 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
De Mello NAST, Brighenti LS, Barbosa FAR, Staehr PA, Bezarra Neto JF. 2017. Spatial variability of methane (CH4) ebullition in a tropical hypereutrophic reservoir: silted areas as a bubble hot spot. Lake Reserv Manage. 35:105—114.
The concentration of methane (CH4) has doubled in the atmosphere over the last 200 yr, raising the need to understand emissions of this potent greenhouse gas from inland waters. CH4 ebullition is the dominant pathway in shallow aquatic environments and is difficult to quantify due to its episodic nature and heterogeneous spatial distribution. We investigated the temporal and spatial variability of CH4 ebullition during 2013 in a shallow hypereutrophic urban reservoir, in Belo Horizonte City, Brazil. The average emission measured during summer was 780 mg CH4/m2/d, ranging from 1 to 3070 (n = 75). During winter, the average emission was 316 mg CH4/m2/d, ranging from 4 to 1253 (n = 75). A strong spatial variation (P < 0.001) was observed across the reservoir in both seasons. Several folds higher (39—58% of the total) emissions were recorded at the mouth of the main tributaries, which therefore was considered to be a hot spot ebullition zone. This was expected due to its shallow area (mean depth 1.30 m) with low hydrostatic pressure and 2 to 6 C (winter and summer, respectively) higher sediment temperatures, which is aggravated by the intense siltation process resulting from insufficient management of the sewage water entering the reservoir. In this article we demonstrate the consequence of siltation as an enhancing factor for CH4 emission from the hot spots ebullition zones.
Methane (CH4) is a potent greenhouse gas (GHG) and has a global warming potential (GWP) 34 times higher than CO2 over a 100-year period. Recently, the atmospheric concentration of CH4 reached 1.87 ppm, which is 2.62 times higher than recorded in the pre-industrial era (Myhre et al. Citation2013). Although inland waters (lakes, rivers, and reservoirs) occupy a small area of the global surface (∼3%; Downing et al. Citation2006), they are considered significant sources of CH4 (93.1 Tg CH4/yr) to the atmosphere, and may offset current continental GHG budgets (Bastviken et al. Citation2011). In aquatic ecosystems, CH4 production in sediments is regulated by several environmental factors, including temperature, the quantity and quality of organic substrate, nutrient availability, and oxygen concentration (Megonigal et al. Citation2005).
The CH4 transport from sediments via the water column to the atmosphere occurs via distinct pathways, including diffusive flux, bubble flux (ebullition), and the liberation of CH4 stored within the oxygen depleted deeper layers during periods of water column destratification. The CH4 can also be transported from the sediment to the atmosphere mediated by emergent macrophytes; this pathway is very significant in wetlands (Bastviken et al. Citation2004). Studies suggest that the ebullition emissions is the dominant pathway in shallow environments (< 50 m), which in many cases may exceed 80% of all CH4 emitted at the open waters of the aquatic ecosystems (Repo et al. Citation2007, Bastviken et al. Citation2011). In addition, it is the most difficult pathway to quantify due to its episodic nature and heterogeneous spatial distribution (Bastviken et al. Citation2008, Ostrovsky et al. Citation2008, DelSontro et al. Citation2011). The bubble releases from sediment are controlled by factors which decrease the hydrostatic pressure, such as variations in water level (Engle and Melack Citation2000), in atmosphericpressure (Eugster et al. Citation2011, Wik et al. Citation2013), in the current speed (Scranton et al. Citation1993), and wind events (Keller and Stallard Citation1994). Increases in temperature also trigger ebullition emissions, since it reduces the solubility (Yamamoto et al. Citation1976) and increases the production of CH4 in sediments (Duc et al. Citation2010).
The accumulation of organic sediment is typically greater in areas close to the mouth of the tributaries. These areas are likely hot spots of CH4 emissions, because sediments of such areas are rich in substrates for methanogenesis and have low hydrostatic pressure and high temperature, favorable factors for CH4 production and release of bubbles (Keller and Stallard Citation1994, Maeck et al. Citation2013). In small reservoirs, areas of sediment accumulation represent a significant portion of the total surface area when compared with large reservoirs. Small reservoirs are capable of quickly accumulating sediments; such accumulation contributes to the volume loss of these environments (Glymph Citation1973, Maeck et al. Citation2013). Moreover, shallow reservoirs are more susceptible to the eutrophication process, due to their relatively low potential for dilution of the external nutrient load (Smith Citation2009). During the eutrophication process, the increased organic matter and nutrient stimulated microbial activity, consuming dissolved oxygen, result in an increase of anoxic conditions near the sediment and in a decrease of aerobic CH4 oxidation (Tranvik et al. Citation2009). Moreover, the eutrophication promotes primary production and autochthonous organic carbon production, which are favorable for methanogens. In this way, the eutrophication process creates perfect conditions for CH4 production, because it generates large quantities of organic matter followed by anoxic conditions (Segers Citation1998, Gonzalez-Valencia et al. Citation2014, Martinez-Cruz et al. Citation2016).
