, the LDS consists of a string of temperature and DO sensors at 0.5 m depths from the surface to the bottom of the lake. Daily DO from the 0.5 m sensor was then used to estimate daily pH from the linear relationship obtained during the week-long sampling. Daily fluctuations in PCO2 were determined using equations Equation1(1) and Equation2
(2) .
Model verification: diurnal PCO2 and δ13C direct measurements
To verify the model used for PCO2 calculations, we performed direct measurements of PCO2 and δ13C diurnally during August 2012. Samples were collected from Lake Fayetteville in acid- washed and rinsed bottles with no headspace, crimped, and placed on ice. Lake Fayetteville was chosen to maximize chances of obtaining drastic swings in PCO2 because of its eutrophic nature. Temperature, pH, and DO were also measured at sample collection using a Yellow Springs 600 XLM multiparameter datasonde. PCO2 and δ13C were measured on a Picarro CO2/CH4 analyzer (G2201-I) at the University of Arkansas Stable Isotope Lab (UASIL). Instrument precision for CO2 was 0.16‰. Isotopic standards ranged from −3.64 to −44.47‰, obtained from Oztech Trading Company, Dallas, Texas. The final C isotope values are expressed relative to V-PBD standard. The quantitative standards for CO2 were from Scott Specialty Gases (Aire Liquide) with a balance of air. Additionally, we estimated PCO2 from the measured pH during sample collection using the same method for the long-term data. Estimated PCO2 was then compared with the instrumentally measured PCO2 using regression to determine the reliability of the model.
Algal response to elevated PCO2
To determine if elevated PCO2 and P increased biomass and altered algal nutrient content, we conducted a semicontinuous enrichment experiment at PCO2 representative of preindustrial, present-day, and 2050 projected levels from the Intergovernmental Panel on Climate Change across a P gradient. We used preindustrial PCO2 (250 µatm) to represent the control and PCO2 of 550 µatm to represent elevated PCO2 conditions. This experiment was conducted for 13 days during summer 2012.
We harvested 70 L of water from the Beaver Lake transition zone, which was placed on ice and transported back to the lab. The transition zone is the area between the riverine and lacustrine zones and is characterized by decreased turbidity and increased phytoplankton productivity relative to the riverine zone (Wetzel Citation2001). We performed the enrichment experiment for the transition zone for several reasons. First, Beaver Water District, the regional domestic water supplier, has been drawing water from the transition zone since the 1970s and had the longest available dataset for the PCO2 reconstructions (1986–2010). Second, the lake undergoes annual taste and odor episodes specifically in the transition zone. Our experiment is part of the research designed to better understand conditions leading to taste and odor episodes in the transition zone.
In the lab, the sample was subdivided over a 63 µm mesh to remove large zooplankton and placed into 3 acid-washed 20 L aquatainers. To buffer against pH changes, 5.5 g of CaCO3/L were added to each aquatainer. Each aquatainer was aerated with CO2 at 250, 400, and 550 µatm for 4 h to allow equilibration. From each aquatainer, 18 acid-washed 1 L cubitainers were filled with 0.95 L of the aerated water. Each cubitainer was amended with 1000 µg/L N as potassium nitrate (KNO3) to prevent N limitation. The cubitainers were subdivided into threes and amended with P as disodium hydrogen phosphate (Na2HPO4) to achieve final concentrations of 5, 10, 25, 50, 75, and 100 µg/L, respectively (initial P concentration 5 µg/L). At the end of the setup, we had 54 cubitainers: 18 cubitainers at each PCO2 level (250, 400, and 550 µatm) along an enrichment gradient of 5–100 µg/L P.
After N and P amendments, cubitainers were placed in a 30 C circulating water bath under artificial lighting. Light exposure was measured to be 500 µmol photons/m2 s2 and controlled by a timer on a 12 h on/off cycle. The conditions were designed to mimic in situ conditions at Beaver Lake during summer, ranging from 400 to 500 µmol photons/m2 s2 at the surface. The cubitainers were shaken manually each day to prevent particles from attaching to the sides. On days 4, 7, 10, and 13, ∼0.7 L of water was collected from each cubitainer to determine particulate C, N, and P content and Chl-a. Each cubitainer was renewed with the same volume of sterilized Beaver Lake water at the respective CO2 concentration and initial concentrations of N and P. Following the experiment, samples were filtered onto Whatman GFF filters for phytoplankton PC, particulate N (PN), Chl-a, and particulate P (PP). Filtrate was also retained for dissolved nutrient analysis.
