ABSTRACT
Effler SW, Prestigiacomo AR, Hairston NG, Auer MT, Kuczynski A, Chapra SC. 2016. Dissolved phosphorus concentrations in Cayuga Lake system and differences from two analytical protocols. Lake Reserve Manage. 32:392–401.
Differences in the concentrations of dissolved forms of phosphorus (P) measured with 2 widely used spectral protocols were documented and evaluated for Cayuga Lake, New York, and 4 of its primary tributaries. The analysis focuses on 2 operationally defined forms of dissolved P, soluble reactive P (SRP) and soluble unreactive P (SUP), which together constitute dissolved P (TDP). Direct comparisons were based on analysis of the results from the 2 protocols of split samples of year-round deep water representative of the entire water column during turnover and the respective dependencies of tributary concentrations on stream flow. Although the TDP concentrations converged for the 2 protocols, there were systematic differences for the contributions of SRP versus SUP (i.e., one protocol yielding lower SRP but higher SUP). The interpretive implications of the differences in the operationally defined concentrations from the 2 analytical protocols were considered in the context of common limnological and bioavailable paradigms for these forms of P, and the needs and structures of mechanistic P-eutrophication models. The lower SRP analytical protocol was favored because of its greater consistencies with the independent bioavailability results, limnological paradigms for dissolved forms of P, and contemporary mechanistic modeling. The differences between the 2 protocols are particularly problematic where contemporary mechanistic models are to be applied, requiring compensating differences in kinetic representations, and likely structure, for cases of higher SRP datasets attributable to the protocol.
Cultural eutrophication remains a major water quality and ecological issue for lake and reservoir management, with phosphorus (P), the limiting nutrient in most of these lacustrine waters (Schindler et al. Citation2008), the primary focus. Early studies often considered only concentrations of total P (TP) with respect to inputs, the in-lake pool, and management of P (Vollenweider Citation1976). Partitioning of multiple forms of P became important in the context of limnological understanding and management as the differences in their potential to support primary production were recognized (DePinto et al. Citation1981, Wetzel Citation2001, Reynolds and Davies Citation2001). P available to support algae and cyanobacteria production is described as bioavailable (DePinto et al. Citation1981, Auer et al. Citation1998).
The chemical composition of the multiple forms of P has not been amenable to routine determination (Hudson et al. Citation2000, Dodds Citation2003). Orthophosphate ion (PO3-4) provides the P necessary to support algal growth (Hudson et al. Citation2000, Wetzel Citation2001). Certain forms of dissolved organic P (DOP) can be converted to PO3-4 from enzymatic activity by phosphatases, especially under PO3-4-deficient conditions (Currie et al. Citation1986, Bentzen et al. Citation1992). Much of the terrigenous particulate P (PP) is not bioavailable (Prestigiacomo et al. Citation2016).
Spectrophotometric procedures continue to be the primary type of P analysis used to monitor fresh waters Three forms of P are commonly measured: TP, total dissolved P (TDP), and soluble reactive P (SRP). Two other forms are calculated from these 3 as residuals: particulate P (PP = TP − TDP) and soluble unreactive P (SUP = TDP − SRP). SUP is usually assumed to be composed primarily of forms of DOP (Baldwin Citation2013). The concentrations of these forms are “operationally defined,” reflecting the analytical protocols specified in a particular methodology. A paradigm has emerged for the differences in the bioavailability of the operationally defined forms of P delivered to lakes that partition TP. SRP has been considered to be completely available in the short-term (Auer et al. Citation1998, Prestigiacomo et al. Citation2016). By contrast, SUP has been described as less bioavailable (Auer et al. Citation1998, Prestigiacomo et al. Citation2016), becoming bioavailable over longer time scales through enzymatic and mineralization processes Currie et al. Citation1986, Bentzen et al. Citation1992). PP is the least bioavailable of these forms (Auer et al. Citation1998, Prestigiacomo et al. Citation2016).
The SRP fraction is often incorrectly described as a quantitative measure of PO3-4 (Dodds Citation2003). Radio-bioassay measurements for more than 40 years have established that PO3-4 represents only a small fraction (e.g., <1%) of SRP in productive layers of P-limited pelagic waters (Hudson et al. Citation2000, Hudson and Taylor Citation2005). Other components included in SRP are inorganic polyphosphates, the more reactive components of DOP (Dodds Citation2003), and reactive small (<0.45 µm) particles (Dodds Citation2003). The multiple analytical methods for SRP (6 in New York) are a potential source of variations in this operationally based concentration (Tarapchak et al. Citation1982, Tarapchak and Rubitschun Citation1981), with complicating implications for limnological interpretations and water quality modeling. Paired or even noncoincident results from multiple analytical protocols for P, particularly in combination with bioavailability assessments, are generally not available from contemporary monitoring programs.
