Abstract
Knopik J, Newman R. 2018. Transplanting aquatic macrophytes to restore the littoral community of a eutrophic lake after the removal of common carp. Lake Reserve Manage. 34:365–375.
Six native submersed aquatic macrophyte taxa were transplanted to a eutrophic lake (Lake Susan, Minnesota) to promote the growth and expansion of native taxa after the removal of common carp (Cyprinus carpio). Muskgrass (Chara spp.), wild celery (Vallisneria americana), northern watermilfoil (Myriophyllum sibiricum), bushy pondweed (Najas flexilis), water stargrass (Heteranthera dubia), and flat-stem pondweed (Potamogeton zosteriformis) were transplanted in a series of shallow (0.5–1.0 m) and deep (1.0–1.5 m) experimental plots around the lake. Survival and expansion of plants were measured over 4 yr and compared against environmental factors. Transplantation of whole plants in shallow water was generally successful, but plants in depths ≥1.4 m failed to persist. Light availability was the most important factor determining success. Water stargrass was the most successful, with high long-term survival and substantial expansion. Wild celery had high survival, but slow and limited expansion. Bushy pondweed had variable survival, but when it survived it generally expanded well. Muskgrass and northern watermilfoil had poor survival and expansion. Transplanting whole submersed aquatic macrophytes can help to restore the littoral community in degraded systems, but ecological stressors such as common carp should first be addressed. Poor mid-summer water clarity will limit the depth and distribution of successful transplants and taxa that survive. Taxa with large perennial structures such as water stargrass and wild celery are more likely to establish and persist, but the annual bushy pondweed was also able to grow and spread.
Aquatic macrophytes play a significant role in maintaining water quality and ecosystem health by reducing sediment re-suspension and providing habitat for aquatic organisms ranging from algae-consuming invertebrates to fish and other wildlife (Horppila and Nurminen Citation2003, Sondergaard et al. Citation2003, James et al. Citation2004). A healthy aquatic macrophyte community consists of a high diversity of native taxa (Madsen Citation1997). High densities of invasive common carp (Cyprinus carpio) uproot aquatic macrophytes and stir up sediments (Weber and Brown Citation2009), encouraging a turbid water state with a low density of macrophytes (Schrage and Downing Citation2004, Bajer et al. Citation2009). Reducing the population of common carp can increase the distribution and abundance of the aquatic plant community (Bajer et al. Citation2009). However, it can take several years for aquatic macrophytes to establish in high densities after fish removal (Hanson and Butler Citation1994), and often the taxa that do establish are nonnative invasive taxa (Lauridsen et al. Citation1994, Lougheed et al. Citation2004, Baker and Newman Citation2014). Manipulation of the littoral plant community may be necessary to promote the establishment of native vegetation rather than nonnative invasive taxa.
Transplanting can be an effective method for introducing submersed aquatic macrophytes into water bodies where few propagules exist (Smart et al. Citation1998, Lauridsen et al. Citation2003, Smiley and Dibble 2006). The distribution of aquatic macrophytes, and subsequent success of transplanting them, is related to a number of environmental factors including light availability (Scheffer Citation1998), temperature (Barko and Smart Citation1981), water level fluctuation (Fleming et al. Citation2012), sediment structure and fertility (Barko and Smart Citation1986, Doyle Citation2001), and timing of transplants (Vanderbosch and Galatowitsch Citation2011). It has also been shown that protection from herbivores and wave energy can be important for transplanting aquatic macrophytes (Smart et al. Citation1996). Previous studies have used barriers such as wire mesh (Smart et al. Citation1996, Lauridsen et al. Citation2003) to protect plants, but these structures can pose a recreation hazard and increase cost to littoral restoration projects. Stands of floating-leaf macrophytes (e.g., water lily stands) may also provide wave energy reduction and protection for transplanted macrophytes until establishment.
Although transplanting submersed macrophytes as a means for littoral community restoration has been shown to be successful, the majority of the case studies were done in reservoirs in southern latitudes of the United States or in more temperate climates of Europe (Doyle et al. Citation1997, Lauridsen et al. Citation2003, Dick et al. Citation2004a, Citation2004b, Fleming et al. Citation2011). Few examples from natural lakes in northern North America exist (Storch et al. Citation1986). Quantifying the growth and expansion of the transplants, along with environmental variables related to submerged macrophyte growth such as depth, light intensity, sediment texture, timing of transplanting, soil organic matter, and available nitrogen, may help further define the most important factors that can lead to success in future transplanting.
The objective of our study was to determine if transplantation can be used to establish new submersed macrophyte species after carp removal in a continental-climate Minnesota lake. In addition, we wanted to compare the environmental variables that may affect the survival and colonization rate, and determine if floating-leaf stands could provide protection from wave activity to enhance transplant success.