Algal P content was determined using the ascorbic acid method following a persulfate digestion (APHA Citation2005). Briefly, the filters were transferred to vials using 7.5 mL of Nanopure water with 7.5 mL of persulfate digestion solution (20g K2S2O8/L) added to each vial. The vials were weighed and then autoclaved at 121 C for 1 h and allowed to cool overnight before being reweighed to calculate the percent recovery to account for water loss. After the digestion, P was determined according to the ascorbic acid method on a Turner Design Trilogy fluorometer with a spectrophotometric adapter. Algal C and N were determined using a Thermo Flash 2000 combustion elemental analyzer. Peach leaf standards were included with the P analyses and peach leaf and aspartic acid standards with the C and N analyses. From these data we calculated molar C:P, C:N, and N:P ratios for all treatments on all sample days.
Chl-a was measured to estimate algal biomass according to Standard Methods, with modifications (APHA Citation2005). Briefly, each filter was transferred into a 15 mL test tube containing 7 mL of 90% acetone solution. The samples were placed in a dark freezer for 24 h to further enhance complete pigment extraction. In a dark room, 3 mL of each sample extract was then transferred into VWR disposable test tubes and analyzed on a Turner Design Fluorometer. To adjust for the chlorophyll degradation product pheophytin, following the first measurement, 0.1 mL of 0.1 M HCl was added to each sample and measured again after 90 s.
Data analysis
All datawere analyzed using SYSTAT 13 (Systat Software Inc, Chicago, IL). Simple linear regression was used to assess the relationship between modeled and measured PCO2. To assess the impact of elevated PCO2 and P on algal biomass and nutrient content, response variables were first averaged over the 13 d period, checked for normality and homoscedasticity, and transformed when appropriate. Simple linear regression was used to determine the relationship between algal biomass (PC, Chl-a) and P. We compared the slopes of the regression lines between biomass and P using analysis of covariance (ANCOVA) to determine if the rate of biomass increase was influenced by the covariate (PCO2 level). A 2-way ANOVA was performed to determine if mean Chl-a, PC, C:N, C:P, and N:P ratios differed significantly across PCO2 levels and to determine the synergistic effects of PCO2 and P on algal biomass. When significant differences were obtained, post-hoc comparisons were performed with the Tukey's HSD test. Significance was set at P < 0.05.
Results
Carbon dioxide (CO2) drawdown on decadal to daily scale and relationship to biomass
Beaver Reservoir was the oldest of the 4 lakes sampled as part of this project, with data representing 25 years (1986–2011) of reconstructed PCO2 (). Reconstructed PCO2 ranged from 10 to 6800 μatm and averaged 1070 ± 46 μatm over the entire period of record. The 25-year summer average was 319 ± 49 μatm. Water column PCO2 was greater than atmospheric PCO2 in ∼79% of the reconstructed values and less than atmospheric PCO2 in 21% of the values. Low water column PCO2 occurred almost exclusively during summer. Chl-a values were low and ranged between 1 and 15 µg/L. Generally, higher Chl-a occurred during summer and coincided with low PCO2 values ().
Figure 1. Reconstructed carbon dioxide partial pressures (PCO2) for (a) Beaver Lake (1966–2010) and Lakes (b) Brittany, (c) Norwood, and (d) Rayburn (2011 and 2012). Note the different scale on (a). The dotted line represents the current global PCO2 level.
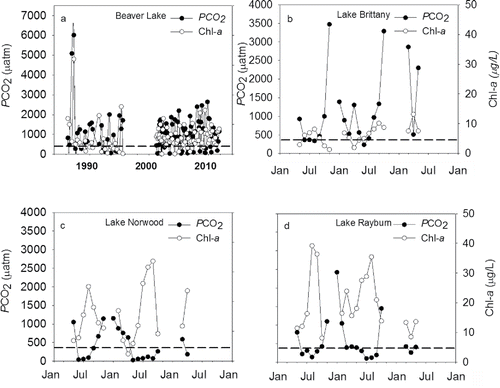
Lakes Brittany, Norwood, and Rayburn illustrate the results of PCO2 reconstructed between 2011 and 2012. Among these lakes, Lake Brittany had the greatest average PCO2 of 989 ± 50 μatm. Reconstructed PCO2 ranged from 63 to 4336 μatm with a median value of 504 μatm. Values were generally greater than atmospheric PCO2 in fall, winter, and spring and less than atmospheric PCO2 during summer (). Lake Norwood had an average PCO2 of 528 ± 26 μatm. Reconstructed PCO2 values ranged from 32 to 3388 μatm with a median value of 273 μatm (). Lake Brittany had water column PCO2 greater than atmospheric levels in fall, winter, and spring and less than atmospheric PCO2 during summer. Lake Rayburn also had higher PCO2 values during fall, winter, and spring and lowest values during summer (). Comparatively, Lake Rayburn had the lowest PCO2 of the younger lakes and averaged 405 ± 28 μatm. Reconstructed PCO2 ranged from 13 to 3780 μatm with a median value of 127 μatm. Values then increased from 19:00 h onward. Chl-a concentrations were lowest at Lake Brittany and ranged from 2 to 10 µg/L (). At Lakes Norwood and Rayburn Chl-a ranged from 2.5 to 35 µg/L and 8 to 40 µg/L, respectively. Similar to Beaver Lake, Chl-a concentrations were highest during summer and generally coincided with low PCO2 values (). For both 2011 and 2012, daily reconstructed PCO2 was typically highest from 23:00 to 07:00 h and lowest from 12:00 to 18:00 h. ().