In contrast to large hydroelectric reservoirs (Barros et al. Citation2011), studies on CH4 emissions in small urban reservoirs are scarce, with few investigations in tropical regions (Martinez-Cruz et al. Citation2016). The spatial coverage of reservoir GHG emission measurements is also often limited, with many studies measuring emissions at fewer than 5 sites and very few studies having more than 10 sites (Deemer et al. Citation2016). Here we evaluated, in detail (15 sites), the spatial variations of CH4 ebullition within a small tropical hypereutrophic urban reservoir with an intense siltation process (Pampulha Reservoir, southeast Brazil). Furthermore, by covering 2 seasons (summer and winter), we aimed to investigate the importance of the main environmental factors (temperature and depth/hydrostatic pressure) regulating CH4 ebullition pathway in small urban reservoirs. We hypothesized that silted areas, especially those in areas near tributary mouths, represent hot spots of CH4 bubble emissions, resulting from the high sediment temperature and low depth found on those areas. This study aimed to demonstrate the importance of proper management of small urban reservoirs in order to prevent the formation and expansion of hot spot ebullition zones.
Study site
The Pampulha Reservoir (WGS84 19°51′09″ S, 43°58′42″W) is part of the “Pampulha Architectonic Complex” in the city of Belo Horizonte in southeast Brazil, recognized by UNESCO in 2016 as an Intangible Cultural Heritage of Humanity (). The reservoir was first established in 1938 and then re-inaugurated in 1958 following a dam rupture. The reservoir was built mainly to serve as a water source for the northern area of the city. However, the rapid and disorganized urbanization which took place in the watershed (97.9 km2) since the 1970s () caused intense eutrophication and siltation processes, with frequent algal blooms resulting in a rapid decay of the water quality (Barbosa et al. Citation1998, Torres et al. Citation2007).
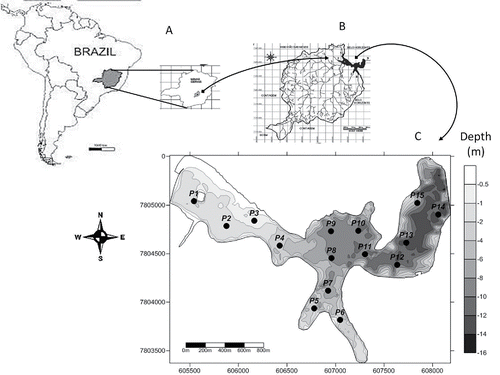
Figure 1. Location (A), watershed (B), and bathymetric map of Pampulha Reservoir indicating the distribution of the sampling points for the present study (C).
Since the 1980s, because of these hypereutrophic conditions and frequent cyanobacteria blooms (Giani Citation1994, Figueredo et al. Citation2016), the reservoir has not functioned as a water source. Although it is appealing to tourists and contributes to flood control, direct contact with its water through aquatic sports or navigation is prohibited. In appreciation of the deteriorated status of the reservoir, the Municipality of Belo Horizonte initiated a project in 2015 aiming to restore the water quality (SMOBI-SUDECAP Citation2016).
The tributaries contribute 1797.7 ton/yr of total solids in suspension to the reservoir (Torres et al. Citation2007), causing a significant loss of volume. The silting process in the Pampulha Reservoir is an old problem. Giani et al. (Citation1988) verified that one-third of Pampulha Reservoir surface area was lost due to siltation processes. Comparing the most recent bathymetric studies it is possible to verify that the reservoir lost 8.4% of its original volume between 2007 and 2010 due to an intense upstream siltation process, particularly at the mouth of the Sarandi and Ressaca streams, which are major tributaries. The silted area comprises an area of 0.62 km2, which is equivalent to 35% of the total area of the reservoir (Bezerra-Neto et al. unpubl. data; , ).
Table 1. Morphometric data of Pampulha Reservoir in 2007 and 2011. Sources: Resck et al. (Citation2007), Bezerra-Neto et al. (unpubl. data).
