ABSTRACT
Schütz J, Rydin E, Huser BJ. 2017. A newly developed injection method for aluminum treatment in eutrophic lakes: Effects on water quality and phosphorus binding efficiency. Lake Reserv Manage. 33:152–162.
Application of aluminum (Al) salts has been used to reduce phosphorus (P) concentrations in lakes since the 1960s. Al is typically applied to the water column where Al-hydroxides form, settle to the sediment surface, and bind P in sediment. Al can be transported to other, non-target areas of the lake, however, potentially limiting treatment effectiveness. To alleviate this problem, a new method has been developed in which the Al salt is injected directly into the sediment as a liquid. In this study, the binding efficiency and application costs were calculated for 2 lakes in Sweden that received injection applications of polyaluminum chloride (PAC). Binding efficiency was similar to previous water column applications, implying there is little difference between the 2 application methods. Other factors, however, such as dissolved organic matter and type of Al salt used (PAC vs. Al sulfate), can also affect binding efficiency. Thus, Al injection may have improved the amount of P bound per unit Al in the study lakes given the in-lake conditions and Al salt used. Treatment cost (cost per kilogram of P bound to Al) for the injection method compared to previous water column treatments was somewhat higher due to increased costs for buffered PAC and time needed for application. Both mobile sediment P and internal loading remained reduced compared to pre-treatment conditions, showing that the Al injection treatments continued to control sediment P release. More study is needed, however, to determine the relative effectiveness of this method in different types of lakes.
When external sources of nutrients are reduced, release of historically accumulated (legacy) phosphorous (P) from lake sediment can maintain excess nutrient levels and sustain high lake productivity. Aluminum (Al) is commonly used to decrease internal P loading in lakes by inactivating easily releasable (i.e., mobile) P in the sediment. Al salts have been used to reduce P in eutrophic lakes for decades with varying results and treatment longevities. Lake Långsjön, south of Stockholm, Sweden, was the first lake to be treated with Al (water column precipitation) in 1968 (Jernelöv Citation1970), and 100s of lakes have since been treated with Al to reduce internal loading and restore water quality (Huser et al. Citation2016b). Al salts are favorable for lake treatment because Al hydroxide (Al(OH)3) binds strongly to inorganic forms of P (Rydin and Welch Citation1998, Lewandowski et al. Citation2003, Cooke et al. Citation2005), and the P is considered permanently bound in the sediment. Treatment longevity has varied substantially, however, with the beneficial effects of treatment lasting from just months to more than 40 years (Welch and Cooke Citation1999, Huser et al. Citation2016b).
One reason for the variation in treatment longevity may be the P binding efficiency of the added Al. Of the data that exist for changes to sediment P pools after Al treatment, substantial variation exists between studies with respect to binding efficiency. Rydin and Welch (Citation1999) showed that Al treatment of Lake Delevan (Wisconsin, USA) resulted in an Al to Al-bound P (Al:PAl) ratio of 5.7:1 (molar), and Retizel et al. (Citation2005) detected an Al:PAl ratio of 7.7:1 in sediment collected 2 years after Al treatment of a polymictic lake in Denmark. Rydin et al. (Citation2000) found a constant relationship between Al added and PAl formed in 6 lakes in Washington state (USA; average 12.6:1 for 6 lakes), whereas ratios between 5.6:1 and 14.5:1 were detected in sediment cores collected from 6 polymictic and dimictic lakes in Minnesota, USA (Huser Citation2012). These studies represent a near 3-fold difference in the amount of P bound per unit Al added. One reason for the variation in P binding efficiency of the added Al may be crystallization (or aging) of the Al floc. Increases in crystallization of freshly precipitated Al(OH)3 are accompanied by a decrease in surface area (Sims and Ellis Citation1983, Berkowitz et al. Citation2005) as well as a decrease in P adsorption (Berkowitz et al. Citation2006), especially when aging occurs in the absence of P (de Vicente et al. Citation2008a).
Various techniques available to apply Al to lakes may also affect binding effectiveness and treatment longevity. The most common method is to apply a liquid slurry to the water column, either just under the water surface or above the sediment–water interface (Welch and Cooke Citation1999). After application of the liquid slurry, hydrolysis reactions occur in the water column, forming amorphous Al(OH)3. In lakes with steep sediment bed slopes or areas prone to wind disturbance of the sediment, the Al floc may be transported to non-treatment areas (Huser Citation2017) by resuspension and sediment focusing processes. The transport of the Al floc may lead to short-term, elevated Al concentrations in the water column (Egemose et al. Citation2013), and excess accumulation of Al in deeper areas of the lake can limit binding efficiency (Huser Citation2012). A number of previous studies have used injection-type treatment to treat sediments directly, although these have generally used other chemicals designed mainly to oxidize sediment organic matter (Ripl Citation1976) or were at the mesocosm scale (Mesner and Narf Citation1987).