Figure 2. Reconstruction of carbon dioxide partial pressures (PCO2) during June, July, and August in (a–c) 2011 and (d–f) 2012 at Beaver Lake, northwest Arkansas. The dotted line represents the current global PCO2 level.
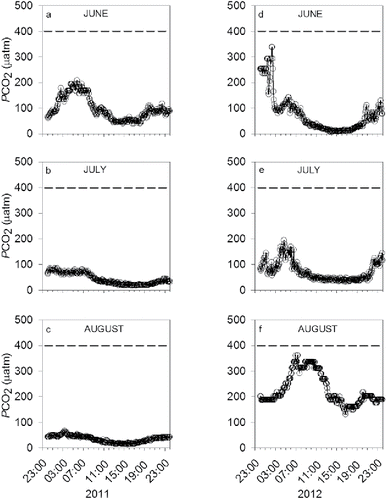
For the 2011 reconstructions, June PCO2 ranged from 40 to 209 μatm, July from 18 to 86 μatm, and August from 14 to 66 μatm (). Mean PCO2 was lowest in August (35 ± 1 μatm) and highest in June (99 ± 3 μatm). For the 2012 reconstructions, June PCO2 ranged from 8 to 339 μatm, July from 32 to 195 μatm, and August from 131 to 361 μatm (). Mean PCO2 was lowest in July (74 ± 2 μatm) and highest in August (228 ± 4 μatm).
Table 2. Summary of CO2 partial pressure (PCO2) for 22 June, 22 July, and 22 August 2011 and 2012.
Diurnal PCO2 and δ13C direct measurements
The diurnal PCO2 measurements were similar in trend to the PCO2 reconstructed for JJA 2011 and 2012 in that maximum and minimum PCO2 had a predictable diurnal variation. At the beginning of the sampling, measured PCO2 was 180 μatm at 20:00 h, which was less than half of atmospheric PCO2 (). By 06:00 h the following day, measured PCO2 increased to a maximum value of 800 μatm, approximately double atmospheric PCO2. The PCO2 then fell to 150 μatm around midday and remained at that level until the end of the sampling at 19:00 h (). The δ13C exhibited a trend opposite that of measured PCO2 (). Minimum δ13C of −12‰ occurred at 06:00 h when PCO2 was at a maximum (). The highest δ13C of 6‰ was measured at 19:00 h when PCO2 was at a minimum ().
Figure 3. Changes in δ13C and measured PCO2 over (a) a 24-hour period at Lake Fayetteville, northwest Arkansas, and (b) relationship between modeled and measured PCO2 for Lake Fayetteville, northwest Arkansas. The dotted line represents the current atmospheric PCO2 level.
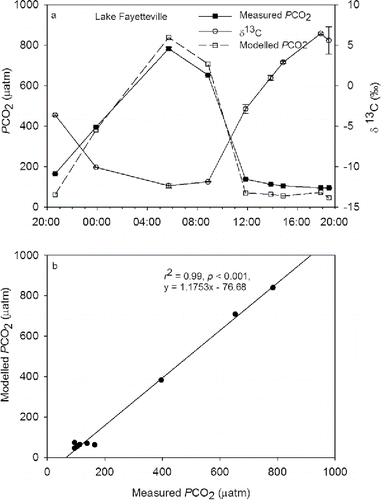
Modeled PCO2 values were typically lower than measured PCO2 values (). Underestimation seemed to be greater at lower PCO2. For example, at the start of the sample collection, measured PCO2 was 180 μatm compared to the modeled value of 62 μatm (). Modeled and measured values were almost identical at 06:00 and 09:00 h, at 800 and 700 μatm, respectively. Despite the underestimation, modeled PCO2 was significantly correlated to measured PCO2 (r2 = 0.99, P < 0.001; ).