The overarching goals of this analysis were to (1) document differences in concentrations of dissolved forms of P for a lake and its tributaries obtained with 2 widely used spectrophotometric protocols, (2) consider their consistencies with parallel bioavailability information for the fractions, and (3) consider the implications for limnological and water quality modeling analyses. This analysis is based on a rare combination (described subsequently) of monitoring and process information available for Cayuga Lake, New York, and its tributaries. It addresses the problems of limnological interpretation and modeling for lacustrine waters where fundamental protocol-based differences in concentrations of P fractions have been reported.
Study site
Cayuga Lake (42°41′30″N; 76°41′20″W) is the fourth easternmost of the 11 New York Finger Lakes (). It has the second largest volume (9.4 × 109 m3) and surface area (172 km2) of the Finger Lakes, with mean and maximum depths of 55 m and 133 m, respectively. This lake has a monomictic stratification regime and a retention time of ∼10 years (Effler et al. Citation2010). Phytoplankton growth is P limited, and the lake is mesotrophic (Effler et al. Citation2010). The entire water column remains oxic throughout the year.
Figure 1. Cayuga Lake, the 11 Finger Lakes, and position in New York. Shown are 4 monitored tributaries, USGS gauges, pelagic site, lake source cooling (LSC) discharge, and shelf portion of the lake at its southern end.
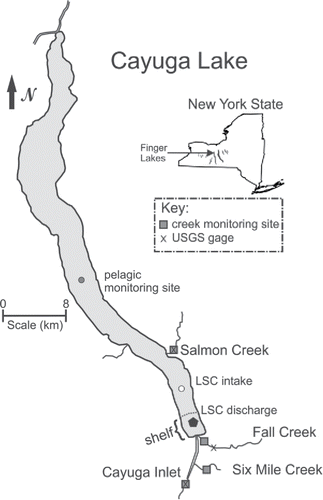
Four of the primary tributaries to the lake were gauged for flow (); 3 enter the shallow southern end (“shelf”; ) and one is positioned 10 km farther north, entering from the east (). Large fractions of the P loads received by these tributaries were delivered during runoff events (Prestigiacomo et al. Citation2016), which justifies a focus on characterizing these events. Agricultural land use varies, representing 22–68% of these watershed areas (). Cornell University, situated near the south end of the lake, installed a lake source cooling (LSC) facility in 2000 that withdraws water from a depth of 73 m (in the hypolimnion) to meet much of the institution's cooling needs and returns the spent cooling water (closed system, no P added within the facility) to the shelf.
Table 1. Specifications for inputs to Cayuga Lake monitored for forms of phosphorus (P); watershed areas, agricultural land use, samples analyzed according to the 2 protocols, and bioavailable fractions for the P forms as analyzed by the standard methods protocol.
Materials and methods
Two widely used methodologies were applied to measure 3 forms of P (TP, TDP, and SRP): Standard Methods No. 4500 PE and 4500-P B(5) (Clesceri et al. Citation1998), hereafter called the S protocol, and USEPA No. 365.3 (USEPA Citation1983), hereafter called the E protocol. Both protocols are based on an ascorbic acid method (e.g., Murphy and Riley Citation1962). Ammonium molybdate and antimony potassium tartrate react in an acid solution with PO3-4 to form a heteropoly acid that is reduced to an intensely colored molybdenum blue by addition of ascorbic acid. The 2 protocols have certain operational differences (selected features in ), detailed elsewhere (Clesceri et al. Citation1998, USEPA Citation1983). Results from the 2 methods are henceforth differentiated by subscripts “S” (e.g., SRPS) and “E” (e.g., SRPE), corresponding to the Standard Methods and EPA protocols, respectively.
Table 2. Comparison of selected analytical features for 2 methodologies for measurements of the concentration of phosphorus (P).
Paired weekly samples from the intake water at the LSC facility, representative of lake water at a depth of 73 m (hereafter “deep lake water”), were collected throughout 2013 for analysis of TP, TDP, and SRP according to both the S and E protocols, and carried out by 2 laboratories. The numbers of paired measurements of TP, TDP, and SRP were 48, 26, and 26 respectively; TDPE discontinued after 26. Measurements of TDPS, TPS, and SRPS were made for samples from a pelagic lake site (laboratory filtered chilled sample) during 1999−2006, biweekly (1 per 2 wk) for the April−October interval (Effler et al. Citation2010).