In a newly developed technique, liquid Al is injected directly into the surficial sediment during a whole-lake treatment. This application method has 2 main advantages: (1) translocation of the Al floc should be limited because Al is directly injected into the sediment, and (2) binding efficiency should improve because Al and dissolved pore water P come in to contact during treatment and subsequent floc formation, in contrast to water application after which P must diffuse from deeper sediment layers to the Al floc over time. To determine Al binding efficiency of Al after injection treatment, sediment cores were collected from 2 lakes in Sweden where this method was used to limit sediment P release. The main hypothesis of this study was that direct injection would lead to improved binding efficiency compared to lakes where Al was added to the water column and allowed to settle to the sediment surface. A cost–benefit analysis was also conducted by comparing treatment costs and total amounts of P inactivated by the added Al.
Materials and methods
Study sites
The 2 lakes chosen for this study were Flaten and Långsjön, both located south of Stockholm, Sweden. Lake Flaten is a 63 ha dimictic lake situated 10 km south from Stockholm city center, 59°25′01″N, 18°15′38″W (). The lake has a catchment area of 403 ha, of which 70% consists of forested and recreational areas. Residential areas are located in the northern part of the catchment. Approximately 10 fish species have been detected in Lake Flaten (Fränstam Citation2011). Lake Långsjön is a 29 ha, shallow polymictic lake located in an old residential area on the border of Stockholm and Huddinge municipality, 59°26′77″N, 17°96′67″W (). The catchment area of Lake Långsjön (243 ha) is mainly residential. Fish species present are representative of eutrophic lakes and include European perch (Perca fluviatilis), common roach (Rutilus rutilus), crucian carp (Carassius carassius), tench (Tinca tinca), and common carp (Cyprinus carpio, Fränstam Citation2011).
Table 1. Background information including morphology and coring locations for lakes Flaten and Långsjön.
Water quality data were collected by Stockholm County from 1995 through 2015. Average growing season (Jun-Sep) total P (TP), chlorophyll a (Chl-a), and water clarity (Secchi disk depth) were determined pre- and post-treatment for both lakes. Secchi depth occasionally reached the bottom of Lake Långsjön after Al treatment, and thus the improvement in water clarity was limited by the maximum depth of the lake (3.3 m).
Al injection treatment
The injection treatment method used in the 2 study lakes was developed by Vattenresurs AB (Sten-Åke Carlsson and Lars Eriksson) to more accurately target the areas of sediment containing excess P. Dissolved polyaluminum chloride (PAC; PAX XL 100, Kemira Kemwater, Sweden) was injected together with lake water and instantly mixed in the sediment, forming the Al floc. GPS navigation enabled a controlled application to the top 0.1–0.2 m of sediment through tubes mounted on a 10 m-wide steel bar mounted on a barge (Supplemental Fig. S1). Side scan sonar was used to map the bottom and find potential obstacles before the treatment. Water column depth was monitored continuously during application as well, and the steel bar was adjusted automatically to follow changes in water depth. Passing over a rock, for example, would simply cause the tube and its nozzle to rise above the rock. The semi-stiff plastic tubes delivered lake water into the sediment under pressure to “fluidize” the sediment. Inside these wider tubes, smaller injection tubes supplied the concentrated PAC that flocculated as it mixed with the lake water and entered the sediment. The hydrated PAC caused a small drop in lake water pH (0.4 units).
Bottom areas deeper than 6 m were treated in Lake Flaten in 2000. Sediments between 6 and 9 m depth were injected with Al at a rate of 30 g/m2, and another 10 g/m2 was added to the water above to precipitate dissolved P. Sediments at depths of 9–10 m were injected with 40 g/m2, and 14 g/m2 was applied in the bottom water. The deepest zone sediment (10–14 m), was injected with 53 g/m2, and an additional 17 g/m2 was dispersed in the bottom water.
In 2006, 25 g/m2 of Al was injected into Lake Långsjön sediments at water depths between 1.5 and 2.0 m, 50 g/m2 between 2.0 and 2.5 m, and 75 g/m2 between 2.5 m and the maximum depth (3.3 m). Bottom water was not treated in Lake Långsjön; only direct sediment injection was used. A ratio of 10:1 Al to mobile sediment P (including labile organic P) was used to calculate doses needed to inactivate the mobile sediment P pool in the upper 10 cm of sediment in both lakes.
Sediment sampling and analysis
Three sediment cores from each lake were collected from the ice at the beginning of February 2015 with a Willner gravity sediment-coring device (). The sediments were sliced on site at 1 cm intervals between 1 and 20 cm sediment depth and at 2 cm intervals from 20 to 40 cm (or to the deepest depth of the cored sediment). Samples were collected at water column depths of 12.9, 11.6, and 8.4 m in Lake Flaten and 2.0, 2.7, and 3.0 m in Lake Långsjön. Samples were stored in the dark at 4 C before being analyzed within a week of collection. Pre-treatment sediment cores were collected in 1999 for Lake Flaten (Rydin Citation1999) and 2005 for Lake Långsjön (Rydin Citation2006) at similar areas and depths to determine Al doses needed to inactive mobile and labile organic sediment P.