Algal response to elevated PCO2 and P
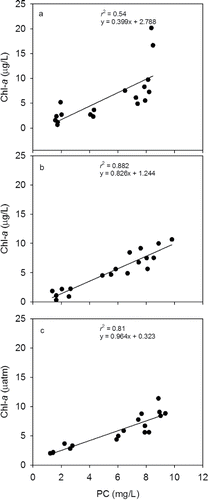
To determine if Chl-a and seston PC were good proxies for algal biomass, we plotted a regression-based relationship between the 2 variables (). PC and Chl-a were significantly correlated to each other at every level of PCO2 (P < 0.001; ). The strength of the relationship, expressed by the r2 value, was strongest at PCO2 values of 400 and 550 µatm (r2 = 0.88 and 0.81, respectively) and weakest at 250 µatm (r2 = 0.54).
Figure 4. The relationship between chlorophyll a (Chl-a) and particulate carbon (PC) at (a) 250 µatm, (b) 400 µatm, and (c) 550 µatm.
Algal PC ranged from a low of 2 mg/L to a maximum of 8 mg/L across all PCO2 treatments (). Lowest PC was measured in cubitainers that received no P treatments, whereas highest PC occurred at P treatments of 100 μg/L. PC was positively related to P concentration irrespective of PCO2 level (). The greatest increases in PC generally occurred when P increased from 10 to 25 μg/L and from 25 to 50 μg/L. Doubling P approximately doubled PC, but the effect was not observed when P increased from 50 to 100 μg/L (). There was a significant response in mean PC to P treatment (F4,10 = 91, P < 0.001). Tukey's post-hoc test values were used to summarize P comparisons ().
Figure 5. Algal biomass response reported as (a, b, and c) particulate carbon (PC) and (d, e, and f) chlorophyll-a to different levels of PCO2 along a phosphorus concentration gradient. The r2 values represent the linear fit with P concentration as the x-variable.
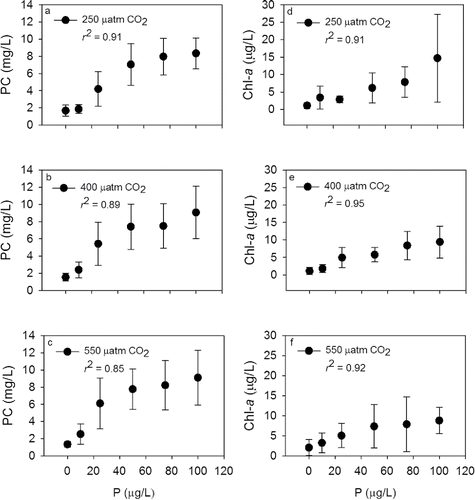
Table 3. Pairwise comparison of phosphorus treatment and corresponding p-values for particulate carbon (PC), chlorophyll a (Chl-a) and carbon to phosphorus (C:P), carbon to nitrogen (C:N), and nitrogen to phosphorus (N:P) ratios. Results were from a 2-way ANOVA, but there were no significant differences in PCO2, so only P treatment values are shown. Values <0.05 represent significant differences.
Increased PCO2 had a small but measurable effect on PC concentration. For example, PC at 25 μg/L P and 250 μatm PCO2 was ∼4 mg/L compared to 6 mg/L at 550 μatm PCO2 (), but the increase was not significant (F2,13 = 0.06, P = 0.94).
Chl-a concentration ranged from 2 to 15 μg/L across all PCO2 levels (). Similar to PC, the lowest Chl-a was measured in no P treatments, whereas highest Chl-a was measured at P levels of 100 μg/L (). Doubling P concentration did not double Chl-a as observed with PC. Chl-a was positively correlated to P levels and increased significantly as P level increased (). Mean Chl-a was significantly different across P treatments (F4,10 = 15, P < 0.001). Tukey's post-hoc test results were used to summarize P comparisons (). Increased PCO2 had no measurable effect on Chl-a concentration. Similar to PC, mean Chl-a was not significantly different across PCO2 levels (F2,12 = 0.03, P = 0.97).
Effect of PCO2 and P on algal nutrient content
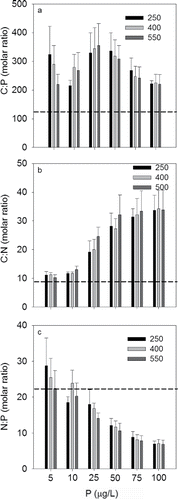
There were no significant differences in algal C:P, C:N, and N:P ratios at individual levels of P for PCO2 treatment (P = 0.069, 0.640, and 0.170 respectively), but significant differences were observed with increasing P levels. For example, between 50 and 100 μg/L, the C:P ratio declined significantly as P increased (; F2,2 = 110, P < 0.001). Tukey's post-hoc test results were used to summarize the pairwise comparisons of P for the C:P ratio (). The C:P ratio indicated P limitation at P concentrations between 5 and 50 μg/L P. Algal C:N increased significantly from about 10 at 5 μg/L P to 33 at 100 μg/L P (). Tukey's post-hoc test results were used to summarize the pairwise comparisons of P for the C:N ratio (). The C:N ratio suggested N limitation at P concentrations >10 μg/L P (). The N:P ratio declined significantly with increasing P concentration (; F2,5 = 52, P = 0.001). The N:P ratio suggested P limitation at P concentrations <25 μg/L P. Tukey's post-hoc test results were used to summarize the pairwise comparisons of P for the N:P ratio ().