The samples supporting comparison of the 2 analytical protocols here for the 4 Cayuga Lake gauged tributaries () were not paired but were collected at positions proximate to the mouths (). A long-term monitoring program has been conducted since 2002 adopting the E analytical protocol to measure TPE and SRPE (i.e., no TDPE). These samples were collected manually on a temporally irregular basis in each year, targeting runoff events (up to 5 times per year) to reflect the effects of a range of stream flow conditions. A shorter-term, but more temporally intensive, sampling program was conducted over the April−October interval of 2013, with analyses of TPS, TDPS, and SRPS (Prestigiacomo et al. Citation2016). This sampling program had 2 components: (1) biweekly (1 per 2 wk) manual collections, and (2) collections for 4 major runoff events with automated sampling equipment (e.g., multiple samples within days of events). The results from the 2 analytical protocols were compared in the common logarithmic concentration versus stream flow (Q, m3/s) format (e.g., Vogel et al. Citation2003). Because the samples used in the tributary protocols were not directly paired, tributary results were compared by analyzing the respective log-transformed flow-concentration relationships for the 2 protocols. The flow-concentration (log) slopes for the E and S relationships were tested using the homogeneity of slopes test (Statsoft Citation2003), and the intercepts were evaluated using an analysis of covariance (ANCOVA; Helsel and Hirsch Citation1992).
The bioavailability of SRPS, SUPS, and PPS in the 4 gauged tributaries to the lake was assessed previously through bioassays on samples from 3 runoff events in 2013 with a P-starved green alga (Selenastrum capricornutum), as described by Prestigiacomo et al. (Citation2016). This procedure provided measurements of the fraction of these forms taken up by the assay alga, defined as the bioavailable fraction, fBAP (). The assays were additionally conducted for SRPS and SUPS for 2 samplings of the LSC facility discharge in 2014. (www.communityscience.org/database/monitoringregions/1; Prestigiacomo et al. Citation2016). These bioavailability results reported by Prestigiacomo et al. (Citation2016) are used here as a basis to evaluate the character of differences in P concentrations reported for the S and E protocols. The paradigm of contrasting bioavailability of the forms of P was supported; SRP completely, SUP diminished, and PP very limited (; Auer et al. Citation1998, Prestigiacomo et al. Citation2016).
Results
Patterns for forms of P in deep and upper lake waters
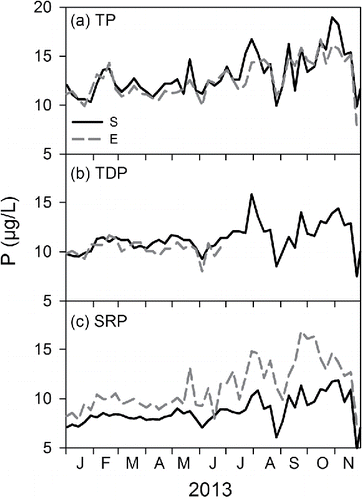
Time series of TP, TDP, and SRP are presented for the paired hypolimnetic samples (LSC discharge) for the 2 analytical protocols for 2013 (; note measurements of TDPE were discontinued at the end of June). Substantial temporal variation occurred for each of these forms according to both protocols. The temporal patterns of the 2 measurement methods for TP, TDP, and SRP were each significantly correlated (P < 0.05; r values of 0.85, 0.55 and 0.78, respectively). The most conspicuous differences in the patterns for the 2 protocols were that SRPE exceeded SRPS (), and there were therefore greater differences in concentrations (SUPS vs. SUPE) between TDPS and SRPS ( and ) than between TDPE and SRPE ( and ). This second feature means that SUPS was consistently higher than SUPE in deep lake water.
Figure 2. Time series for the LSC discharge for 3 forms of phosphorus (P) for 2013, according to 2 analytical protocols, standard methods (S) protocol (subscript S), and EPA protocol (subscript E): (a) TP, (b) TDP, and (c) SRP.
Results from 2 analytical protocols were compared using the distributions of the ratios of the paired results for both the directly measured and calculated forms of P (). The 2 methods produced similar results for TP (i.e., TPE:TPs ≈ 1.0; ) and TDP (); ∼70 and 75% of the observations of these forms of P were within 10% of each other. Accordingly, a general convergence was observed for the calculated PP (; median ratio of 0.95). The modest contribution of PP to TP, together with the fact that it was calculated by difference (PP = TP − TDP), probably contributed to the PP ratio variance.
Figure 3. Representations of extent of closure of forms of P in the LSC discharge from split samples for the S and E protocols as distributions of ratio values: (a) TP, (b) TDP, (c) PP, (d) SRP, (e) SUP, and (f) the ratio SRPE:TDPS.