Methods
Site description
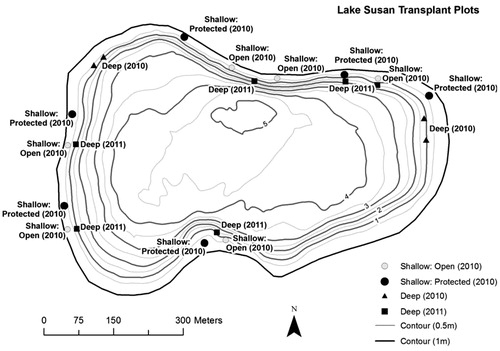
Lake Susan is a natural, eutrophic lake in Carver County, Minnesota, USA. The oval lake (shoreline development = 1.15) has a surface area of 36 ha, a mean fetch of 600 m (390–850 m), and maximum depth of 5.2 m (). Lake Susan had low summer Secchi depths (0.6 m), few aquatic macrophyte taxa in very low abundance, and a high abundance of common carp (Bajer and Sorensen Citation2012, Citation2015). Using under-ice seining in the winter of 2009, approximately 78% of the carp were removed from the lake (carp standing stock reduced from 307 kg/ha to 65 kg/ha) in an attempt to determine if removal of carp would enhance water clarity and improve the native plant community (Bajer and Sorensen Citation2015).
Transplant plot methodology
It was suspected there were few viable aquatic plant propagules in the benthos, due to sustained high carp densities for decades. Starting in 2010, 6 native submersed aquatic taxa were transplanted from nearby Lake Ann, Carver County, Minnesota, into Lake Susan to promote the growth and expansion of native plants. The source lake (Lake Ann) is located about 3 km upstream of Lake Susan. It is within the same watershed and had good water clarity (summer Secchi depths of 2.5–4 m) and high species richness (25 submersed, emergent, and floating-leaf species).
Transplant selection criteria considerations included that all species were native to Minnesota, expected to have high likelihood of success as determined by Smart et al. (Citation1996), in adequate abundance in the source lake, and not already found in Lake Susan. The 6 species were muskgrass (Chara spp.), wild celery (Vallisnaria americana), northern watermilfoil (Myriophyllum sibiricum), bushy pondweed (Najas flexilis), water stargrass (Heteranthera dubia), and flat-stem pondweed (Potamogeton zosteriformis). Water stargrass was transplanted in only shallow plots (<1 m) and flat-stem pondweed was transplanted in only deeper plots (∼1.4 m), matching their distribution in the source lake.
Transplants were collected from Lake Ann by snorkeling and wading in 0.5–1.5 m depths. A small garden spade was used to dig up the sediment around the roots of mature plants; care was taken to not damage roots and rhizomes and to ensure only the preselected species were collected. The roots were gently rinsed of sediment and whole plants were placed in a large cooler with like taxa and lake water, stored at room temperature overnight, and transplanted into Lake Susan the next day.
Transplant plots were located away from developed areas and locations were recorded by GPS. Each plot contained 5 transplant sites, spaced 2 m apart, and marked with a small labeled PVC pipe pushed into the substrate to aid future monitoring. One species was randomly selected to be transplanted at each site, where 10 individual plant stems were planted within a 0.25 m2 area. Existing vegetation, if present, was removed by hand prior to transplanting. Bare roots were placed in a small hand-dug hole (approximately 5 cm deep) and a 10 cm steel sod staple was used to hold the roots in place, which were then covered with sediment. Muskgrass, which lacks roots, was transplanted as a cluster approximately 500 cm3 (1 cluster was considered 1 stem) and held to the substrate with a sod staple. Sediment at transplant sites tended to be sandy with a mean bulk density of 1.26 g/mL±0.09 SE () and mean organic matter of 1.66% ± 0.25 SE (Knopik Citation2014).
Table 1. Summary of mean (±1 SE) sediment bulk density, mean percent organic matter, and mean total nitrogen in soil samples taken July 2011.
Shallow plots
Twelve plots of 5 taxa (muskgrass, water stargrass, northern watermilfoil, bushy pondweed, and wild celery) were transplanted at shallow (0.5–0.75 m depth) locations distributed around the lake on 1 August 2010. Six plots were planted shoreward of lily stands (protected), and 6 plots were open to wave action (open) to evaluate whether stands of floating-leaf species, white water lily (Nymphea odorata), yellow waterlily (Nuphar variegata), and water lotus (Nelumbo lutea), provided adequate wave protection. Transplant plot locations were distributed around the undeveloped stretches of shorelines to minimize human interference and to maximize potential for lake-wide colonization. One “protected” plot and 1 “open” plot were located near each other in an attempt to have similar fetch, shading, and substrate characteristics ().
Deeper plots
Transplant success in deeper water was tested at 4 additional plots (2 plots on the east side and 2 on the west side of the lake). The plants were transplanted in depths ranging from 1.2 m to 1.6 m on 22 July 2010 (). The 5 species transplanted were muskgrass, flat-stem pondweed, northern watermilfoil, bushy pondweed, and wild celery.
Six additional deeper plots were transplanted on 24 June 2011 using the same 5 taxa listed above to determine if earlier planting improved the success of the deeper transplants. The transplanting was timed to allow the plants at least 3–4 weeks to establish in Lake Susan before Secchi transparency dropped below the transplanting depth of 1.6 m.