Discussion
Results from our study suggest elevated PCO2 is not likely to increase algal growth by alleviating algal C limitation or alter carbon:nutrient ratios in NWA reservoirs. This result was contradictory to our expectations because the field-based PCO2 reconstructions and δ13C analysis suggested inorganic carbon limitation during summer. For the 4 lakes where PCO2 was reconstructed, summer concentrations were consistently below atmospheric PCO2. The decline in PCO2 was simultaneous with Chl-a increase, suggesting active CO2 drawdown during primary production to potentially limiting levels. During fall, winter, and spring, reconstructed PCO2 was generally greater than atmospheric PCO2.
The mean PCO2 calculated for Beaver Lake (1070 µatm) in this study is consistent with the mean value for global lakes (1036 µatm) reported by Cole et al. (Citation1994) and for about a 1000 lakes across the United States (1043 µatm) reported by McDonald et al. (Citation2013). Lakes Brittany, Norwood, and Rayburn were all less than the global average, possibly due to their smaller watershed sizes and lower inputs of allochthonous C from their tributaries (McDonald et al. Citation2013). Nevertheless, the temporal variations in PCO2 were similar to that of the larger Beaver Lake. Our results suggest that NWA reservoirs are sources of CO2 during fall, winter, and spring, but during summer, the reverse occurs, and the reservoirs become a sink for CO2. Because the number of lakes similar in size to Lakes Brittany, Norwood, and Rayburn are more globally abundant, low summer PCO2 is possibly much more widespread than currently known. Our study further demonstrated that long-term studies are needed to understand the seasonal role individual lakes play in CO2 dynamics given their importance in regulating regional climate and global C cycling (Alin and Johnson Citation2007, Battin et al. Citation2008, Balmer and Downing Citation2011).
Interpretations from our analysis of PCO2 limitation depended on the validity of the PCO2 reconstructions. Algal abundance through CO2 uptake and pH could both lead to lower PCO2 values. Quiñones-Rivera et al. (Citation2015) showed that when lake water pH fell below 8.6 in Lake Diefenbaker, Canada, CO2 was released. The pH in all the NWA lakes was <8.6, but, interestingly, Lake Brittany had the lowest pH and also the highest annual average PCO2. This finding suggests that degassing due to pH was not a factor in this study, and variations in reconstructed PCO2 were reflective of water column PCO2.
We used 2 techniques to validate the model: direct measurement of PCO2 in the water column and measurement of δ13C-CO2. The strong agreement between the measured and modeled values suggests that the model accurately predicted water column PCO2 based on the input values. Values of δ13C have been used to track source and transformation of C in numerous studies (Friedli et al. Citation1986, Clark and Fritz Citation1997, Karlsson et al. Citation2007, Winston et al. Citation2014). Typically, as CO2 is utilized for primary production, concentrations will decrease, whereas the δ13C-CO2 will increase due to the preferential incorporation of the lighter C (12C) into biomass. Based on previous work, atmospherically derived CO2 has a δ13C range from −6 to 0‰, respiration from −28 to −31‰, and ground water from −5 to −25‰ (Friedli et al. Citation1986, Clark and Fritz Citation1997, Karlsson et al. Citation2007).
Variations in δ13C during the study corroborated the reconstructions, and the range in δ13C values indicated potential C sources. During the night, water-column PCO2 was almost twice atmospheric PCO2 with a concomitant decrease in δ13C. Unexpectedly, the δ13C values were higher than respiration-derived δ13C and ranged from −12 to 7‰, reflecting a C source dominated by atmospheric and ground water inputs. In a recent study of 1000 lakes, 12% had C for primary production subsidized with hydrologic DIC inputs (McDonald et al. Citation2013). The results suggest that NWA reservoirs maybe supplemented by hydrologic C inputs given the vast array of caves, tributaries, and preferential flow paths present in the watershed.