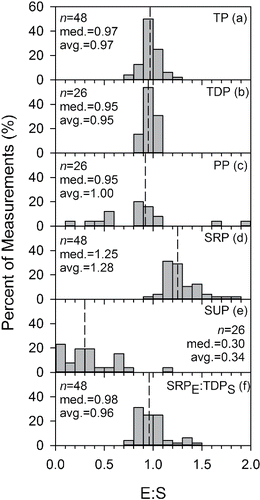
Values of SRPE were systematically greater than SRPS; the mean and median of SRPE:SRPS were 1.28 and 1.25 (), respectively. SRP was the dominant form of P in these deep lake waters according to both protocols, although the contribution of SRPE (88%) was greater than that of SRPS (67%). These differences in SRPE and SRPs, together with the near equivalence of TDP values from the 2 protocols, caused SUPS to exceed SUPE. According to the analytical results from the S protocol, SUPS represented 23% of TP for the January−June interval and 21% for the entire year, substantially more than the 7% contribution indicated by the E protocol for the period January−June (TDPE and SUPE were not determined for July−December). The mean and median SUPE:SUPS values were 0.34 and 0.30 (), respectively. The average values of the SRPE:TDPE and SRPE: TDPS () ratios were 0.94 and 0.96, indicating SRPE approached TDP for deep lake water.
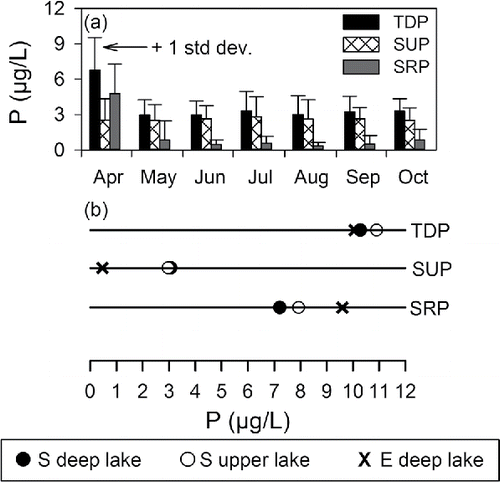
Seasonal patterns of TDPS, SRPS and SUPS in the upper waters of Cayuga lake are presented as monthly mean values for the pelagic monitoring site for the April–October interval for 1999−2006 and 2013 (). Despite interannual differences (1 standard deviation, vertical bars), recurring patterns were noted. The highest TDPS and SRP concentrations were observed during turnover in April (Effler et al. Citation2010); both decreased, but particularly SRPS. Although SUPS was somewhat lower at turnover, it increased modestly by midsummer. SRPS concentrations remain mostly <1 µg/L over the May–October interval. The measured SRPS concentrations (as P) were often near a detection limit of 0.4 µg/L. SUPS was the dominant component of TDPS over the May–October interval; the average summertime SUPS:TDPS ratio value has been ∼0.8. The 8 April 2013 observations () corresponded to spring turnover, as shown by the near equivalence of the TDPS and SRPS measurements and SUPS calculated values for samples from the upper waters and the LSC discharge (). The substantial differences in the deep-water LSC E protocol values (SRPE and SUPE) from the reported S protocol observations for that day are shown. These differences would also have been observed in the upper lake waters on this day of turnover.
Figure 4. Lake P patterns and (a) time series of monthly average TDPS, SRPS, and SUPS in the upper waters of Cayuga Lake for the April-October 1999–2006 interval and 2013; vertical bar corresponds to ±1 standard deviation, (b) comparison of TDP, SUP, and SRP concentrations in early April (during turnover) 2013 for deep wake Water and from the S and E protocols and upper lake water from S protocol.
Patterns for forms of P in tributaries
Comparisons of tributary P concentrations measured by the 2 analytical protocols were made using the commonly adopted log-transformation format (Vogel et al. Citation2003), log10 concentration versus log10 Q (daily average flow rate; ). These comparisons are supported, despite differences in the temporal features of the associated monitoring programs, by a lack of any systematic changes in the concentration versus Q relationships over the long-term monitoring program (www.communityscience.org/database; Prestigiacomo et al. Citation2016). The comparisons presented here for the mouth of Fall Creek () are generally representative for the 4 gauged tributaries (Prestigiacomo et al. Citation2016).
Figure 5. Phosphorus (P) concentration versus stream flow (Q) relationships for Fall Creek that compare results from the 2 analytical protocols: (a) dependencies of TPS and TPE on Q, (b) dependencies of SRPS and SRPE on Q, and (c) dependencies of TDPS and SRPE on Q.
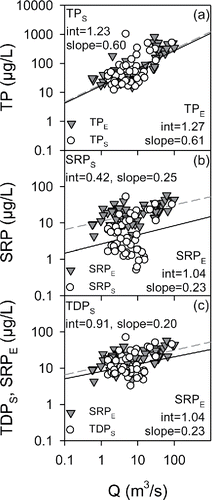
The relationships between TP and Q () were similar for the longer-term TPE and the 2013 TPS datasets (P < 0.05; homogeneity of slopes test for slopes; ANCOVA for intercepts). The situation for SRP was strikingly different. SRPE values shifted higher than the SRPS values, with greater variation in the SRPS dataset (); the intercept of the power law relationship for SRPE was significantly greater than for SRPS (P < 0.001). By comparison, the SRPE versus Q and TDPS verus Q relationships, and thereby the concentrations, were similar (), although the intercepts were significantly different (P = 0.003). Although SUPE was not assessed in these streams (no TDPE measurements), the analyses of the available information () indicates it would have been low by comparison to SUPS (e.g., note convergence of SRPE and TDPS relationships; ). This analysis of the datasets for the streams indicates that the same diverging characteristics in the concentrations of SRP and SUP determined by the 2 analytical protocols for deep lake waters were also manifested for the streams entering the lake (i.e., SRPE > SRPS, and SUPE < SUPS.