The sediment material of the Pampulha Reservoir is very fine-grained, consisting mainly of fine silt (<63 μm) and clay (<2 μm). Near the main tributaries, which are responsible for the main sediment load in the reservoir, the silt content is predominant, while near the dam, clay predominates (Friese et al. Citation2010). From the tributaries toward the dam, the water content in the sediment surface (0–5 cm) varies from 70 to 90%, while the total organic carbon content varies from 4.3 to 5.2% of the dry weight (SMOBI-SUDECAP Citation2016).
Materials and methods
Sampling summary
The sampling points were selected based on recent bathymetric maps to efficiently cover horizontal and vertical gradients in the reservoir. The silted areas were defined from the bathymetric studies conducted in 2010 and in 2007 (Bezerra-Neto et al, unpubl. data). These studies defined the silted areas as the ones that lost the largest volume between 2007 and 2010, being located in the riverine zone and mouth of the main tributaries of the reservoir. A total of 15 sampling points were selected approximately 350 m apart from each other. Of these 15 points, 6 were located at the mouth of the major tributaries, an area subjected to an intense siltation process (average depths of 1.5 m); 5 were located at the transition zone (average depth of 6 m); and 4 points were located within the pelagic zone close to the dam (average depth of 10 m) (). On each field day, the following parameters were measured at the sampling points: water depth, dissolved oxygen and the temperature of surface sediment using a multiparameter probe (Hydrolab DS5, Hach Inc.), and the altimetric water levels of the reservoir measured at the dam. A total of 10 days were sampled, 5 consecutive days in the wet summer (Feb) and 5 consecutive days during the dry winter (Jul) of 2013.
Ebullition emissions
The gases emitted by bubbles (ebullition) were collected at each sampling point () using an inverted funnel with a diameter of 0.70 m, connected to a graduated cylinder collector. The funnels and respective collection cylinders were filled with water, submerged to prevent contamination with atmospheric air, and then anchored. The anchors (3 kg each) were previously thrown into the water and, after they were settled in the sediment, we moved about 2 m from the anchoring point before deploying the funnels. This way, we avoided having the funnels remain right above the anchorage point. The funnels remained in this position for 24 h, and at the collection time, the volume of the collected gas and the depth of each sampling point were registered. Aliquots of 30 mL of the harvested gas were transferred to gasometric ampoules, protected from the sun, and refrigerated for later analysis in a gas chromatograph (GC; model Shimadzu 2014). The GC was equipped with a 30 m capillary column (PLOT Carboxen 1006) and a flame ionization detector (FID), calibrated using CH4 standards at concentrations of 1 ppmv, 50 ppmv, 250 ppmv, 500 ppmv, and 900 ppmv. The gas chromatograph analysis was completed within 48 h of collecting the funnels. This procedure was repeated over 5 d in each season.
The ebullition flux was calculated according to the following equation (UNESCO-IHA Citation2010):
(1)
(1)
Where, FE is the flux of CH4 via bubble (CH4/m2/d), C is the CH4 concentration in the sample (mg/m3), V is the volume of gas collected (m3), A is the funnel area (m2), and D is the sampling intervals (days).
Diffusive emissions
Diffusive CH4 emissions were measured using floating chambers at 3 locations in Pampulha Reservoir, also covering different zones and water depths: in the lotic zone–silted area (P02); in the transition zone (P08); and 3 in the pelagic zone, near the dam (P14; ). The active area of a chamber was 0.047 m2 and trapped a headspace of 1500 mL. It was equipped with a subsurface shield with a diameter of 0.5 m, which was hung 0.5 m below the chamber to avoid influence of bubbles. One gas sample was taken from the chamber after 0, 1, 2, and 4 min, counting from the initial moment when the chamber was placed on the water–air interface. The air samples inside the chambers (30 mL) were collected with 60 mL polyethylene syringes via 3-way valves and transferred to glass gasometric ampoules. All samples were taken between 08:00 and 12:00 h local time. CH4 concentrations were determined in laboratory within 24 h after collection, using a gas chromatograph, as described for the ebullition emissions.
The diffusive flux was calculated according to the following equation (UNESCO-IHA Citation2010):
(2)
(2)
Where FD is the CH4 diffusive flux (mg/m2/d), slope is the slope (a) from graph of [CH4] ppm vs. time (min), F1 is a conversion factor of ppm to μg/m3 (655.47 for CH4), F2 is a conversion factor from minutes to days (1440), V is the volume of air trapped in the chamber (m3), S is the surface of the floating chamber over the water (m2), and F3 is a conversion factor from μg to mg (1000). Only linear regressions with correlation coefficient (r2) above 0.90 were used in the calculations.