Plot assessment
Transplants were assessed at each site for survival and area of coverage during the growing season. Survival was based on the presence of any stems of the transplanted taxa growing within 1 m of the transplant site post and expressed as the proportion of sites with transplants present. Expansion of coverage at the transplant sites was measured to further understand if the transplanted taxa would expand coverage within the littoral zone. Coverage was calculated by measuring the area of homogenous growth (cm2) as well as the area of presence. The area of homogenous growth was defined as the area in which approximately ≥90% of the plants were made up of the transplanted taxa (). A large area of homogenous growth would indicate monotypic stand development of the taxa. The area of presence was defined as the area in which the species was present, but not necessarily dominant (). A large area of presence would indicate the taxon is colonizing the littoral area as part of a more heterogeneous plant community. Mean area of presence and homogenous growth were calculated based on sites where plants were present (zeros were not included) to indicate expansion where plants survived. The summer maximum mean area for each taxa was summarized per year. Shallow transplants were assessed every 3–4 weeks during the summers of 2010 through 2013, and once in 2014. Deep transplants were assessed about every 4 weeks during the growing seasons of 2010 and 2011, twice in 2012, and once in 2013.
Figure 2. Example of plant growth assessment with area of presence and homogenous area of growth indicated. This site was planted with wild celery (Vallisneria americana).

To monitor natural recruitment of aquatic macrophytes after carp removal, point-intercept surveys were conducted in the summers of 2009–2014 to determine the frequency of occurrence of taxa present. Following methods outlined by Madsen (Citation1999), GIS software was used to generate 146 survey points in a 50 m systematic square grid across the lake. A GPS was used to navigate a boat within 5 m of each point. At each point, depth was recorded and vegetation was sampled with a weighted double-headed rake (0.33 m wide) attached to a rope, tossed into the lake, and dragged along the lake bottom for approximately 3 m to cover 1 m2. Vegetation on the rake was identified to species level and frequency of occurrence was determined for the points sampled within the littoral zone (≤4.6 m depth). Borman et al. (Citation2014) was used as the taxonomic authority.
Environmental variables
In situ limnological variables were measured at 0.5 m depth intervals in the water column at the deepest part of the lake biweekly between April and October. Water temperature (C) and dissolved oxygen (mg/L) were measured with a YSI 50B electronic meter. Photosynthetically active radiation (PAR) was measured with a LI-COR 189 digital meter and LI-192 quantum sensor. The depth at which surface PAR declined to 5% was used to compare PAR among years. Secchi depth was recorded to the nearest 0.1 m. PAR was also measured in August 2011 at 0.1 m intervals at 2 sites per plot, and PAR attenuation was compared between shallow open, shallow protected, and deep plots.
In August 2011, a sediment sample was collected with a 5 cm diameter PVC core tube at each end of the transplant plots, resulting in 2 samples per plot (∼8 m apart). The top 10 cm of sediment was subsampled and analyzed for sediment bulk density, organic matter, and total nitrogen.
Bulk density (g/mL) was determined from a 10 mL subsample of homogenized sediment. The percent organic matter was determined by combusting the sample for 4 h at 550 C. Ammonium-N was measured in the pore water centrifuged from a 50 mL subsample of homogenized sediment using an ammonia electrode (Orion model 9512). The exchangeable ammonium-N was extracted from the remaining subsample with a 2 molar KCL solution (Keeney and Nelson Citation1982), then measured with the ammonia electrode. The pore water nitrogen and exchangeable nitrogen were combined for macrophyte-available N.
The potential influence of fetch and wind direction was tested by comparing transplant survival by year among plots with similar fetch/direction. Summer prevailing wind direction is from the WSW (NOAA Citation2012), so plots along the northern and eastern shoreline (with higher fetch/direction) were in one group, and plots along the southern and western shoreline were in the other group ().
Statistical analysis
Statistical analyses were completed using R statistical software version 2.13.1 (R Development Core Team Citation2011) and Microsoft Excel. Survival was evaluated separately from expansion. Single-factor ANOVA analysis was used to compare survival to year and fetch. For expansion analysis, only sites with surviving plants were analyzed to assess expansion in the sites where the taxa survived. Single-factor ANOVA analysis of expansion was performed separately for shallow sites and deep sites, with homogenous area, and again with area of presence. A single-factor ANOVA was conducted for each taxa to compare mean area of presence for open vs. protected sites.
Results
Shallow transplant plots
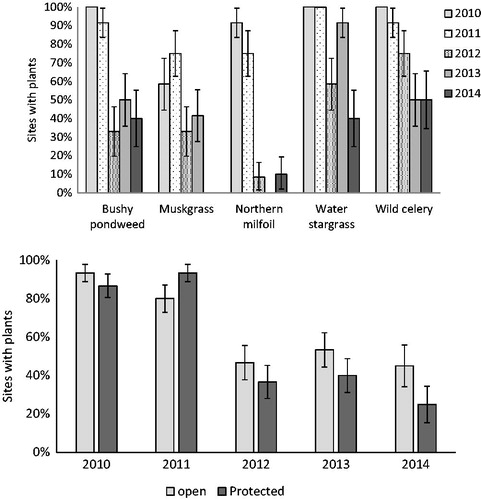
By the end of the first growing season (2010), 100% of the water stargrass, wild celery, and bushy pondweed sites contained viable plants, and 92% of northern watermilfoil sites and 58% of muskgrass sites contained viable plants (). Survival over the 5 yr decreased (P = 0.005), especially after 2012. By 2014, bushy pondweed, water stargrass, and wild celery were found in at least 40% of sites, but northern watermilfoil was found at only 10% of sites and muskgrass was not found ().