Discussion
Protocol-based differences in P concentrations
Analyses of split samples for deep Cayuga Lake water showed definitively that values of SRPE were consistently greater than those for SRPS and conversely that SUPS was consistently greater than SUPE (). The differences in SUP were driven by the differences in SRP as TDP values from the 2 protocols converged. Analogous differences between the 2 SRP protocols for the lake's tributaries is supported by the significantly higher intercept of the SRPE versus Q relationship compared with that for SRPS versus Q (). The case that SUPS would have systematically exceeded SUPE in the tributaries had TDPE been measured is also strong, based on (Equation1(1) ) the similarity of the SRPE versus Q and TDPS versus Q relationships for the tributaries (), (2) the similarity of TDPS and TDPE for deep lake water samples, and (3) the equivalence of the results () for total unfiltered (TP) and filtered (TDP) P analyses for both the E and S protocols.
The lower values of SRPS compared with SRPE indicates more P-containing components other than ionic PO3-4 were converted (i.e., hydrolyzed) to PO3-4 in the E protocol than the S protocol. This paper does not present a position on which specific methodological differences between the 2 protocols (e.g., ) caused the observed disparities in the SRP (and thereby SUP) results; that should be addressed elsewhere. Rather our focus was on the implications of such differences for limnological interpretation and utilizing contemporary mathematical models. It is noteworthy, however, that Tarapchak et al. (Citation1982) reported that such method-specific differences in SRP results were not necessarily proportional to differences in the acid strength or exposure time of the protocols, but also varied with changes in the chemical composition of the SRP pool (e.g., sample origin). The differences in SRPE and SUPE from SRPS and SUPS probably reflect disparities in apportioning the diverse DOP pool between these 2 operationally defined forms of dissolved P (Baldwin Citation2013). In a recent review of organic P in the aquatic environment, Baldwin (Citation2013) identified 5 important classes of dissolved organic P compounds: nucleic acids, other nucleotides, inositol phosphates, phospholipids, and phosphonates, some of which are known to have agricultural origins. SUP has often been equated to DOP, a position identified as flawed, in part because of the inclusion of certain of the forms of DOP in the SRP measurements (Baldwin Citation2013). Apparently more of the DOP pool was included within the SRPE measurements than was included in SRPS. The divergent SRPS and SUPS concentrations observed in Cayuga Lake and its tributaries, and elsewhere, represent a signature consistent with the widely held understanding of the metabolic sink effect of lacustrine primary production on DOP (Wetzel Citation2001) and the observed differences in the bioavailability of these 2 forms of P (Prestigiacomo et al. Citation2016).
Tributary levels of both SRPS and SUPS (represented as Q-weighted concentrations; ) exceeded Cayuga Lake concentrations. The depletion of the SRPS pool within the upper lake layers by early summer (), despite tributary inputs, is consistent with the Reynolds (Citation2006, p. 154) description of the SRP pool “as demonstrably bioexhaustible and freely bioavailable.” The higher SRPS maintained in deep lake water relative to the upper waters () reflects the absence of photosynthesis-based depletion and likely some mobilization from the lake bottom during stratification (e.g., mussels; Prestigiacomo et al. Citation2016). Vertical oscillations of stratified layers from seiche activity (Effler et al. Citation2010) contribute to the irregular temporal dynamics in SRPS (and SRPE) and the other forms of P in these layers (). The higher SUPS concentrations in the upper productive layers during summer compared with SRPS () reflect the effects of (1) continuing tributary inputs of SUPS, along with SRPS (Prestigiacomo et al. Citation2016); (2) the diminished bioavailability of SUPS relative to SRPS (); and (3) the slower utilization kinetics of the bioavailable fraction of SUPS (Auer et al. Citation1998). This dominance of SUPS in the epilimnetic dissolved P pool in summer, as observed in Cayuga Lake (), is widely observed (; e.g., Wetzel Citation2001, Lampert and Sommer Citation2007). The dynamics of the epilimnetic SUPS pool () can be viewed as reflecting temporal imbalances in sources (tributary inputs; Prestigiacomo et al. Citation2016) and sinks (enzymatic conversions of DOP toPO3-4; Currie et al. Citation1986, Bentzen et al. Citation1992). Decreases of SUPS are observed when SRPS is severely depleted, a temporal pattern attributed to the sustaining of phytoplankton growth by enzymatic conversions within the SUPS pool (Connors et al. Citation1996 Effler et al. Citation2010).