P fractions in sediment were determined according to Psenner et al. (Citation1988) and modified by Hupfer et al. (Citation1995), in which sediment P is separated into pore-water, loosely bound, iron-bound, Al-bound, calcium-bound, and organic P. The fractions typically released to the water column during internal loading events, herein termed mobile P, included pore-water, loosely bound, and iron-bound P. All extracted fractions were then analyzed in a spectrophotometer at 880 nm using the ammonium molybdate blue method (Murphy and Riley Citation1962). Water content was determined by freeze-drying the sediments at −70 C for ∼24 h. Sediment density was determined after loss on ignition at 550 C for 2 h (Håkanson and Jansson Citation1983). Total Al was determined by acid digestion with 7 M HNO3 in an autoclave at 120 C. The samples were then analyzed using an ICP-AES, and Al was measured at a wavelength of 396 nm. Background concentrations of Al and PAl for each core were estimated using sediment layers that were not affected by treatment. To determine Al:PAl ratios (g/m2), the above-background mass of Al and PAl in the sediment layers affected by treatment were summed.
Results
Changes to sediment chemistry after treatment
The concentrations of added Al and PAl formed varied substantially in most cores collected from Lake Flaten (). Substantial peaks of PAl and Al in the sediments between 5 and 7 cm sediment depth were detected in core F1, and sediment density was substantially lower here compared to surrounding sediment layers (1.006 to 1.009 g/cm3 vs. 1.022 to 1.036 g/cm3). The sum of excess (i.e., added) Al was 52 g/m2, which resulted in the formation of 5.0 g/m2 PAl and an Al:PAl ratio of 10.4:1 (by weight). Distinct peaks of PAl and Al occurred at sediment depths between 4 and 6 cm in core F2 (). The sum of added Al and PAl formed was 29 g/m2 and 3.3 g/m2 respectively, resulting in an Al:PAl ratio of 8.9:1. Similar differences in sediment density between peak layers (1.013–1.017 g/cm3) and sediment layers directly above (1.023 g/cm3) and below (1.036 g/cm3) these layers were found. Core F3 was collected at a shallower depth (8.1 m), and lower amounts of sediment Al and PAl were detected relative to cores F1 and F2 (). The resulting Al:PAl ratio in core F3 was 12.4:1 based on the sum of added Al (9.0 g/m2) and PAl formed (0.7 g/m2). Recovery of Al in the 3 cores collected from Lake Flaten ranged from 23% to 74% ().
Figure 1. Concentrations (dry weight) of aluminum (Al) and Al-bound P (PAl) in (a–c) cores F1–F3, respectively, collected from Lake Flaten. Short- and long-dashed lines represent background concentrations for PAl and Al, respectively.
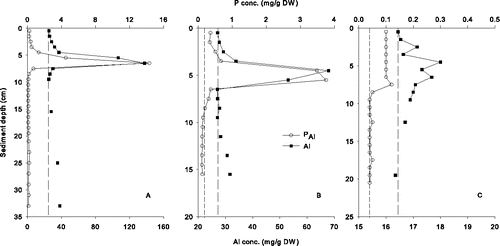
Table 2. Sediment chemical data and Al:PAl ratios for lakes Flaten and Långsjön. Al recovered based on only the amount of Al injected in the sediment (only Lake Flaten received partial water application of Al) is shown in parenthesis.
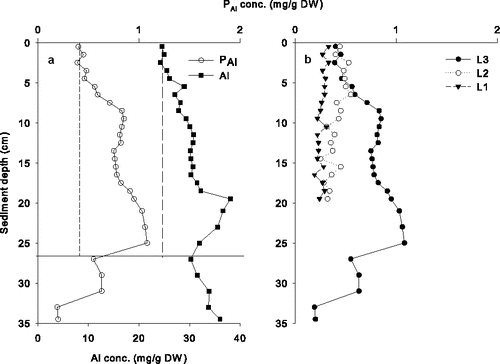
Evidence of PAl formed was also detected in core L3 collected from Lake Långsjön () but not for cores L1 and L2 (). In contrast to the distinct concentration peaks generally seen in Lake Flaten cores, the results for Lake Långsjön were less clear (i.e., no distinct peak), which indicated both sediment redistribution and mixing. Increases in Al and PAl were detected throughout the upper 25 cm of sediment in Lake Långsjön (core L3), probably due to mixing of sediment. The sum of Al detected in the sediment (71 g/m2) and formed PAl (4.5 g/m2) resulted in an Al:PAl ratio of 15.9:1 (). Because of elevated concentrations of Al and PAl at the bottom of the core, likely due to Al treatments conducted from 1968 to 1970, background concentrations were calculated using the uppermost 4 cm of sediment where both Al and PAl had apparently stabilized since treatment as new sediment accumulated on the lake bottom.
Figure 2. (a) Concentrations (dry weight) of aluminum (Al) and Al-bound P (PAl) in core L3 and (b) PAl for all 3 cores (L1–L3) collected from Lake Långsjön. Short- and long-dashed vertical lines represent background concentrations for PAl and Al, respectively (a). The horizontal line indicates separation of the 1968 Al treatment and the more recent treatment (above the line in (a)).