Data analysis
Data normality was checkedby the Shapiro–Wilk test. The spatial variation of ebullition emissions was tested using a nonparametric Kruskal–Wallis H test. The seasonal variation of surface sediment temperature was tested using the nonparametric 2-tailed Mann–Whitney U test. The correlation among parameters was investigated using linear regression analysis in SigmaPlot for Windows version 12.0 (Systat Software, Inc.).
A spatial model was applied to determine spatial gradients in the ebullition emissions and diurnal differences in point emissions using Surfer version 10.0 software. Modeling the spatial structures was carried out through variogram analysis. Variograms were used both to describe the spatial correlation among samples of a variable acquired in different locations and to spatially interpolate them using the kriging method (Bailey and Gatrell Citation1995).
Results and discussion
During the sampling period, the reservoir remained hypereutrophic according to the Trophic State Index for reservoirs (Carlson Citation1977), exhibiting anoxic conditions all along the water–sediment interface (Bezerra-Neto et al. unpubl. data). As the Pampulha Reservoir is not used to generate power, the water level is only modestly regulated and varied by less than 0.5 m with the maximum water levels registered (800.6 m above sea level) during the summer period and the lowest (800.1 m) during the winter period ().
Table 2. Parameters of water quality and altimetric water levels of Pampulha Reservoir during the studied period. Total phosphorous (TP); Total nitrogen (TN); Chlorophyll-a (Chl-a); Total suspended solids (TSS). Average values; minimum and maximum values in parentheses; n = number of samples. Source: Bezerra-Neto et al. (unpubl. data).
The ebullition fluxes varied significantly across the reservoir during both summer (H = 52.254, df 14, P < 0.001) and winter (H = 60.965, df 14, P < 0.001). The highest ebullition fluxes were measured in the summer (3071 mg CH4/m2/d) and in the winter (1253 mg CH4/m2/d) in sampling points with an average depth of 1.30 m (; , , ) and located near the mouth of the major tributaries of the Sarandi and Ressaca streams. Where siltation was observed, by comparing bathymetric maps from 2007 and 2010 (Bezerra-Neto et al. unpubl. data). Tributary mouths were considered as bubble hot spots as the ebullition emissions for these areas corresponded to 58% and 39% of the total ebullition emissions in summer and winter, respectively. This demonstrates the importance of the detailed spatial sampling approach to estimate emissions, even in a small reservoir. Assuming a sample design where only the central parts of the reservoir were sampled, this would have led to an underestimation of CH4 emissions by 70% in summer and 45% in winter. It is therefore important to apply a proper sampling design to represent even small reservoirs, to ensure that appropriate depth and temperature gradients are captured in each system, thus improving also the global estimations of CH4 emissions, by first reducing the uncertainties in the local scales.
Table 3. Ebullition and diffusive emissions, temperature of surface sediment, and depth of sampling points in Pampulha Reservoir. Average values; minimum and maximum values in parentheses; n = number of samples.
Figure 2. Variograms indicating the spatial tendencies of ebullition emissions during summer (A) and winter (B); diurnal variation (standard deviation (SD)) of ebullition emissions during 5 sampled days in summer (C) and winter (D) of 2013. Spatial variation of ebullition emissions at Pampulha Reservoir during summer (black circles) (H = 52.254, df 14, P < 0.001) and winter (open circles) (H = 60.965, df 14, P < 0.001) (E). Correlation between standard deviation of ebullition emissions and depth at sampling point (r2 = 0.13, P < 0.05) (F).
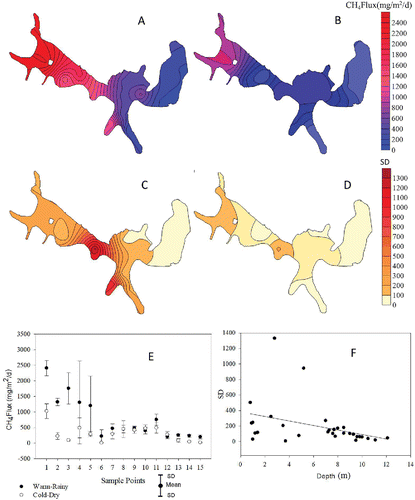
Our findings are supported by recent studies (DelSontro et al. Citation2011, Maeck et al. Citation2013). DelSontro et al. (Citation2011) recorded higher ebullition emissions at the mouth of tributaries to the Kariba Reservoir, and Maeck et al. (Citation2013) recorded a similar pattern in distinct reservoirs along the Saar River in Germany, also reporting a significant correlation between the sedimentation rate and ebullition emissions.