The existence of a recalcitrant portion of SUPS (P: 2–3 µg/L) in the lake was indicated by the return to the initial spring SUPS concentration in the upper waters by fall (), the temporal uniformity of SUPS in the hypolimnion in 2013 (), and the long-term uniformity of SUPS in the hypolimnion since monitoring of deep lake water commenced in 1998. This position is supported by 2 features of the bioassay results: the incomplete bioavailability of SUPS in the major tributaries, and the low bioavailability of SUPS in the deep waters of the lake (fBAP = 0.08 for LSC discharge; ). The latter point is consistent with the extended exposure of the lake pool of SUPS to enzymatic loss processes associated with the long water retention time in Cayuga Lake (Prestigiacomo et al. Citation2016). Estimates of the recalcitrant SUPS concentrations of the tributaries (P: 1.4–2.5 µg/L), as the product of [1 − fBAP] and SUPS (i.e., the nonbioavailable fraction of SUP; ), are generally consistent with the observed deep lake concentration (SUPS = TDPS − SRPS; and ). SUPE underrepresents SUP.
Consistency with the bioavailability paradigm
The partitioning of the dissolved P pool by the S analytical protocol reasonably represents the distinct differences in bioavailability reported for SRPS versus SUPS (). Prestigiacomo et al. (Citation2016) found both lake and tributary SRPS to be nearly completely available on the short-term (), reflecting the utilization of PO3-4 and other forms of P that can be rapidly converted to PO3-4 (Baldwin Citation2013). SUPS successfully differentiates those fractions of the dissolved P pool that either are not available () or become available only over a longer time frame. SUPS is imperfect as a measure of unavailable soluble P in Cayuga Lake (and no doubt in other lakes), however, because a uniform quantitative relationship with fBAP does not prevail. The data for the 4 Cayuga Lake tributaries () suggest a positive dependence of fBAP on tributary SUPS concentration.
Different conditions are suggested by the E protocol results. The observed approach of SRPE to TDP (i.e., SUPE approaches zero) in both deep lake waters and tributaries indicates that the E protocol yields greater inconsistencies with the bioavailability paradigm. Despite the general expectation in limnological studies that SRP represents P readily available for phytoplankton uptake, it seems SRPE would not be completely available because a substantial portion of SUPS (effectively included in SRPE) is not available (). Moreover, this effective inclusion of SUPS in SRPE would preclude a differentiation of short-term and longer-term available fractions (within different kinetics) based on the E protocol. External loading estimates of SRPE would overestimate the loading of dissolved bioavailable P (particularly short-term) to the lake (Prestigiacomo et al. Citation2016) from both the tributaries and the LSC facility. In studies where no TDP measurements are made (i.e., neither SUPS nor SUPE: not recommended), however, SRPE would be a more useful measure than SRPS because it would more closely approach the total load of dissolved bioavailable P.
Implications for mechanistic P-eutrophication modeling
Mechanistic mass balance P-phytoplankton (or eutrophication) models are widely used to guide management deliberations for systems with excessive eutrophication and are critical tools supporting P management analyses (Cooke et al. Citation2005). The “mechanistic” descriptor described earlier implicitly reflects an effort to utilize realistic process-based representations of lacustrine systems in model structure. A broad range of model complexity (e.g., number of processes and interactions considered) has been adopted in contemporary modeling efforts (Chapra Citation1997, Arhonditsis and Brett Citation2004, Robson Citation2014), most of which represent the patterns of the various operationally defined forms of P addressed in this paper. Despite myriad differences in structural detail, contemporary models generally share certain converging features, including representation of the multiple forms of P. Whether partitioning of the dissolved P pools of the tributaries and lake is based on the E or the S analytical protocol would have important implications for a Cayuga Lake modeling initiative, as well as for similar efforts for other systems.
The bioavailability of P in external loads is increasingly incorporated in mechanistic modeling initiatives (Effler et al. Citation2012) and has been adopted for a Cayuga Lake modeling effort to avoid the gross overestimates using TP loads instead (Prestigiacomo et al. Citation2016). Systematic errors in these estimates inevitably lead to compensating misrepresentation of in-lake source and/or sink processes for P as part of model calibration. The focus on control of external loads as the primary management option to abate cultural eutrophication (Chapra Citation1997, Cooke et al. Citation2005) further establishes the need for representative loading estimates that account for bioavailability. SRPE data for the lake inputs are problematic in that regard.