Comparison of mobile sediment P before and after treatment
Pre-treatment sediment cores were collected in 1999 (Flaten) and 2005 (Långsjön) to determine Al doses needed to inactive mobile P in the sediment of both lakes. Cores were collected in 2015 from similar areas in both lakes so that pre- and post-treatment concentrations of mobile P could be compared. Average pre-treatment concentrations of mobile P in the upper 5 cm of sediment in Lake Flaten ranged from 0.134 to 0.208 mg/g prior to treatment with Al (). Similar results were found for Lake Långsjön, where average mobile P concentrations in surficial sediment (0–7 cm) ranged from 0.139 to 0.210 mg/g. Mobile P concentrations in sediment collected after treatment ranged from 0.055 to 0.143 mg/g for Lake Flaten and 0.11 to 0.147 mg/g for Lake Långsjön (). All differences between pre and post-treatment concentrations of mobile P were significant except for core L3 in Lake Långsjön.
Table 3. Differences in mobile sediment P content pre- and post-treatment. Sediment was collected the year before treatment in both lakes Flaten (1999) and Långsjön (2005).
Mobile P concentrations detected in 2015 in Lake Flaten were nearly half (mean = 43%) of those measured before treatment in 1999. The results may be conservative because anaerobic conditions occurred in Lake Flaten at water column depths of 9 m and greater during sampling in 1999, indicating that a portion of the mobile P pool had already been released from the sediment. Dissolved oxygen was 7 mg/L or greater across all water column depths during sediment collection in 2015. A similar decline in mobile P occurred in Lake Långsjön at site L3 between 2005 (pre-treatment) and 2015 (48%). Mobile P in core L2 was also lower (22%) compared to pre-treatment data, even though a significant increase in PAl was not detected in sediment. No significant difference between pre- and post-treatment mobile P concentration was found in core L1.
Treatment costs
The total cost (in 2014 US dollars) for injection application of Al was $352,000 for Lake Flaten and $388,000 for Lake Långsjön. To compare cost efficiency for the 2 application methods, the average amount of PAl formed was used along with the Al:PAl ratio for each lake (). The total cost of the treatment was then divided by total amount of formed PAl to determine the cost per kilogram of sediment P bound to Al after treatment. Cost per kilogram P inactivated by Al was between $152,000 and $268,000 for the 2 injection treatments, whereas previous water application treatment costs ranged from $18,000 to $114,000 ().
Table 4. Dose, total amount of formed PAl, and treatment costs for lakes Flaten and Långsjön compared with lakes treated with surface application. An 8.5:1 exchange rate was used to convert Swedish kronor to US dollars (US$).
Water quality improvement
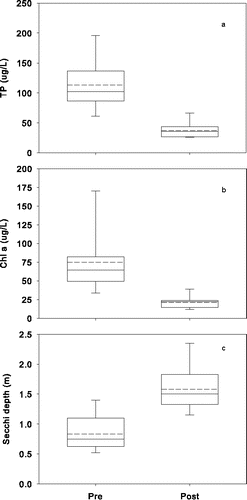
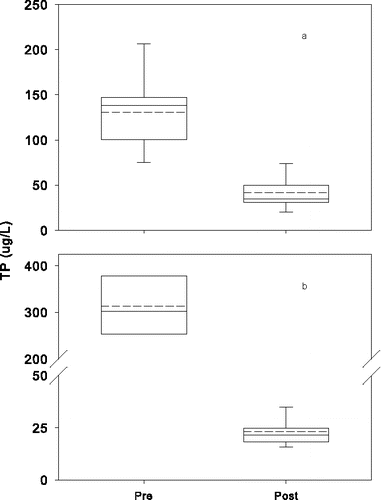
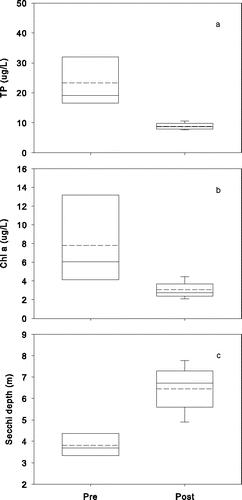
Water quality improved substantially in both lakes after addition of Al to the sediment (). The annual growing season epilimnetic means (Jun–Sep) for TP concentration before treatment generally ranged from 16 to 36 μg/L (overall mean of 23 μg/L) in Lake Flaten and decreased to <10 μg/L after treatment (). Annual mean epilimnetic TP concentration in Lake Långsjön decreased as well, from a pre-treatment mean of 115 μg/L (range 87 to 209 μg/L) to a mean of 37 μg/L after Al injection (). Mean growing season epilimnetic Chl-a decreased from 8 to 3 µg/L in Lake Flaten and from 79 to 22 µg/L in Lake Långsjön ( and ). The decrease in algal biomass caused Secchi disk depth to increase in both lakes ( and ) after Al the treatments. All pre- and post-treatment differences for TP, Chl-a, and Secchi disk depth were significant (P-value < 0.05). P declines in bottom waters of both lakes were even greater (), with mean growing season concentrations dropping from 315 μg/L (peak of 658 μg/L) to 23 µg/L in Lake Flaten (10–11.5 m water column depth) and from 131 μg/L (peak of 271 μg/L) to 41 µg/L in Lake Långsjön (2.5 m water column depth).