The highest day-to-day variation (highest standard deviation) was also registered in the silted area with a negative relationship between depth and daily variation of the emissions (r2 = 0.13, P < 0.05; , , ). Shallow areas are subject to greater effects of wind on bubble release from sediment of these areas. First, by inducing the formation of waves, which leads to short-term reductions in hydrostatic pressure. Secondly, waves transmit turbulent energy, which increases the shear stress on the sediment surface. The combination of these 2 effects triggers a release of CH4 bubbles from shallow sediments (Keller and Stallard Citation1994). Thus, episodic wind events may therefore explain elevated daily variations in these shallow areas. The granulometric characteristic of sediment is another factor that can regulate the release of bubbles. Sediments with higher porosity and connection between pores (sandy sediment) release the bubbles after their formation, while fine-grained sediments with lower porosity (silt and clay) retain the bubbles after formation. In very fine-grained sediments, as in the Pampulha Reservoir, the bubbles are released sporadically, when the accumulated gas pressure exceeds the pressure exerted by the fine sediment (Martinez and Anderson Citation2013, Ramirez et al. Citation2015).
The large spatial heterogeneity in ebullition fluxes recorded in this study seems related to spatial differences in sediment temperature and water depth. The sediment temperature differed significantly (U = 1.696, P < 0.001) between summer and winter. Moreover, sediment temperatures were higher in the shallower areas with a clear inverted relationship between water depth and sediment temperature in summer (r2 = 0.88, P < 0.0001) and winter (r2 = 0.51, P < 0.0001) (). We accordingly found strong evidence of the importance of 2 environmental conditions (temperature and depth) well known to affect rates of CH4 ebullition (Bastviken et al. Citation2004). Temperature and depth are expected to influence CH4 ebullition through absorption and loss of heat, which is greater within shallow sediments compared to deeper ones (Keller and Stallard Citation1994, Wik et al. Citation2014). In agreement with this we found a positive and significant correlation between sediment temperature and ebullition emissions both in summer (r2 = 0.74, P < 0.0001) and in winter (r2 = 0.28, P < 0.0001) (). Moreover, ebullition emissions were negatively related to depth (P < 0.001) in summer (r2 = 0.62), while not in winter (P > 0.05) when the spatial range in temperature was much lower (). While sediment temperatures were significantly higher in summer, sampling depths were similar between seasons (P > 0.05).
Figure 3. Linear regression between temperature of surface sediment and depth (summer r2 = 0.88, P < 0.0001 and winter r2 = 0.51, P < 0.0001) (A); ebullition emissions and temperature of surface sediment (summer r2 = 0.74, P < 0.001 and winter r2 = 0.28, P < 0.001) (B); and depth (summer r2 = 0.62, P < 0.001 and winter not significant P > 0.05) (C) at Pampulha Reservoir during summer (black circles) and winter (open circles).
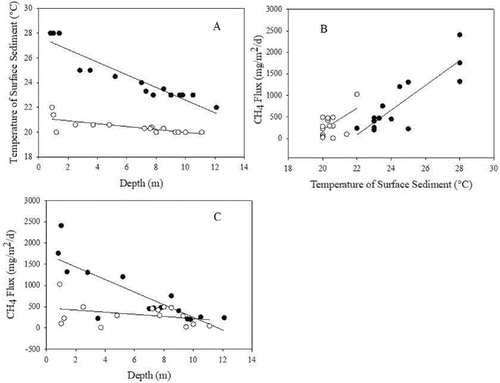
The ebullition emission is known to be higher when the CH4 formation within sediment is high and the hydrostatic pressure, which must be surpassed to allow for bubble liberation, is low (Eugster et al. Citation2011, Wik et al. Citation2013). As methanogenesis and the solubility of the CH4 are both strongly temperature dependent (Chanton and Martens Citation1988) it is not surprising that the highest emissions were found during summer. Furthermore, as temperature and depth covaried within the reservoir, especially during summer, and the hydrostatic pressure is dependent on the depth in both seasons, the ebullition emissions are related to a combination of both parameters (Joyce and Jewell Citation2003, Bastviken et al. Citation2004).