During the day, as PCO2 in the water column decreased from supersaturated to undersaturated levels, δ13C increased. Values ranged from −3 to 0‰ at 12:00 h and then increased to 7‰ at 18:00 h. Values of δ13C >0‰ suggested that utilization of CO2 during primary production exceeded supply of CO2 into the water column (Falkowski and Raven Citation1997) leaving the residual pool enriched in δ13C. This result was consistent with conclusions from Shapiro (Citation1973) and Barnese and Schelske (Citation1994), who suggested that atmospheric CO2 solution may not fully supply phytoplankton carbon demand during primary production.
Chl-a and PC are both used as proxies for phytoplankton biomass. Chl-a is more frequently used because it is easier to measure (Felip and Catalan Citation2000), but it may underestimate biomass due to species-specific differences. By comparison, PC may overestimate biomass by incorporating detritus, primarily from recently deceased planktonic organisms. The relationship between the 2 variables was significantly correlated at all PCO2 levels (P < 0.001), including at 250 μatm where the strength of the relationship seemed weaker (r2 = 0.54). Overall, the 2 variables suggest they were good surrogates of each other and potentially biomass, particularly at the higher PCO2 levels.
We observed no significant differences in biomass or ratios of C:P, C:N, C:P with elevated PCO2. These results contradicted studies by (Hein Citation1997), Urabe et al. (Citation2003), and Jansson et al. (Citation2012) but were similar to results obtained by Verschoor et al. (Citation2013). Algal community composition and nutrient limitation might account for the differences.
Phytoplankton taxa differ in their ability to uptake CO2 and in their growth response to elevated PCO2 (Rost et al. Citation2003, Riebesell Citation2004). Cyanobacteria tend to grow faster at lower PCO2, whereas chlorophytes grow faster at higher PCO2 (Birmingham and Colman Citation1979, Low-Décarie et al. Citation2011). Additionally, the competitive response of cyanobacteria decreases as PCO2 increases, whereas that of chlorophytes steadily increases (Low-Décarie et al. Citation2011). Diatom growth responses are intermediate and tend to show little change with elevated PCO2 (Low-Décarie et al. Citation2011). Although the algal community was not assessed microscopically, previous work suggests the community would have been dominated by diatoms at the time of sample collection (Winston et al. Citation2014). Diatom indifference toward elevated PCO2 (Low-Décarie et al. Citation2011) might account for the lack of significant relationships among PCO2, biomass, and nutrient content, suggesting that responses to elevated PCO2 might be dependent on the in situ algal community and species-specific CO2 affinities (Gradoville et al. Citation2014).
Verschoor et al. (Citation2013) suggests the lack of a significant increase in biomass was because elevated PCO2 relieved C limitation and drove phytoplankton toward nutrient limitation. Nutrient limitation was also likely in this study. Using limitation lines of Healey and Hendzel (Citation1979), C:P and N:P ratios indicated P limitation between 5 and 50 µg/L, whereas the C:N ratio indicated N limitation at P >10 µg/L. PCO2-driven limitation, however, does not seem to be the mechanism driving limitation in this study. Parallel line analysis indicates that the slope of the regression between biomass and P level were not significantly different among PCO2 treatments.
Micronutrient co-limitation, specifically iron (Fe), likely played a role in dampening the increases in biomass. North et al. (Citation2007) showed that the addition of Fe reduces N limitation by allowing algal communities to uptake more nitrate. Because nitrate was the source of N in this study, uptake was possibly limited by low Fe concentration (North et al. Citation2007) because micronutrients were not part of the enrichment regime. This could further explain why N limitation occurred above 10 µg/L P, despite enriching cubitainers with 1000 µg/L N.
The most significant increases in biomass, C:N, and N:P occurred with elevated P, an expected finding because P, or combinations of N and P, have been shown to control phytoplankton biomass (Schindler Citation1977, Dzialowski et al. Citation2005, Scott and McCarthy Citation2010). Thus, even under conditions of elevated PCO2, nutrients provide primary control on phytoplankton biomass. Several researchers have suggested that sound nutrient management remains the most feasible method for curtailing eutrophication symptoms (Conley et al. Citation2009, Esten and Wagner Citation2010, Wagner Citation2010) under global climate change scenarios (Paerl and Paul Citation2012).
Conclusion
Overall, our results suggest that the simultaneous increase in C and P in NWA reservoirs is not likely to increase algal biomass and alter algal nutrient content any more than by increasing P alone. Our results showed significant changes in biomass and nutrient content only with increasing P. We agree with recommendations from several researchers that sound nutrient management remains the best method for curtailing eutrophication symptoms under climate change scenarios. The study also provided a cost-effective way to obtain long-term data on C dynamics. Given the abundance of reservoirs globally, this approach could provide insight into CO2 fluctuations on a more refined spatial and temporal scale and help better constrain the modern global carbon budget.