Representing the extent of P-limitation of algal growth is a fundamental feature of these mechanistic models, often described by the Monod relationship (Chapra Citation1997):
(1)
where G(N) is the fraction of the maximum specific growth rate (with a value of 1 corresponding to nutrient saturation); Kp is the half-saturation coefficient, a measure of the algal community's efficiency in acquiring P; and SRP is the concentration of this operationally defined form of P, assumed to be entirely available for algal uptake and growth (other forms of P are assumed to not be immediately available). Substantial nutrient (P) limitation (i.e., low G(N) levels) prevails in most north temperate lacustrine systems (for trophic states less than hypereutrophy) in summer (Reynolds Citation2006). Most of the Kp values adopted in contemporary models fall in the range of 0.5–1.5 µg/L (P as SRP; Connors et al. Citation1996, Doerr et al. Citation1998, Tomlinson et al. Citation2010). A threshold of SRP ≤1 µg/L has been used to identify conditions that correspond to distinct P limitation of algal growth (Effler et al. Citation2010), supported by accompanying increases in the activity of the enzyme alkaline phosphatase (indicator of P limitation; Connors et al. Citation1996). Reynolds (Citation2006) described SRP concentrations of >3 µg/L as “scarcely” limiting, which is generally consistent with the above range of KP values from the contemporary literature and the hyperbolic dependency of G(N) on SRP described in equation Equation1(1) . The indicated systematically higher levels of SRPE compared to SRPS, that doubtless extend into the upper productive layers (e.g., and ), could be problematic for representing the extent of P-limitation in the upper productive layers because it would depict (e.g., Kp = 0.5−1.5 µg/L; equation Equation1
(1) ) only a minor, instead of substantial, degree of limitation.
The SUP pool is represented as a separate state variable in most contemporary P-eutrophication models (Arhonditsis et al. Citation2006, Robson Citation2014), that together with SRP corresponds to the overall dissolved P pool. In general, SUP (or a fraction of SUP) is represented as a source of SRP. The kinetics of the conversion of DOP to SRP are generally specified to be slow relative to the uptake of SRP by phytoplankton (Doerr et al. Citation1998). Accordingly, the DOP pool can, along with other recycling pathways, contribute to sustained phytoplankton growth under severely depleted SRP conditions (Connors et al. Citation1996, Effler et al. Citation2010). The apportionment of TDPS according to SRPS and SUPS can support such model representations of the P cycle. A shift to higher SRP and lower SUP levels that would occur using the E protocol would necessarily change this representation. Accordingly, in the context of contemporary P-eutrophication models (but not the older models; e.g., Vollenweider Citation1976), the selection between these 2 analytical protocols for P has the unfortunate likely effect of influencing the model structure choices that can be implemented and perhaps the associated level of realism represented.
The extent to which SRPE and SUPE datasets would influence the success of a P-eutrophication modeling initiative is uncertain. At the least, the differences between SRPE and SRPS reported here would require different values for model coefficients (model parameterization describing the P cycle), and perhaps differences in model structure, to achieve calibration using respective datasets from the 2 protocols. For example, use of an SRPE dataset would require an increase in Kp (equation Equation1(1) ) to maintain appropriate P-limitation of algal growth and higher transformation rates of the (smaller) DOP (SUPE) pool to SRPE. Such analytical protocol-driven differences in model parameterization would translate to an undesirable increase in the degrees of freedom of related mechanistic modeling initiatives. Great care is needed to recognize which measurement protocols have been adopted in datasets to be used in contemporary P-eutrophication modeling initiatives and in related research to be drawn upon from the literature. Limnological and modeling expertise in P-eutrophication should be included in the data collection parts of the programs used for development and testing of related management modeling.
Acknowledgments
This is contribution no. 326 of the Upstate Freshwater Institute.
Funding
Funding for this work was provided by Cornell University.
References
- Arhonditsis GB, Adams-VanHarn BA, Nielsen L, Stow CA, Reckhow KH. 2006. Evaluation of the current state of mechanistic aquatic biogeochemical modeling: citation analysis and future perspectives. Environ Sci Technol. 40:6547–6554.
- Arhonditsis GB, Brett MT. 2004. Evaluation of the current state of mechanistic aquatic biogeochemical modeling. Mar Ecol Prog Ser. 271:13–26.
- Auer MT, Tomasoski KA, Babiera MJ, Needham M, Effler SW, Owens EM, Hansen JM. 1998. Phosphorus bioavailability and P-cycling in Cannonsville Reservoir. Lake Reserv Manage. 14:278–289.
- Baldwin DS. 2013. Organic phosphorus in the aquatic environment. Environ Chem. 10:439–454.
- Bentzen E, Taylor WD, Millard ES. 1992. The importance of dissolved organic phosphorus to phosphorus uptake by limnetic plankton. Limnol Oceanogr. 37:217–231.
- Chapra SC. 1997. Surface water-quality modeling. New York: McGraw-Hill. 844 p.
- Clesceri LS, Greenberg AE, Eaton AD. 1998. Standard methods for the examination of water and wastewater. Washington (DC): American Public Health Association, American Water Works Association, Water Environment Federation.