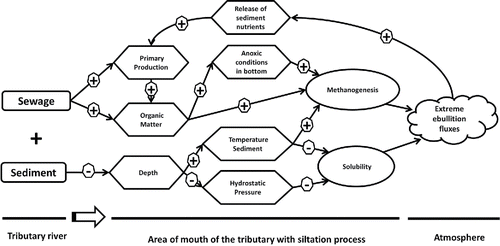
In urban, shallow, and hypereutrophic reservoirs such as Pampulha, accumulation of organic matter in sediments is caused by inadequate watershed management, which therefore stimulates CH4 emissions. According to Torres et al. (Citation2007), the tributaries of the Pampulha Reservoir contribute with the input of 360.7 ton/yr of particulate organic carbon. Figueredo et al. (Citation2016) estimated that the average phytoplankton biomass in this reservoir is 29.2 mm3/L, with more than 80% composed of cyanobacteria. Discharge of poorly treated domestic sewage and the reoccurring blooms of cyanobacteria in Pampulha Reservoir (Figueredo et al. Citation2016) provide high concentrations of organic matter in low oxygen sediments,creating a rich substrate for methanogenesis under anoxic conditions (Segers Citation1998, Gonzalez-Valencia et al. Citation2014, Martinez-Cruz et al. Citation2016). In addition, the accumulation of sediments in areas near the mouth of tributaries, due to suppression of riparian vegetation, results in a decrease in depth, thus reducing the hydrostatic pressure and increasing the temperature of the local sediment. These conditions enhance the production and decrease the solubility of CH4. This array of interconnected factors results in a hot spot of CH4 emissions caused by siltation. Additionally, hot spots of ebullition emissions, apart from contributing with significant amounts of greenhouse gases to the atmosphere, also have the potential to release nutrients and other elements stored into the sediment by resuspension during the release of bubbles (Bussman Citation2005), thus increasing primary production. In this way, the high ebullition events contribute to the eutrophication process, thus creating an ongoing cycle between these 2 processes ().
Figure 4. Conceptual model on effects of sewage discharges and siltation on creation of hot spots of CH4 emissions by bubbles.
Considering the discharge of domestic sewage, reoccurring algal blooms, high temperatures, and shallow waters, it is therefore not surprising that Pampulha Reservoir is a significant source of CH4 emission to the atmosphere. The mean areal surface emissions (diffusion plus ebullition) recorded was 804 (1.3–3070.5) mg CH4/m2/d in summer and 380 (3.7–1253.1) mg CH4/m2/d in winter, with annual average of 592 mg CH4/m2/d. As expected, ebullition was the predominant pathway, representing 97% and 83% compared to 3% and 17% diffusive emissions of CH4 emitted at the surface in summer and winter, respectively (). According to a recent estimate the global mean CH4 emissions from reservoirs are 120 CH4/m2/d (Deemer et al. Citation2016), compared to tropical reservoirs estimated to be 300 (20–1500) mg CH4/m2/d (St. Louis et al. Citation2000). Thus, the Pampulha Reservoir presents a considerably higher CH4 surface emission, exceeding the global mean and tropical region estimate in both seasons. These results reinforce the need for proper management not only to reduce the hypereutrophic condition, but also to reduce the siltation process, which in tropical reservoirs with low depth and warm sediments provide a CH4 emissions hot spot.
Conclusions
From our study in a eutrophic tropical reservoir, it appears that shallow areas with high siltation are hot spots of CH4 emissions, especially during warm summer conditions. Studies on inventories of CH4 emissions from reservoirs must therefore be aware of bathymetric conditions, which may cause high spatial heterogeneity of the major emission process (ebullition). Our results reinforce the important role of small eutrophic urban reservoirs, which under inadequate management are likely to become regional important sources of CH4 emissions. As the studied region is experiencing both increasing air temperatures and prolonged droughts (Jiménez Cisneros et al. Citation2014), enhanced CH4 emissions, through reduced water levels and higher water and sediment temperatures, will likely occur with ongoing climate changes. Proper water treatment is therefore essential to stop the deadly spiral (high temperature – eutrophication – ebullition events – climate change), along with implementation of restoration measures to reduce accumulation of sediments at the tributary mouths which are currently hot spots of CH4 emissions.
Acknowledgments
The authors thank the staff of LIMNEA laboratory for their technical assistance and help with logistics during the study. The authors are also thankful to Professor John Melack for the considerations and suggestions given to the manuscript. Finally, the authors thank an anonymous reviewer for suggestions and improvements on this manuscript.
Funding
This work was financially supported by the Support Foundation of the State of Minas Gerais Research (FAPEMIG) and the Higher Education Personnel Improvement Coordination Foundation (CAPES), which granted a scholarship to the first author. The authors LS Brighenti and PA Staehr were funded by CAPES (Proc. no. 88887.059683/2014–00).
References
- Bailey TC, Gatrell AC. 1995. Interactive spatial data analysis. Essex: Longman Higher Education.
- Barbosa F, Garcia FC, Marques MMGSM, Nascimento FA. 1998. Nitrogen and phosphorus balance in a eutrophic reservoir in Minas Gerais: a first approach. Rev Bras Biol. 58:233–239.