Funding
Special thanks to the Arkansas Water Resources Center for funding this project, USGS 104 B.
Acknowledgments
Special thanks to the Scott Biogeochemistry and University of Arkansas Stable Isotope Labs (UASIL) for analyses, and Aaron Jackson, Hal Halvorson, Amie West, Erin Scott, and Maysill Pascal for comments on the manuscript. Special thanks to Dr. Douglas Lepley for his invaluable contribution to the manuscript.
References
- Alin SR, Johnson TC. 2007. Carbon cycling in large lakes of the world: a synthesis of production, burial, and lake-atmosphere exchange estimates. Global Biogeochem Cy. 21:1–12.
- [APHA] American Public Health Association. 2005. Standard methods for the analysis of water and wastewater. 20th edition. Washington (DC): American Public Health Association, American Water Works Association, Water Environment Federation.
- [ADEQ] Arkansas Department of Environmental Quality. 2004. Lake assessment report for 2004. Arkansas Department of Environmental Quality, Little Rock, Arkansas. (Available from: http://www.adeq.state.ar.us/water/default.htm)
- Balmer MB, Downing JA. 2011. Carbon dioxide concentrations in eutrophic lakes: undersaturation implies atmospheric uptake. Inland Waters. 1:125–132.
- Barnese LE, Schelske CL. 1994. Effects of nitrogen, phosphorus and carbon enrichment on planktonic and periphytic algae in a softwater, oligotrophic lake in Florida, USA. Hydrobiologia. 259:159–170.
- Battin TJ, Kaplan LA, Findlay S, Hopkinson CS, Marti E, Packman AI, Newbold JD, Sabater F. 2008. Biophysical controls on organic carbon fluxes in fluvial networks. Nat Geosc. 1:95–100.
- Birmingham BC, Colman B. 1979. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 64:892–895.
- Burkhardt S, Riebesell U, Zondervan I. 1999. Effects of growth rate, CO2 concentration, and cell size on the stable carbon isotope fractionation in marine phytoplankton. Geochim Cosmochim Ac. 63:3729–3741.
- Clark ID, Fritz P. 1997. Environmental isotopes in hydrogeology. Boca Raton (FL): Lewis Publishers.
- Cole JJ, Caraco NF, Kling GW, Kratz TK. 1994. Carbon dioxide supersaturation in the surface waters of lakes. Science. 265:1568–1570.
- Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot E, Likens GE. 2009. Controlling eutrophication: nitrogen and phosphorus. Science. 323:1014–1015.
- Croneis C. 1930. Geology of the Arkansas Paleozoic area: Bulletin 3 Arkansas Geological Commission.
- Dzialowski A, Wang SH, Lim N, Spotts WW, Huggins DG. 2005. Nutrient limitation of phytoplankton growth in central plains reservoirs, USA. J Plankton Res. 27:587–595.
- Esten EM, Wagner KJ. 2010. Investigation of benthic phosphorus flux controls in Lake Waco, Texas. Lake Reserv Manage. 26:114–122.
- Falkowski PG, Raven JA. 1997. Aquatic photosynthesis. Oxford (UK): Blackwell Science.
- Felip M, Catalan J. 2000. The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: decoupling in their spatial and temporal maxima. J Plankton Res. 22:91–105.
- Friedli H, Lotscher H, Oeschger H, Siegenthaler U, Stauffer B. 1986. Ice core record of the 13C/12C ratio of atmospheric CO2 in the past centuries. Nature. 324:237–238.
- Gervais F, Riebesell U. 2001. Effect of phosphorus-limitation on elemental composition and stable isotope carbon fractionation in a marine diatom growing under different CO2 concentrations. Limnol Oceanogr. 46:497–504.
- Galloway JM, Green WR. 2007. Application of a two-dimensional reservoir water quality model of Beaver Lake, Arkansas, for the evaluation of simulated changes in input water quality, 2001–2003. US Geological Survey Scientific Investigations Report 2006–5302.
- Gradoville MR, White AE, Böttjer D, Church MJ, Lettelier RM. 2014. Diversity trumps acidification: lack of evidence for carbon dioxide enhancement of Trichodesmium community nitrogen or carbon fixation at station ALOHA. Limnol Oceanogr. 59:645–659.
- Haley BR, Glick EE, Busch WV, Clardy BF, Stone CG, Woodward MB, Zachary DL. 1993. Geologic map of Arkansas. Arkansas Geological Commission, scale 1:500,000.
- Healey FP, Hendzel LL. 1979. Indicators of phosphorus and nitrogen deficiency in five algae in culture. J Fish Res Board Can. 36:1364–1369.