Figure 3. Survival of plant taxa in shallow plots by year (top) and overall survival by protection treatment (bottom).
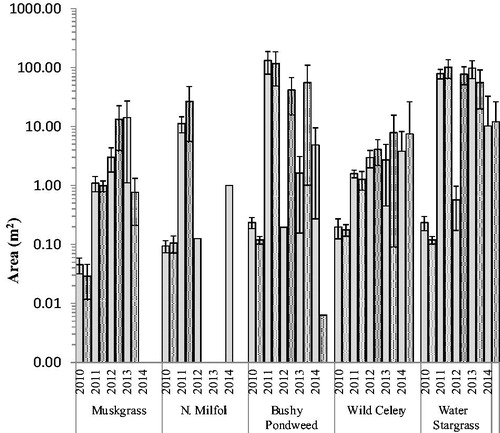
None of the transplanted taxa grew into sizable monotypic stands as measured by the homogenous area of growth (). Across all years, water stargrass in 2011 had the largest mean homogenous area of 3.1 m2 (). During the initial (2010) growing season, no plants expanded beyond the original planting area of 0.25 m2 for either homogenous area () or area of presence ().
Figure 4. Mean homogenous area of growth at open (hollow) and protected (stippled) sites by year of transplant in shallow plots. Initial planted area (indicated by dashed line) was 0.25 m2. Note area axis is in logarithmic scale of base 10. Error bars are ±1 SE.
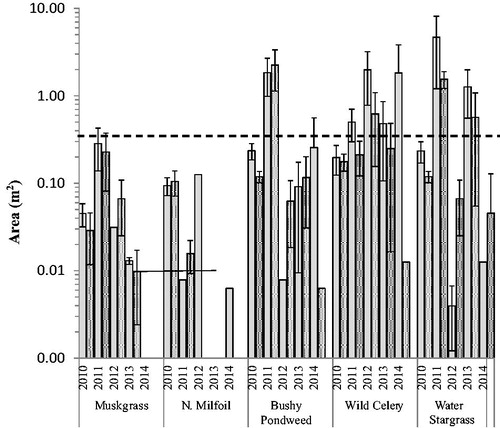
Figure 5. The mean area of presence of plants at open (hollow) and protected (stippled) sites by year of transplant in shallow plots. Initial area planted (indicated by dashed line) was 0.25 m2. Note area axis is in logarithmic scale of base 10. Error bars are ±1 SE.
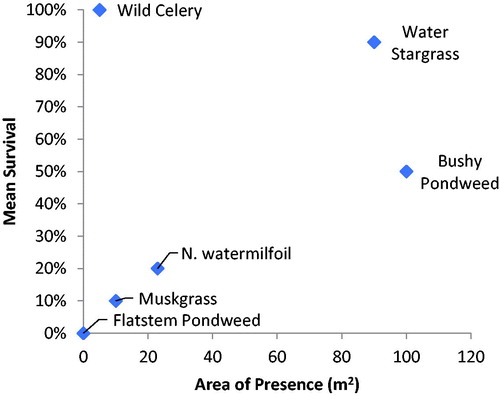
There was wide variation between taxa in the mean area of presence. Comparing across all years, water stargrass generally had the greatest area of presence, ranging from 89.6 m2 ± 24.8 SE in 2011 to 11.1 m2 ± 9.0 SE in 2014 (). Bushy pondweed also had large but more varied areas of presence, ranging from 124.1 m2 ± 61.2 SE in 2011 to 2.33 m2 ± 1.8 SE in 2014. Wild celery had slower, but consistent increases in area of presence, ranging from 1.4 m2 ± 0.3 SE in 2011 to 5.6 m2 ± 5.1 SE in 2014. Northern watermilfoil showed an initial increase of area of presence to 18.9 m2 ± 12.2 SE in 2011, but it was not found in 2013 and only 0.49 m2 ± 0.48 SE of coverage in 2014. Muskgrass had highly varied areas of presence ranging from less than 1.0 m2 in 2011 to 8.1 m2 ± 5.3 SE in 2012, but it was not found at all in 2014.
Fetch did not influence transplant survival as there was no difference in survival by site location (P = 0.60). There was also no difference in survival between open and protected sites (P = 0.67; ). Between open and protected sites, there was no difference in homogenous area (P = 0.928; ) nor area of presence (P = 0.097; ), and no individual taxa showed a difference in homogenous area or area of presence between open and protected. There was high variation in expansion among open and protected sites between years and taxa. For example, in 2012 the area of coverage in protected sites containing bushy pondweed and water stargrass increased in both homogenous area () and area of presence (). However, the opposite was true for northern watermilfoil. Comparing both survival and the area of presence there appeared to be 2 general categories (): taxa that survived and/or expanded well (wild celery, water stargrass, bushy pondweed), and taxa that had lower survival and expansion (muskgrass, northern watermilfoil).