Table 5. Summer means (Jun–Sep) for water quality and chemistry pre- and post-treatment (1995–2015) in lakes Långsjön and Flaten. Data for year of treatment (2000 for Flaten and 2006 for Långsjön) were not included in the averages.
Figure 3. Changes in nutrient-related water quality variables (growing season means Jun–Sep) after Al injection treatment in Lake Flaten: (a) TP, (b) Chl-a, and (c) Secchi depth. Dashed and solid lines in the box plots represent means and medians, respectively.
Discussion
Binding efficiency between Al and P was assessed after Al application using a new sediment injection method designed by Vattenresurs AB (Sweden) to more accurately apply Al to desired treatment locations and to limit resuspension and movement of the added Al after treatment. Water quality in both lakes improved after treatment with substantial and long-lasting declines in TP and Chl-a and increases in Secchi disk depth ( and ). Mobile P was also generally lower in the sediment of both lakes 9 (Långsjön) and 15 years (Flaten) after treatment (), and internal loading remained greatly reduced ().
Al binding efficiency
One main reason for using injection treatment is to limit the movement of the Al floc and improve binding efficiency by reducing the time needed for P to come in contact with Al in the sediment. Al:PAl ratios in Lake Flaten varied between 8 and 12 for the 3 sediment cores analyzed in this study, which were within the range shown in previous studies of whole-lake treatments (Rydin et al. Citation2000, Reitzel et al. Citation2005, Huser et al. Citation2011, Huser Citation2012). The Al:PAl ratio of 15.9 detected in Lake Långsjön, however, is at the upper end of binding ratios reported in the studies cited earlier. Although the results for Lake Långsjön, and potentially Lake Flaten, seem to negate the hypothesis that sediment injection would lead to more efficient binding of P by Al, several confounding factors may have affected the results.
The majority of Al treatments have been conducted in the United States, where Al sulfate (alum) is the most commonly applied Al compound. PAC, however, is the most common Al compound currently used in Europe, and it was used in the study lakes. The difference in chemical reaction between alum and PAC after application in water may explain some anomalies found in this study. Hydrolysis reactions occur when alum is applied to water, and several monomeric Al species form before the solid phase Al(OH)3 forms and precipitates. PAC compounds, by contrast, generally come “pre-hydrolized” with OH, and thus the hydrolysis that occurs with alum is largely complete. Direct AlPO4 formation, which is a 1:1 binding between Al and P, is limited because PAC compounds are pre-hydrolyzed.
Although this type of binding between Al and P is only dominant at high PO4 concentrations (Jenkins et al. Citation1971), it may occur in sediment porewater where PO4 concentrations are much higher relative to those found in the water column (Enell and Löfgren Citation1988, Lewandowski et al. Citation2002). The use of PAC may be one reason why 4 of 6 lakes in a study by Jensen et al. (Citation2015) had ratios similar to, or even higher, than those observed in Lakes Flaten and Långsjön (range 16 to 18). Thus, the use of pre-hydrolized Al compounds may decrease overall binding efficiency after treatment because of limited AlPO4 formation.
Dissolved organic matter (e.g., humic compounds) may be another reason for lower binding efficiency in the study lakes. Average water color before treatment in lakes Långsjön and Flaten was 42 and 15 mg/L Pt units, respectively. de Vicente et al. (Citation2008b) showed that dissolved organic material in water (indicated by water color) could interfere with P binding by Al. Using the data from de Vicente and a power equation relating percent P removal from water by Al to water color (P removal = 49.5*water color [Pt units]−0.208, R2 = 0.58), predicted a 19% reduction in P binding efficiency in Lake Långsjön relative to Lake Flaten. Comparing Lake Långsjön to less humic lakes like those found in Huser et al. (Citation2016c) with water color 8.5 to 9 Pt units, potential binding reduction in Lake Långsjön increases to nearly 30%. Thus the negative effect of organic matter on binding between Al and P in Lake Långsjön is another possible reason for limited binding efficiency.
Yet another reason for reducing binding efficiency is that greater Al doses are generally used to reduce internal loading in lakes. As more Al is added relative to the amount of potentially available sediment P, the binding efficiency of the added Al will decrease because the chance for contact between Al and P decreases (i.e., Le Chatelier's principle). Given enough time, however, excess binding sites may be saturated by P (e.g., Lewandowski et al. Citation2003). Dosing of Al to reduce internal loading in sediments improved after mobile sediment P was incorporated into the dosing scheme (Rydin and Welch Citation1999). Thus, comparing the higher Al doses calculated using the Rydin and Welch method to those based on older methods may show that more recent treatments have been less efficient, simply due to the higher ratio of added Al to sediment P. The resulting binding ratios in the lake reported by Huser (Citation2012) with 70 g/m2 Al applied (similar to both study lakes receiving injection treatment) ranged from 13.3 to 14.5, which are 30% to 40% higher than those from Lake Flaten and similar to that from Lake Långsjön.