- Barros N, Cole JJ, Tranvik LJ, Prairie YT, Bastviken D, Huszar VLM, Del Giorgio P, Roland F. 2011. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat Geosci. 4:593–596.
- Bastviken D, Cole J, Pace M, Tranvik L. 2004. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Glob Biogeochem Cycl. doi:10.1029/2004GB002238.
- Bastviken D, Cole JJ, Pace ML, Van de Bogert MC. 2008. Fates of methane from different lake habitats: connecting whole-lake budgets and CH4 emissions. J Geophys Res Biogeosci. doi:10.1029/2007JG000608.
- Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. 2011. Freshwater methane emissions offset the continental carbon sink. Science. 331:50.
- Bussman I. 2005. Methane release through resuspension of littoral sediment. Biogeochemistry. 74:283–302.
- Carlson RE. 1977. Trophic state index for lakes. Limnol Oceanogr. 22:361–369.
- Chanton JP, Martens CS. 1988. Seasonal variations in the isotopic composition and rate of methane bubble flux from a tidal freshwater estuary. Glob Biogeochem Cycl. 2:289–298.
- Deemer BR, Harrison JA, Li S, Beaulieu JJ, DelSontro T, Barros N, Bezerra-Neto JF, Powers SM, Santos MA, Vonk JA. 2016. Greenhouse gas emissions from reservoir water surfaces: a new global synthesis. BioScience. doi:10.1093/biosci/biw117.
- DelSontro T, Kunz MJ, Kempter T, Wuest A, Wehrli B, Senn DB. 2011. Spatial heterogeneity of methane ebullition in a large tropical reservoir. Environ Sci Technol. 45:9866–9873.
- Downing JA, Prairie YT, Cole JJ, Duarte CM, Tranvik LJ, Striegl RG, McDowell WH, Kortelainen P, Caraco N F, Melack JM, et al. 2006. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol Oceanogr. 51:2388–2397.
- Duc NT, Crill P, Bastviken D. 2010. Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry. 100:185–196.
- Engle D, Melack JM. 2000. Methane emissions from an Amazon floodplain lake: enhanced release during episodic mixing and during falling water. Biogeochemistry. 51:71–90.
- Eugster W, DelSontro T, Sobek S. 2011. Eddy covariance flux measurements confirm extreme CH4 emissions from a Swiss hydropower reservoir and resolve their short-term variability. Biogeosciences. 8:2815–2831.
- Figueredo CC, Pinto-Coelho RM, Lopes AMM, Lima PH, Gücker B, Giani A. 2016. From intermittent to persistent cyanobacterial blooms: identifying the main drivers in an urban tropical reservoir. J. Limnol. doi:10.4081/jlimnol.2016.1330.
- Friese K, Schmidt G, de Lena JC, Nalini HA, Zachmann DW. 2010. Anthropogenic influence on the degradation of an urban lake–the Pampulha Reservoir in Belo Horizonte, Minas Gerais, Brazil. Limnologica. 40:114–125.
- Giani A. 1994. Limnology of Pampulha Reservoir: some general observations with emphasis on the phytoplankton community. In: Pinto-Coelho RMP, Giani A, Sperling E, editors. Ecology and human impacts on lakes and reservoirs in Minas Gerais with special reference to future development and management strategies. 1st ed. Belo Horizonte: Segrag. p. 151–163.
- Giani A, Pinto-Coelho RM, Oliveira SJM, Pelli A. 1988. Ciclo sazonal de parâmetros físico-químicos da água e distribuição horizontal de nitrogênio e fósforo no reservatório da Pampulha (Belo Horizonte, MG, Brasil). Ciên. Cult. 40:69–77.
- Glymph LM.. 1973. Summary: sedimentation of reservoirs. Geophys Monogr Ser. 17:342–348.
- Gonzalez-Valencia R, Sepulveda-Jauregui A, Martinez-Cruz K, Hoyos-Santillan J, Dendooven L, Thalasso F. 2014. Methane emissions from Mexican freshwater bodies: correlations with water pollution. Hydrobiologia. 721:9–22.
- Jimenez Cisneros BE, Oki T, Arnell NW, Benito G, Cogley JG, Doll P, Jiang T, Mwakalila SS. 2014. Freshwater resources. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, et al., editors. Climate change 2014: impacts, adaptation and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. p. 229–269.
- Joyce J, Jewell PW. 2003. Physical controls on methane ebullition from reservoirs and lakes. Environ Eng Geosci. 9:167–178.
- Keller M, Stallard RF. 1994. Methane emission by bubbling from Gatun Lake, Panama. J Geophys Res Atmos. 99:8307–8319.