Deeper transplant plots
Plants transplanted in 2010 at deeper depths (1.4 m) generally failed to establish. The plants all looked dead 1 mo after planting. Evaluation of these plots after 1 yr found a few individual stems of flat-stem pondweed at 3 of the 4 sites, and 1 wild celery plant at 1 site. None of the transplanted taxa were found growing near any of the sites in 2012.
The earlier timing of deep transplants in 2011 showed better initial results compared to the later plantings of 2010. Many of the flat-stem pondweed and northern watermilfoil plants showed some initial growth and development of adventitious roots. Flat-stem pondweed and wild celery both had a 67% survival, with at least 1 plant found in 4 of the 6 sites in August 2011. However, the average homogenous area and area of presence (0.01 m2 and 0.05 m2, respectively) was less than 0.25 m2, the initial area planted. A few individual plants survived, but most of them failed. After 1 mo, bushy pondweed was found growing in only 1 site. Muskgrass survived in none of the sites. Overwinter survival was poor and assessments in spring 2012 found only a single rosette of wild celery growing at 2 locations (33% of the sites). Subsequent assessments in 2012 and 2013 failed to locate any surviving deep transplants.
Environmental factors
Temperature and dissolved oxygen profiles indicate the lake typically became stratified in June and remained so throughout the summer. A thermocline generally formed below 2 m and the hypolimnion became hypoxic (≤ 2 mg/L) below 3.5 m (Knopik Citation2014, Bajer and Sorenson 2015).
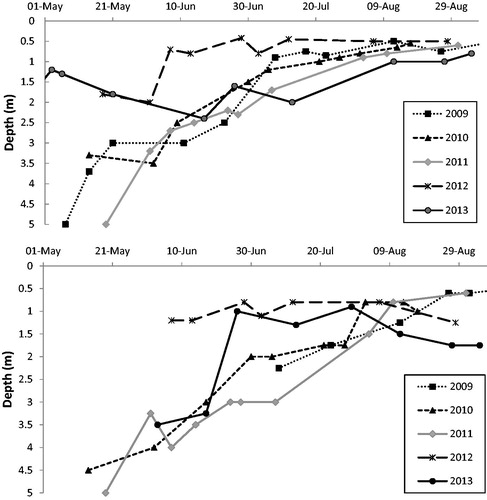
There was an increase in clarity during spring and early summer (May–Jun) 2010 and 2011, after the removal of carp in 2009. However, the increased clarity did not persist for the duration of the summer as Secchi depth and subsurface PAR declined around mid-July (). PAR at 1.5 m was usually <5% of surface light by early August, with the exception of 2010 and 2012 when PAR at 1.5 m was <5% in early and mid-June, respectively (). This decrease in clarity was driven by increased chlorophyll a (Bajer and Sorenson 2015). There was no difference in PAR (P > 0.05) just above the sediment in shallow sites between open (1.8% ± 0.4 SE) and protected (2.1% ± 0.3 SE) sites, nor between deep transplant locations (1.6% ± 0.3 SE) when measured in August 2011.
The available sediment N varied little among sampling locations, with a mean of 0.0037 mg/g ± 0.001 SE (), and no difference in available sediment N between protected and open sites, or deep sites (P = 0.13).
Lake-wide plant surveys
In 2008, prior to carp removal, only 2 submersed taxa were reported from Lake Susan, coontail (Ceratophyllum demersum) and Eurasian watermilfoil (Myriophyllum spicatum) (Bajer and Sorensen Citation2015). In 2009, after carp removal, 5 additional submersed taxa were found: Canada waterweed (Elodea canadensis), curlyleaf (Potamogeton crispus), narrow leaf (P. pusillus), sago (Stukenia pectinata), and horned pondweed (Zannichellia palustris). After this initial appearance of new taxa, all but 2 of the 6 new taxa that appeared in surveys, Illinois pondweed (P. illinoensis) and white water buttercup (Ranunculus aquatilis), were transplants. By 2014, 4 of the transplanted taxa (bushy pondweed, water stargrass, wild celery, and flatstem) were found in point intercept surveys near the areas where they were transplanted. Transplanted taxa accounted for the remaining increase in taxa diversity.
Plant frequency of occurrence increased from 40% in 2009 to almost 60% in 2010 and remained above 45% in 2011 but declined to 30% in 2012 and subsequent years. The number of submersed plants per point also declined from an average of ≥1.27 in 2009, 2010, and 2011 to 0.56 ± 0.14 in 2012 and subsequent years. The maximum depth of plant occurrence decreased from ≥3.1 m in 2009–2011 to 2.0 m in 2012 with poor water clarity, but increased to 2.9 m in 2013 and 2014.