Changes to mobile P pools in sediment
Mobile P remained lower in both lakes relative to levels before treatment with Al (), indicating that the Al treatments exerted continued control of mobile sediment P in both lakes. Reduced mobile P in Lake Flaten was clear 15 years after Al treatment, with an average 43% reduction compared to pre-treatment levels. Mobile P was also generally lower (between 0% and 48% 9 years after treatment) in Lake Långsjön; however, sediment was heavily affected by bioturbation (see below), making quantification of background conditions difficult. Given the continued reduction of mobile sediment P in both lakes, it was not unexpected that internal release of P from the sediment also remained lower after treatment () because mobile sediment P is directly related to sediment P release (Pilgrim et al. Citation2007). The only core that did not show a statistically significant difference in mobile sediment P concentrations between pre- and post-treatment was core L1 in Lake Långsjön. This core, however, was collected close to the boundary of the treatment area and may have not received much, if any Al.
Translocation/bioturbation of Al after treatment
The core collected at the deepest sampling point in Lake Flaten (F1) had the highest mass of PAl, and large concentration peaks of both Al and PAl corresponded to the treatment conducted in 2000. A substantial difference in peak concentration of PAl occurred between core F1 (14.3 mg/g), core F2 (3.8 mg/g), and core F3 (0.12 mg/g), possibly because of the morphology of Lake Flaten. The steep bottom slopes could have contributed to a transportation of low density, organic-rich sediment, along with the added Al floc to deeper parts of the lake (Håkanson and Jansson Citation1983). Huser (Citation2012) showed the importance of lake morphology as a significant contributor of Al translocation and transport of Al and PAl from littoral to profundal zones in lakes, which was shown to occur in a matter of weeks after treatment (Huser Citation2017). In cores F1 and F2, the sediment density where peak concentrations of Al and Al-P were found was low (1.006 to 1.017 g/cm3). Even sediment density in non-treated layers, although greater than that generally found in treated layers, was low enough to be considered easily transported along the lakebed (Håkanson and Jansson Citation1983).
Based on the amount of Al injected into the sediment, Al recovery was near 100% for core F1 but less in cores F2 (55%) and F3 (30%; ). When the total dose was used (both Al injected to the sediment and applied to the water column), recovery was less, ranging from 74% to 23% for the 3 cores. Unfortunately, the deepest area of the lake (a relatively small area near core F1) could not be sampled due to a lack of ice. Greater than 100% Al accumulation likely occurred here but was not detected due to limited access. Further, it may also be possible that some of the Al added to the hypolimnion was transported to shallower areas that were not sampled, but this seems unlikely because of the strong thermal stratification in the lake during the growing season and during treatment. Although Al was injected into the sediment, it seems natural movement or translocation of existing sediment resulted in movement of the Al floc as well. Because less dense, organic-rich sediment generally contains high concentrations of mobile P, the movement of both Al floc and sediment may not be a concern and, in fact, may increase binding efficiency (Huser Citation2017), but further investigation is needed. We also recommend that additional cores be collected from non-treated areas in future studies to better determine the lake-wide distribution of Al because this is not the first study to determine Al recoveries of <100% (e.g., Rydin et al. Citation2000).
As previously suggested, treatment longevity for Al application in shallow, polymictic lakes is generally (but not always) lower compared to deeper dimictic lakes (Welch and Cooke Citation1999, Huser et al. Citation2016b). The management of trophic state is also usually more difficult in shallow lakes (Scheffer et al. Citation1993, Cooke et al. Citation2005), but Huser et al. (Citation2016a) showed that increased sediment mixing might improve the binding efficiency of P by Al in shallow lakes. The results for core L3 collected from Lake Långsjön, a lake with moderate populations of benthic feeding fish, were more ambiguous when compared to Lake Flaten. Major redistribution of surface sediment over the decade since treatment was detected in Lake Långsjön, explaining the lack of PAl significantly greater than natural background concentrations at 2 of 3 sampling sites (L1 and L2).
Other studies have had similar difficulties in shallow lakes where sediment mixing seems to redistribute the amount of Al applied throughout a greater depth of sediment, making detection of newly formed PAl or added Al above that naturally present in sediment difficult (Welch et al. Citation2017). One reason for the difficulty in determining added Al above natural background sediment concentrations is bioturbation of sediment caused by benthic feeding fish such as the common carp (Huser and Bartels Citation2015, Huser et al. Citation2016a). Because of this mixing and lack of definitive background concentrations deeper in the sediment, the upper few centimeters of sediment were instead used to calculate background concentrations of Al and PAl. This method resulted in mass of 71 g/m2 of added Al to Lake Långsjön, nearly the same as the amount applied (75 g/m2). We cannot be certain, however, that the estimation of background level is correct because of bioturbation and the potential impact of the previous Al treatments in the late 1960s. It seems clear, however, that although sediment injection was used, wave-induced water currents and/or bioturbation in Lake Långsjön were strong enough to redistribute the treated sediment layers.
Finally, although injection of Al occurred at similar rates at sediment treatment depths (e.g., 0–10 cm), peaks in Al concentration were more concentrated in some cores (e.g., Lake Flaten core F1), likely due to physical processes that occur during fluidization of the sediment and Al injection/flocculation. The fluidization process possibly did not affect deeper sediment layers equally because of increased pressure and sediment density (i.e., lower water content), potentially limiting the depth penetration of the Al. Thus, although the goal of treatment was to create an “even” treatment layer in the sediment, Al accumulation was still greater near the sediment surface in some cases. The results were still improved over a typical water column application, but more work is needed to improve efficiency of the injection process.