- Hecky RE, Kilham P. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr. 33:796–822.
- Hein M. 1997. Inorganic carbon limitation of photosynthesis in lake phytoplankton. Freshwater Biol. 37:545–552.
- [IPCC] Intergovernmental Panel on Climate Change. 2007. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate change 2007: the physical science basis. Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
- [IPCC] Intergovernmental Panel on Climate Change. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the 5th Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Meyer LA, editors.] Geneva (Switzerland): IPCC. 151 p.
- Jansson M, Karlsson J, Jonsson A. 2012. Carbon dioxide supersaturation promotes primary production in lakes. Ecol Lett. 15:527–532.
- Jansson M, Karlsson J, Jonsson A. 2007. Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol Oceanogr. 52:603–608.
- Jones JR, Knowlton MF. 1993. Limnology of Missouri reservoirs: an analysis of regional patterns. Lake Reserv Manage. 8:17–30.
- Karlsson J, Janssons M, Jonssons A. 2007. Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol. Oceanogr. 52:603–608.
- Low-Décarie E, Fussman GF, Bell G. 2011. The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Global Change Biol. 17:2525–2535.
- Mash CA, Winston B, Mients DA, Pifer AD, Scott JT, Zang W, Fiarey JL. 2014. Assessing trichloromethane formation and control in algal-stimulated waters amended with nitrogen and phosphorus. Environ Sci: Process Impacts. 16:1159–1168.
- McDonald CP, Stets EG, Striegl RG, Butman D. 2013. Inorganic carbon loading as a primary driver of dissolved carbon dioxide concentrations in the lakes and reservoirs of the contiguous United States. Global Biogeochem Cy. 27:285–295.
- North RL, Guildford SJ, Smith REH. 2007. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnol Oceanogr. 52:313–328.
- Paerl HW, Huisman J. 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Reports. 1:27–37.
- Paerl HW, Paul VJ. 2012. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 46:1349–1363.
- Quiñones-Rivera ZJ, Finlay K, Vogt RJ, Leavitt PR, Wissel B. 2015. Hydrologic, metabolic and chemical regulation of water-column metabolism and atmospheric CO2 exchange in a large continental reservoir during spring and summer. J Great Lakes Res. 41:144–154.
- Riebesell U. 2004. Effects of CO2 enrichment on marine phytoplankton. J Oceanogr. 60:719–29.
- Rost B, Riebesell U, Burkhardt S, Sultemeyer D. 2003. Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr. 48:55–67.
- Schindler DW. 1977. Evolution of phosphorus limitation in lakes. Science. 195:260–262.
- Schindler DW, Curtis PJ, Bayley SE. 1997. Climate-induced changes in the dissolved organic carbon budgets of boreal lakes. Biogeochemistry. 36:9–28.
- Scott JT, Grantz E. 2013. N2 fixation exceeds internal nitrogen loading as a phytoplankton nutrient source in perpetually nitrogen-limited reservoirs. Freshwater Sci. 32:849–861.
- Scott JT, McCarthy MJ. 2010. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol Oceanogr. 55:1265–1270.
- Shapiro J. 1973. Blue-green algae: why they become dominant. Science. 179:382–384.
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL. 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
- Sterner RW, Elser JJ. 2002. Ecological stoichiometry. The biology of elements from molecules to the biosphere. Princeton (NJ): Princeton University Press.
- Stumm W, Morgan JJ. 1996. Aquatic chemistry: chemical equilibria and rates in natural waters. New York (NY): Wiley Interscience.
- Urabe J, Togari J, Elser JJ. 2003. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Global Chang Biol. 9:818–825.
- Van de Waal DB, Verschoor AM, Verspagen JMH, Van Donk E, Huisman J. 2010. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front Ecol Environ. 8:145–152.
- Verschoor AM, Van Dijk MA, Huisman J, Van Donk E. 2013. Elevated CO2 concentrations affect the elemental stoichiometry and species composition of an experimental phytoplankton community. Freshwater Biol. 58:597–611.
- Wagner C, Adrian R. 2009. Cyanobacteria dominance: quantifying the effects of climate change. Limnol Oceanogr. 54:2460–2468.
- Wagner K. 2010. Loading of phosphorus and nitrogen to Lake Waco, Texas. Lake Reserv Manage. 26:123–146.
- Wetzel R. G. 2001. Limnology: Lake and River Ecosystems. Academic Press. San Diego (CA).
- Winston B, Hausmann S, Scott TJ, Morgan R. 2014. The influence of rainfall on taste and odor production in a south-central USA reservoir. Freshwater Sci. 33:755–764.