Discussion
Transplanting was successful in establishing plants in the lake. All of the transplanted taxa planted in shallow sites were found in the lake after 1 yr, 4 of the taxa persisted at transplant sites through 2014, and 4 expanded enough to be found in point-intercept surveys (bushy pondweed, water stargrass, wild celery, and flat stem). Natural recruitment was not found to be the main factor in increasing the submersed species diversity in Lake Susan beyond the year after carp removal, as only 2 taxa naturally recruited (Illinois pondweed and white water buttercup) after 2010. Natural recruitment appeared to respond quickly to carp removal, which suggests that waiting a year or two before transplanting will allow for strategic addition of new taxa. The transplants failed to persist at deeper (>1.4 m) sites; only a few stems of flat-stem pondweed and wild celery were found in the year after planting and these did not further persist, although flat-stem did appear in a later point-intercept survey. Transplants should be monitored for several years after planting to assess establishment and expansion. Few studies have reported assessments beyond the first year after transplanting (Storch et al. Citation1986, Lauridsen et al. Citation2003).
Light availability was an important factor in survival of taxa, illustrated by the success of transplants at shallow locations vs. the failure at deeper locations and the reduced survival and expansion in 2012 when clarity was especially poor. By early August of most years, PAR was generally ≤5% of surface light at 1.5 m depth. Madsen (Citation1991) found that, although light compensation point varied between species, an average of 7% surface PAR was needed for most species to survive. The early and persistent low water clarity during 2012 likely inhibited establishment of the deep transplants from 2011 and suppressed and reduced the expansion of the shallow transplants. The depth of 5% surface PAR in 2012 was less than 1.25 m from early June and was 0.5 m for most of the summer. In addition to reducing transplant success, the lake-wide abundance of plants was reduced in 2012 and the maximum depth of plant occurrence was reduced to 2 m. Transplant survival in 2012 was decreased for all taxa; bushy pondweed, muskgrass, and water stargrass showed a rebound in survival in 2013 with better clarity. The better persistence of flat-stem pondweed and wild celery at deeper sites may be due to their lower light compensation points. Wild celery plants provided 5% of surface light were able to produce tubers (Kimber et al. 1995), however, plants did not survive at 2% of surface light. Still, the poor water clarity and low light penetration in Lake Susan may explain the lower than expected area of presence of wild celery in shallow sites.
Water stargrass and wild celery, both with large perennial structures, exhibited the highest survival in this study. Taxa with large vegetative structures have larger carbohydrate reserves, which allows for faster initial growth and carbohydrate levels generally sufficient to overwinter (Madsen Citation1991). Perennial submersed macrophytes often produce twice as many vegetative propagules than seeds, and vegetative propagules are generally better at overwintering than seeds (Madsen Citation1991). Taxa with annual life history traits such as muskgrass and flat-stem pondweed generally had lower survival (). The primary means of overwintering by annual plants is through the production of seeds or spores. Although annuals were noted as having lower survival rates, seed production can be a better means of broadcasting propagules greater distances (Madsen Citation1991). This explains why bushy pondweed, with variable survival, can be considered successful; where it did survive, it grew and expanded well. Northern watermilfoil, however, did not fit the pattern of either perennial or annual groups in our study.
Factors influencing expansion were not clear. Taxa using fragmentation strategies, such as water stargrass and bushy pondweed, had the greatest area of presence (). Water stargrass had good survival and most sites had high expansion. The use of runners by water stargrass likely led to increased expansion and success, as noted by Smart et al. (Citation1996). Wild celery had very good overwinter survival each year, but less expansion of coverage. The slow rate of expansion in wild celery was unexpected as it has both a large rhizome and the use of runners. Smart et al. (Citation1998) also noted that wild celery generally expanded rapidly. Low Secchi transparency and PAR penetration may have limited wild celery expansion in our study as none of the transplanted taxa developed large dense homogenous stands. When wild celery transplants expanded beyond their original planted areas, they generally grew in scattered low-density clusters.
Managers should anticipate the possible effects of year-to-year variability in water clarity (James et al. Citation2004). Additional actions, such as alum treatment, may be needed to maintain clarity and truly restore the submersed plant community. All of the taxa we transplanted may do well in lakes with good summer-long water clarity, but in lakes with lower clarity, bushy pondweed, water stargrass, and wild celery may be the best choices. Maximum successful transplanting depth will be influenced by minimum summer clarity and extreme years should be anticipated.
Protection by floating-leaf stands did not significantly affect the outcomes of transplanting during this study. This may have been because the study lake was small, with a maximum fetch of only 850 m. There was also no apparent effect of fetch or wind direction on survival. During a 2009 pilot study, wire fence barriers were installed around 2 of 4 trial transplant plots. There was no significant difference between fenced and unfenced plots after 1 yr (Knopik Citation2014). Protective barriers may not have been as beneficial on Lake Susan once the carp abundance was greatly reduced and there were few benthivorous fish to uproot plants. The density of other macro-herbivores such as waterfowl or muskrats was not quantified, although personal observation indicated very low abundance of each, which is consistent with similar suburban area lakes. Lakes with uncontrolled herbivores or large fetches may need protective exclosures to be effective (e.g., Smart et al. Citation1998, Lauridsen et al. Citation2003, Fleming et al. Citation2011).