Cost effectiveness
Cost per kilogram P bound (or formed PAl) calculations indicate that treatment costs for lakes Flaten and Långsjön were somewhat higher compared to lakes where Al was applied to the water column (). This difference is not unexpected because the additional worker-hours needed to complete an injection treatment and the buffered PAC compound used would increase overall treatment cost. Unfortunately, costs (per kilogram P bound) for water application treatments using higher cost, buffered Al compounds were not available for comparison. If binding efficiency increases because of injection treatment, however, these cost differences can be offset by a greater amount of P bound per unit Al applied.
Factors other than direct costs were not included in the above calculation. A potential for reduced negative effects (e.g., short-term stress to biota) exists in the water column, although these are mainly related to short-term reduction of plankton due to physical settling. Permitting these treatments may be less intensive because of their lower potential for negative effects, potentially reducing treatment cost. In addition, Al compounds such as alum contain sulfur, which under anoxic conditions can lead to formation of iron sulfides, potentially decreasing the overall binding capacity of the sediment (Hansen et al. Citation2003). Although if productivity in treated lakes is reduced substantially, oxygen conditions can improve, and increased binding by Fe will occur whether PAC or alum is used.
Summary
Our results are the first detailing changes to water quality and sediment after a newly developed sediment injection method for Al treatment was used. From our study and others, we can conclude that both sediment injection and water column application of Al can successfully reduce water column P through inactivation of mobile sediment P and a subsequent reduction in sediment P release. Binding efficiency of both methods was similar, and the hypothesis that binding efficiency would be greater using the injection method was thus rejected. Confounding factors such as the chemistry of PAC versus alum, the interference of organic matter on binding between Al and P, as well as higher doses recently being used, however, make comparisons between treatments difficult. Results also showed the difficulties in determining binding efficiency in shallow lakes where sediment mixing can redistribute the added Al into a much greater mass of sediment, making detection of added Al or PAl above that found naturally in sediment difficult. With the few lakes included in this study (i.e., the two injection treated study lakes and the four lakes previously treaded with water application), the cost per kilogram PAl formed was somewhat greater than previous water-column–based treatments. Further work comparing treatment methods, as well as potential confounding factors, should be conducted to determine if the sediment injection method is a cost-effective alternative to water column application of Al.
ulrm_a_1318418_sm7190.zip
Download Zip (3.5 MB)Acknowledgments
The authors are grateful to Fred Erlandsson at Stockholm Vatten AB for sharing water chemical data for the study lakes, and to Vattenresurs AB (Sten-Åke Carlsson and Lars Eriksson) for sharing detailed Al application information. We are also grateful to 3 anonymous reviewers whose comments substantially improved the manuscript.
References
- Berkowitz J, Anderson MA, Graham RC. 2005. Laboratory investigation of aluminum solubility and solid-phase properties following alum treatment of lake waters. Water Res. 39:3918–3928.
- Berkowitz J, Anderson MA, Amrhein C. 2006. Influence of aging on phosphorus sorption to alum floc in lake water. Water Res. 40:911–916.
- Cooke GD, Welch EB, Peterson SA, Nichols SA. 2005. Restoration and management of lakes and reservoirs. Boca Raton (FL): CRC Press.
- de Vicente I, Huang P, Andersen FO, Jensen HS. 2008a. Phosphate adsorption by fresh and aged aluminum hydroxide. Consequences for lake restoration. Environ Sci Technol. 42:6650–6655.
- de Vicente I, Jensen HS, Andersen FO. 2008b. Factors affecting phosphate adsorption to aluminum in lake water: implications for lake restoration. Sci Total Environ. 389:29–36.
- Egemose S, Reitzel K, Andersen FO, Jensen HS. 2013. Resuspension-mediated aluminium and phosphorus distribution in lake sediments after aluminium treatment. Hydrobiologia. 701:79–88.
- Enell M, Löfgren S. 1988. Phosphorus in interstitial water: methods and dynamics. Hydrobiologia. 170:103–132.
- Fränstam T. 2011. Standardized net fishing in Långsjön, Trekanten and Flaten 2011. Sportfiskarna report. Swedish.
- Hansen J, Reitzel K, Jensen HS, Andersen FO. 2003. Effects of aluminum, iron, oxygen and nitrate additions on phosphorus release from the sediment of a Danish softwater lake. Hydrobiologia. 492:139–149.
- Hupfer M, Gachter R, Giovanoli R. 1995. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci. 57:305–324.
- Håkanson L, Jansson M. 1983. Principals of lake sedimentology. Berlin (Germany): Springer-Verlag.
- Huser BJ. 2012. Variability in phosphorus binding by aluminum in alum treated lakes explained by lake morphology and aluminum dose. Water Res. 46:4697–4704.