- Maeck A, DelSontro T, McGinnis DF, Fischer H, Flury S, Schmidt M, Fietzek P, Lorke A. 2013. Sediment trapping by dams creates methane emission hot spots. Environ Sci Technol. 47:8130–8137.
- Martinez D, Anderson MA. 2013. Methane production and ebullition in a shallow, artificially aerated, eutrophic temperate lake (Lake Elsinore, CA). Sci Total Environ. 454:457–465.
- Martinez-Cruz K, Gonzalez-Valencia R, Sepulveda-Jauregui A, Plascencia-Hernandez F, Belmonte-Izquierdo Y, Thalasso F. 2016. Methane emission from aquatic ecosystems of Mexico City. Aquat Sci. 78:1–11.
- Megonigal JP, Mines ME, Visscher PT. 2005. Linkages to trace gases and aerobic processes. Biogeochemistry. 8:350–362.
- Myhre G, Shindell D, Breon FM, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, et al. 2013. Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner G, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. p. 659–740.
- Ostrovsky I, McGinnis DF, Lapidus L, Eckert W. 2008. Quantifying gas ebullition with echosounder: the role of methane transport by bubbles in a medium-sized lake. Limnol Oceanogr Meth. 6:105–118.
- Ramirez JA, Baird AJ, Coulthard TJ, Waddington JM. 2015. Ebullition of methane from peatlands: does peat act as a signal shredder? Geophys Res Lett. 42:3371–3379.
- Repo ME, Huttunen JT, Naumov AV, Chichulin AV, Lapshina ED, Bleuten W, Martikainen PJ. 2007. Release of CO2 and CH4 from small wetland lakes in western Siberia. Tellus B. 59:788–796.
- Resck R, Bezerra-Neto JF, Pinto-Coelho RM. 2007. Nova batimetria e uma avaliação ecológica de parâmetros morfométricos da Lagoa da Pampulha (Belo Horizonte, Brasil) [New bathymetry and an ecological evaluation of morphometric parameters of the Pampulha Reservoir (Belo Horizonte, Brazil)]. Geografias. 3:24–37.
- Scranton MI, Crill P, Angelis MA, Donaghay PL, Sieburth JM. 1993. The importance of episodic events in controlling the flux of methane from an anoxic basin. Glob Biogeochem Cycl. 7:491–507.
- [SMOBI-SUDECAP] Secretaria Municipal de Obras e Infraestrutura – Superintendência de Desenvolvimento da Capital. 2016. Recuperação da Qualidade da Água da Lagoa da Pampulha [Recovery of the water quality of the Pampulha Reservoir]; [cited 18 Oct 2016]. Available from: http://smma.pbh.gov.br/sgcedocs/formulario.html.
- Segers R. 1998. Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry. 41:23–51.
- Smith VH. 2009. Eutrophication. In: Likens G, editor. Biogeochemistry of inland waters: a derivative of encyclopedia of inland waters. 1st ed. San Diego (CA): Academic Press. p. 617–629.
- St. Louis VL, Kelly CA, Duchemin É, Rudd JW, Rosenberg DM. 2000. Reservoir surfaces as sources of greenhouse gases to the atmosphere: a global estimate. Reservoirs are sources of greenhouse gases to the atmosphere, and their surface areas have increased to the point where they should be included in global inventories of anthropogenic emissions of greenhouse gases. Bioscience. 50:766–775.
- Torres IC, Resck RP, Coelho RMP. 2007. Mass balance estimation of nitrogen, carbon, phosphorus and total suspended solids in the urban eutrophic, Pampulha Reservoir, Brazil. Act Limnol Brasil. 19:79–91.
- Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, Dillon P, Finlay K, Fortino K, Knoll LB, et al. 2009. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr. 54:2298–2314.
- [UNESCO–IHA] United Nations Educational, Scientific, and Cultural Organization – International Hydropower Association. 2010. GHG measurement guidelines for freshwater reservoirs; [cited 25 Aug 2013]. Available from: www.hydropower.org/ghg-measurement-guidelines.
- Wik M, Crill PM, Varner RK, Bastviken D. 2013. Multisource measurements of ebullition methane flux from three subarctic lakes. J Geophys Res Biogeosci. 118:1307–1321.
- Wik M, Thornton BF, Bastviken D, MacIntyre S, Varner RK, Crill PM. 2014. Energy input is primary controller of methane bubbling in subarctic lakes. Geophys Res L. 41:555–560.
- Yamamoto S, Alcauskas JB, Crozier TE. 1976. Solubility of methane in distilled water and seawater. J Chem Eng Data. 21:78–80.