Further research is needed to provide greater understanding of the factors that predict expansion and colonization of plants in the littoral zone. More focus on the timing of transplanting may be beneficial for increasing the successfulness of transplanting in deeper areas. Earlier planting may allow more time to overcome transplant shock and adapt to the available light conditions. Size of plant or perennial structure can influence plant health and ability to withstand transplant shock (Zimmerman et al. Citation1995), however this variable was not measured during our study.
Our study showed that transplanting submersed aquatic macrophytes can be successful as a means of restoring the littoral community in northern climates. However, before an aquatic macrophyte restoration is attempted, the ecological stressors that prohibit the natural recruitment of macrophytes, the population of carp in our case, need to first be addressed (Smart et al. Citation2005). Success should be evaluated over several years as success during the initial year of transplanting did not ensure long-term establishment. Sustained water clarity throughout the growing season is needed to maximize the survival and expansion of transplants and the depth of successful transplants.
Acknowledgments
This research was funded by the Riley Purgatory Bluff Creek Watershed District with additional support from the USDA National Institute of Food and Agriculture, Hatch grant MIN-41-074. We thank James Johnson, Ajay Jones, Jonathan JaKa, Holly Sigler, and Teran Smith for assistance with data collection and Dr. Peter W. Sorensen, Dr. David Biesboer, Dr. Przemek Bajer, and the watershed district for sharing data, lab, and field resources. Drs. Susan Galatowitsch and Peter Sorensen provided helpful input on early versions of this manuscript. Comments by the reviewers and Associate Editor helped us improve the manuscript.
References
- Bajer PG, Sorenson PW. 2012. Using boat electrofishing to estimate the abundance of invasive common carp in small Midwestern lakes. N Am J Fish Manage. 32:817–822. doi:10.1080/02755947.2012.690822.
- Bajer PG, Sorensen PW. 2015. Effects of common carp on phosphorus concentrations, water clarity, and vegetation density: a whole system experiment in a thermally stratified lake. Hydrobiologia. 746:303–311. doi:10.1007/s10750-014-1937-y.
- Bajer PG, Sullivan G, Sorenson PW. 2009. Effects of a rapidly increasing population of common carp on vegetation cover and waterfowl in a recently restored Midwestern shallow lake. Hydrobiologia. 632:235–245. doi:10.1007/s10750-009-9844-3.
- Baker LA, Newman RM. 2014. Managing the biological, economic, and social aspects of sustainability of lake ecosystems. In: Ajuha S, editor. Comprehensive water quality and purification Volume 4. Amsterdam: Elsevier. p. 76–86.
- Barko JW, Smart RM. 1981. Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecol Monogr. 51:219–235. doi:10.2307/2937264.
- Barko JW, Smart RM. 1986. Sediment related mechanisms of growth limitation in submerged macrophytes. Ecology. 67:1328–1340. doi:10.2307/1938689.
- Borman S, Korth R, Temte J. 2014. Through the looking glass: a field guide to aquatic plants. 2nd ed. Wisconsin Lakes Partnership, Stevens Point, WI.
- Dick GO, Smart RM, Gilliland ER. 2004a. Aquatic vegetation restoration in Arcadia Lake, Oklahoma: a case study. ERDC/EL TR-04-7. Lewisville Aquatic Ecosystem Research Facility. Lewisville (TX): U.S. Army Engineer Research and Development Center.
- Dick GO, Smart RM, Smith JK. 2004b. Aquatic vegetation restoration in Cooper Lake, Texas: a case study. ERDC/EL TR-04-5. Lewisville Aquatic Ecosystem Research Facility. Lewisville (TX): U.S. Army Engineer Research and Development Center.
- Doyle RD 2001. Effects of waves on the early growth of Vallisneria americana. Freshwat Biol. 46:389–397. doi:10.1046/j.1365-2427.2001.00668.x.
- Doyle RD, Smart RM, Guest C, Bickel K. 1997. Establishment of native aquatic plants for fish habitat: test plantings in two north Texas reservoirs. Lake Reserv Manage. 13:259–269. doi:10.1080/07438149709354317.
- Fleming JP, Madsen JD, Dibble ED. 2011. Macrophyte re-establishment for fish habitat in Little Bear Creek Reservoir, Alabama, USA. J Freshwat Ecol. 26:105–114. doi:10.1080/02705060.2011.553925.
- Fleming JP, Madsen JD, Dibble ED. 2012. Development of a GIS model to enhance macrophyte re-establishment projects. Appl Geogr. 32:629–635. doi:10.1016/j.apgeog.2011.07.013.
- Hanson M, Butler M. 1994. Responses of plankton, turbidity, and macrophytes to biomanipulation in a shallow prairie lake. Can J Fish Aquat Sci. 51:1180–1188. doi:10.1139/f94-117.
- Horppila J, Nurminen L. 2003. Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland). Water Res. 37:4468–4474. doi:10.1016/S0043-1354(03)00405-6.