- Huser BJ. 2017. Aluminum application to restore water quality in eutrophic lakes: maximizing binding efficiency between aluminum and phosphorus. Lake Reserv Manage. doi.org/10.1080/10402381.2016.1235635
- Huser BJ, Bajer PG, Chizinski CJ, Sorensen PW. 2016a. Effects of common carp (Cyprinus carpio) on sediment mixing depth and mobile phosphorus mass in the active sediment layer of a shallow lake. Hydrobiologia. 763:23–33.
- Huser B, Bartels P. 2015. Feeding ecology of carp. In: Pietsch C, Hirsch PE, editors. Biology and ecology of carp. CRC Press; p. 217–243.
- Huser B, Brezonik P, Newman R. 2011. Effects of alum treatment on water quality and sediment in the Minneapolis Chain of Lakes, Minnesota, USA. Lake Reserv Manage. 27:220–228.
- Huser BJ, Egemose S, Harper H, Hupfer M, Jensen H, Pilgrim KM, Reitzel K, Rydin E, Futter M. 2016b. Longevity and effectiveness of aluminum addition to reduce sediment phosphorus release and restore lake water quality. Water Res. 97:122–132.
- Huser BJ, Futter M, Lee JT, Perniel M. 2016c. In-lake measures for phosphorus control: the most feasible and cost-effective solution for long-term management of water quality in urban lakes. Water Res. 97:142–152.
- Jenkins D, Ferguson JF, Menar AB. 1971. Chemical processes for phosphate removal. Water Res. 5:369–389.
- Jensen H, Reitzel K, Egemose S. 2015. Evaluation of aluminum treatment efficiency on water quality and internal phosphorus cycling in six Danish lakes. Hydrobiologia. 751:189–199.
- Jernelöv A. 1970. Phosphate reduction in lakes by precipitation with aluminum sulphate. In: Jernelöv A, editor. 5th International Water Pollution Research Conference. San Francisco (CA): Pergamon Press.
- Lewandowski J, Rüter K, Hupfer M. 2002. Two-dimensional small-scale variability of pore water phosphate in freshwater lakes: results from a novel dialysis sampler. Environ Sci Technol. 36:2039–2047.
- Lewandowski J, Schauser I, Hupfer M. 2003. Long term effects of phosphorus precipitations with alum in hypereutrophic Lake Susser See (Germany). Water Res. 37:3194–3204.
- Mesner N, Narf R. 1987. Alum injection into sediments for phosphorus inactivation and macrophyte control. Lake Reserv Manage. 3:256–265.
- Murphy J, Riley JP. 1962. A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta. 26:31–36.
- Pilgrim KM, Huser BJ, Brezonik PL. 2007. A method for comparative evaluation of whole-lake and inflow alum treatment. Water Res. 41:1215–1224.
- Psenner R, Boström B, Dinka M, Pettersson K, Puckso R, Sager M. 1988. Fractionation of phosphorus in suspended matter and sediment. Arch Hydrobiol Suppl. 30:98–103.
- Reitzel K, Hansen J, Andersen FO, Hansen KS, Jensen HS. 2005. Lake restoration by dosing aluminum relative to mobile phosphorus in the sediment. Environ Sci Technol. 39:4134–4140.
- Ripl W. 1976. Biochemical oxidation of polluted lake sediment with nitrate: a new lake restoration method. Ambio. 5:132–135.
- Rydin E. 1999. Aluminum dosing for mobile sediment phosphorus in lakes Flaten and Kyrksjön. Uppsala, Sweden, SVAB report 990726. Swedish.
- Rydin E. 2006. Mobile phosphorus in Långsjön's sediment. Norrtälje (Sweden): Naturvatten I Roslagen. Report 2006:9.
- Rydin E, Welch EB. 1998. Aluminum dose required to inactivate phosphate in lake sediments. Water Res. 32:2969–2976.
- Rydin E, Welch EB. 1999. Dosing alum to Wisconsin lake sediments based on in vitro formation of aluminum bound phosphate. Lake Reserv Manage. 15:324–331.
- Rydin E, Huser B, Welch EB. 2000. Amount of phosphorus inactivated by alum treatments in Washington lakes. Limnol Oceanogr. 45:226–230.
- Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E. 1993. Alternative equilibria in shallow lakes. Trends Ecol Evol. 8:275–279.
- Sims JT, Ellis BG. 1983. Changes in phosphorus adsorption associated with aging of aluminum hydroxide suspensions. Soil Sci Soc Am J. 47:912–916.
- Värnhed B. 2005. Restoration of Flaten – September to October 2000. Stockholm Vatten report. Swedish.
- Värnhed B. 2009. Långsjön – reduction of internal phosphorus loading though aluminum treatment of bottom sediment. Stockholm Vatten report. Swedish.
- Welch EB, Cooke GD. 1999. Effectiveness and longevity of phosphorus inactivation with alum. Lake Reserv Manage. 15:5–27.
- Welch EB, Gibbons HL, Brattebo SK, Corson-Rikert HA. 2017. Distribution of aluminum and phosphorus fractions following alum treatments in a large shallow lake. Lake Reserv Manage. doi.org/10.1080/10402381.2016.1276653