- Connors SD, Auer MT, Effler SW. 1996. Phosphorus pools, alkaline phosphatase activity, and phosphorus limitation in hypereutrophic Onondaga Lake, NY. Lake Reserv Manage. 12:47–57.
- Cooke GD, Welch EB, Peterson SA, Nichols SA. 2005. Restoration and management of lakes and reservoirs. Boca Raton (FL): Taylor and Francis, CRC Press.
- Currie DJ, Bentzen E, Kalff J. 1986. Does algal-bacterial partitioning vary among lakes? A comparative study of orthophosphate uptake and alkaline phosphatase activity in freshwater. Can J Fish Aquat Sci. 43:311–318.
- DePinto JV, Young TC, Martin SC. 1981. Algal-available phosphorus in suspended sediments from lower Great Lakes tributaries. J Great Lakes Res. 7:311–325.
- Dodds WK. 2003. Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J N Am Benthol Soc. 22:171–181.
- Doerr SM, Owens EM, Gelda RK, Auer MT, Effler SW. 1998. Development and testing of a nutrient-phytoplankton model for Cannonsville Reservoir. Lake Reserv Manage. 14:301–321.
- Effler SW, Auer MT, Peng F, Perkins MG, O'Donnell SM, Prestigiacomo AR, Matthews DA, DePetro PA, Lambert RS, Minott NM. 2012. Factors diminishing the effectiveness of phosphorus loading from municipal waste effluent: critical information for TMDL analyses. Water Environ Res. 84:254–264.
- Effler SW, Prestigiacomo AR, Matthews DA, Gelda RK, Peng F, Cowen EA, Schweitzer SA. 2010. Tripton, trophic state metrics, and near-shore versus pelagic zone responses to external loads in Cayuga Lake, New York. Fund Appl Limnol. 178:1–15.
- Helsel DR, Hirsch RM. 1992. Statistical methods in water resources. Volume 49. Elsevier.
- Hudson JJ, Taylor WD, Schindler DW. 2000. Phosphate concentrations in lakes. Nature. 406:54–56.
- Hudson JJ, Taylor WD. 2005. Rapid estimation of phosphate at picomolar concentrations in freshwater lakes with potential application to P-limited marine systems. Aquat Sci. 67:316–325.
- Lampert W, Sommer U. 2007. Limnoecology: the ecology of lakes and streams. New York (NY): Oxford University Press. 324 p.
- Mayer LM, Gloss SP. 1980. Buffering of silica and phosphate in a turbid river. Limnol Oceanogr. 25:12–22.
- Murphy J, Riley J. 1962. A modified single solution for the determination of phosphate in natural waters. Anal Chim Acta. 27:31–36.
- Prestigiacomo AR, Effler SW, Matthews DA, Auer MT, Downer BE, Kuczynski A, Walter MT. 2016. Apportionment of bioavailable phosphorus loads entering Cayuga Lake, New York. J Am Wat Resour Assoc. 52:31–47.
- Reynolds CS. 2006. Ecology of phytoplankton. Cambridge (UK): Cambridge University Press.
- Reynolds CS, Davies PS. 2001. Sources and bioavailability of phosphorus fractions in freshwaters: a British perspective. Biol Rev Camb Philos. 76:27–64.
- Robson BJ. 2014. State of the art in modelling of phosphorus in aquatic systems: review, criticisms and commentary. Environ Model Softw. 61:287–296.
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. P Nat Acad Sci. 105:11254–11258.
- StatSoft. 2003. STATISTICA (data analysis software system), version 6. Available from: www.statsoft.com
- Tarapchak SJ, Chambers RL, Bigelow SM. 1982. Soluble reactive phosphorus measurements in Lake Michigan: causes of method-specific differences. J Great Lakes Res. 8:700–710.
- Tarapchak SJ, Rubitschun C. 1981. Comparisons of soluble reactive phosphorus and orthophosphorus concentrations at an offshore station in southern Lake Michigan. J Great Lakes Res. 7:290–298.
- Tomlinson LM, Auer MT, Bootsma HA, Owens EM. 2010. The Great Lakes Cladophora Model: development, testing, and application to Lake Michigan. J Great Lakes Res. 36:287–297.
- [USEPA] United States Environmental Protection Agency. 1983. Methods for chemical analysis of water and wastes. Cincinnati (OH): Environmental Monitoring and Support Laboratory. EPA-600/4-79-020.
- Vogel RM, Stedinger JR, Hooper RP. 2003. Discharge indices for water quality loads. Water Resour Res. 39:1273.
- Vollenweider RA. 1976. Advances in defining critical loading levels for phosphorus in lake eutrophication. Mem Ist Ital Idrobiol. 33:53–83.
- Wetzel RG. 2001. Limnology: lake and reservoir ecosystems. 3rd ed. New York: Academic Press. 1006 p.