- James WF, Best EP, Barko JW. 2004. Sediment resuspension and light attenuation in Peoria Lake: can macrophytes improve water quality in this shallow system? Hydrobiologia. 515:193–201. doi:10.1023/B:HYDR.0000027328.00153.b2.
- Keeney DR, Nelson DW. 1982. Nitrogen–inorganic forms. In: Page AL et al., editor. Methods of soil analysis: part 2, chemical and microbiological properties. 2nd ed. Madison (WI): American Society of Agronomy and Soil Science Society of America. p. 643–698.
- Knopik JM 2014. Aquatic macrophyte response to carp removal and the success of transplanting aquatic macrophytes to restore the littoral community. MS thesis. University of Minnesota: Minneapolis (MN).
- Lauridsen TL, Jeppesen E, Sodergaard M. 1994. Colonization and succession of submerged macrophytes in shallow Lake Vaeng during the first five years following fish manipulation. Hydrobiologia. 275:233–242. doi:10.1007/BF00026714.
- Lauridsen TL, Sandsten H, Moller PH. 2003. The restoration of a shallow lake by introducing Potamogeton spp.: the impact of waterfowl grazing. Lakes Reserv Res Manage. 8:177–187. doi:10.1111/j.1440-1770.2003.00224.x.
- Lougheed VL, Theysmeyer TS, Smith T, Chow-Fraser P. 2004. Carp exclusion, food-web interactions, and the restoration of Cootes Paradise Marsh. J Great Lakes Res. 30:44–57. doi:10.1016/S0380-1330(04)70328-7.
- Madsen JD 1991. Resource allocation at the individual plant level. Aquat Bot. 41:67–86. doi:10.1016/0304-3770(91)90039-8.
- Madsen JD 1997. Methods for management of nonindigenous aquatic plants. Assessment and Management of Plant Invasions. SectionIII:145–171. doi:10.1007/978-1-4612-1926-2_13.
- Madsen JD 1999. Point intercept and line intercept methods for aquatic plant management. Vicksburg (MS): US Army Engineer Research and Development Center, Aquatic Plant Control Technical Note (TN APCRP-M1-02).
- NOAA. 2012. Local climate data, Minneapolis, Minnesota, May and June 2012. https://www.ncdc.noaa.gov/IPS/lcd/lcd.html. Asheville (NC): National Climate Data Center.
- R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0 URL http://www.R-project.org.
- Scheffer M 1998. Ecology of shallow lakes. New York (NY): Chapman and Hall.
- Schrage LJ, Downing JA. 2004. Pathways of increased water clarity after fish removal from Ventura Marsh; a shallow, eutrophic wetland. Hydrobiologia. 511:215–231. doi:10.1023/B:HYDR.0000014065.82229.c2.
- Smart RM, Dick GO, Doyle RD. 1998. Techniques for establishing native aquatic plants. J Aquat Plant Manage. 36:44–49.
- Smart RM, Dick GO, Snow JR. 2005. Update to the propagation and establishment of aquatic plants handbook. ERDC/EL TR-05-4. Lewisville Aquatic Ecosystem Research Facility. Lewisville (TX): U.S. Army Engineer Research and Development Center.
- Smart RM, Doyle RD, Madsen JD, Dick GO. 1996. Establishing native submersed aquatic plant communities for fish habitat. Am Fish Soc Symposium. 16:347–356.
- Smiley PC, Dibble ED. 2006. Evaluating the feasibility of planting aquatic plants in shallow lakes in the Mississippi delta. J Aquat Plant Manage. 44:73–80.
- Sondergaard M, Jensen J, Jeppesen E. 2003. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia. 506(1–3):135–145. doi:10.1023/B:HYDR.0000008611.12704.dd.
- Sponberg AF, Lodge DM. 2005. Seasonal below ground herbivory and a density refuge from waterfowl herbivory for Vallisneria americana. Ecology. 86:2127–2134. doi:10.1890/04-1335.
- Storch TA, Winter JD, Neff C. 1986. The employment of macrophyte transplanting techniques to establish Potamogeton amplifolius beds in Chautauqua Lake, New York. Lake Reserv Manage. 2:263–266. doi:10.1080/07438148609354640.
- Vanderbosch DA, Galatowitsch SM. 2011. Factors affecting the establishment of Schoenoplectus tabernaemontani (C.C Gmel.) Palla in urban lakeshore restorations. Wetlands Ecol Manage. 19:35–45. doi:10.1007/s11273-010-9198-7.
- Weber MJ, Brown ML. 2009. Effects of common carp on aquatic ecosystems 80 years after “Carp as a Dominant”: ecological insights for fisheries management. Rev Fish Sci. 17:524–537. doi:10.1080/10641260903189243.
- Zimmerman RC, Reguzzoni JL, Alberte RS. 1995. Eelgrass (Zostera marina L.) transplants in San Francisco Bay: role of light availability on metabolism, growth, and survival. Aquat Bot. 51:67–86. doi:10.1016/0304-3770(95)00472-C.