Abstract
Preece EP, Moore BC, Skinner MM, Child A, Dent S. A review of the biological and chemical effects of hypolimnetic oxygenation. Lake Reserv Manage. 35:229–246.
The technology of hypolimnetic oxygenation (HO) as a management tool continues to mature; at least 30 HO systems have been deployed worldwide in lakes and reservoirs to combat water quality issues that can flow from hypolimnetic hypoxia/anoxia. HO has been shown to decrease internal nutrient loading, decrease soluble metals (i.e., manganese, iron and mercury), reduce cyanobacteria blooms, and increase cold-water fish and zooplankton habitat. In this article, we review available case studies to examine how HO affects the biological and chemical components of lakes and reservoirs. Because HO is typically implemented with other restoration activities it is difficult to discern the exact impacts of HO in many waterbodies. Nevertheless, evidence from the reviewed case studies suggests that HO can be an important tool for restoring water quality when used properly.
Expanding human populations and associated increased nutrient loads have multiplied the number of culturally eutrophic waterbodies worldwide with hypolimnetic hypoxia/anoxia; these are expected to accelerate in numbers, geographic distribution, and intensity in upcoming decades, especially in the context of global climate change. Hypoxia/anoxia at the sediment–water interface (SWI) facilitates release of sediment phosphorus (P), nitrogen (N), methylmercury (MeHg), hydrogen sulfide (H2S), manganese (Mn), Iron (Fe), and other elements and compounds into the overlying water (). Anoxic/hypoxic sediment nutrient release can stimulate algal growth and the incidence of nuisance algal blooms (Beutel and Horne Citation1999, Nürnberg Citation1984; ). Algal bloom cells eventually precipitate and the resulting organic load produces additional respiratory oxygen demand in the water column and at the SWI (Beutel and Horne Citation1999, Dodds and Whiles Citation2010). Molecular diffusion of oxygen is exceptionally slow; without eddy diffusive processes, the hypolimnia of thermally stratified lakes are essentially isolated from atmospheric oxygen. Therefore, the increased respiratory oxygen demand from organic load associated with sestonic algae essentially depletes oxygen faster than it can possibly be replenished. Anoxia at the SWI allows P to accumulate in the hypolimnion, where it can eventually reenter the photic zone at lake turnover or be used by low-light-tolerant algae near the thermocline. Recycled P stimulates more algae growth in what develops as a self-reinforcing feedback (Beutel and Horne Citation1999, Dodds and Whiles Citation2010).
Figure 1. Conceptual diagram of hypolimnetic oxygenation control on nutrients, metals, phytoplankton, fish, and zooplankton.
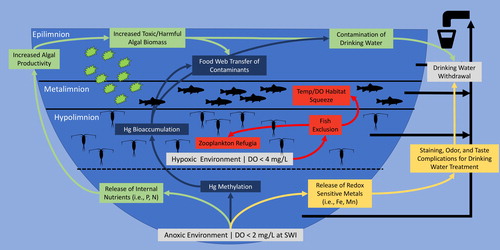
Besides regulating internal P, mitigation of hypoxia/anoxia is necessary to address other water quality problems (). Anoxic conditions produce soluble forms of metals, such as Fe and Mn that cause taste, odor, and color issues in drinking-water supplies, necessitating higher treatment costs (Stauffer Citation1986, AWWA Citation1987). Low oxygen at the SWI can also impact aquatic food webs by decreasing hypolimnetic and benthic habitats. For example, many large-bodied zooplankton species have developed diurnal migration patterns to avoid predators like fish, especially sight-feeders, in the daytime. These zooplankton utilize darker hypolimnetic waters during the day, migrating into the photic zone at night where fish that are sight-predators, such as trout, have difficulty locating prey in the darkness (Lampert Citation1987, Stirling et al. Citation1990). However, dissoved oxygen (DO) concentrations less than 2 mg/L remove the available refugia, forcing zooplankton higher in the water column, which cause zooplankton to become more susceptible to fish predation (Klumb et al. Citation2004). Increased predation on zooplankton can reduce their populations, subsequently reducing grazing rates on algae. This type of “trophic cascade” can result in higher algae biomass.
Similarly, anoxic conditions can also decrease cold-water fish habitat, forcing fish into less suitable warm surface water and preventing access to necessary food supplies (Aku and Tonn Citation1999, Coutant Citation1985, Swales Citation2006). A typical result can be a “temperature/DO squeeze” in which suitable habitat for trout and other cold-water salmonids is confined to a narrow stratum encompassing the thermocline, where temperature and DO are suitable but less than optimal for fish growth and survival (Coutant Citation1985). Loss of cold-water fish habitat and prey access often results in increased summer fish mortality, negatively impacting lake ecology and economic viability of both sport and commercial fisheries (Dodds et al. Citation2009).
Control and management of eutrophic waterbodies is a complex issue, in part due to the complexity and wide range of environmental impacts that may follow hypolimnetic anoxia. Multiple water quality management objectives have spawned a variety of restoration techniques to mitigate eutrophication and resulting anoxia (Søndergaard et al. Citation2000). The foundation for use of oxygen to prevent P release from sediments traces back to some of the most influential and cited papers in limnology, the classic works of Clifford H. Mortimer on “The Exchange of Dissolved Substances Between Mud and Water in Lakes” (Mortimer Citation1942, 1943). Mortimer’s works should still be read today; his outline of the basic chemical–physical–biological interplay in redox control of trivalent and divalent Fe compounds in controlling phosphate movement between sediments and the water column has been confirmed by an especially extensive literature. The basic model of redox-sensitive Fe phosphates (and metal hydroxide–oxide compounds) in sequestration or mobilization of P and metals in sediments was suggested by Einsele (Citation1938). Mortimer cited Hewitt (Citation1931) and Kusnetzow and Kusnetzowa (Citation1935) for reviews of the bacterial relationships to redox potential and generation of specific redox couples to various reactions, respectively.
The application of artificially raising hypolimnetic oxygen to counter anoxia and to control internal P followed relatively quickly on the heels of Mortimer’s seminal work with an Austrian aeration system (Mercier and Perret Citation1949). This seems especially remarkable considering the turmoil following World War II, and was also premised on the importance of maintaining lake stratification to maintain fish habitats. Hypolimnetic aeration (HA) technology received more sustained development in the late 1960s and 1970s (Bernhardt Citation1967, Fast et al. Citation1973, Fast and Lorenzen Citation1976), and 1980s (Ashley Citation1983, Ashley et al. Citation1987, Citation1985, Lean et al. Citation1986, McQueen and Lean Citation1986), as well as the first commercial HA vendors (e.g., Atlas Copco Inc., Kobe Designs Inc.). The concept of using pure oxygen, known as hypolimnetic oxygenation (HO), also emerged in this time frame (Fast et al. Citation1975, Citation1977).
In the early years of HO, results were often variable, in part due to availability of pure oxygen, design and performance issues, and oxygen storage logistics. As research on HO systems continued, technology improved, and pure oxygen became more readily available, HO has proven to be an effective technique with more predictive results. However, costs for HO treatment remain high. Most success with HO, often in tandem with other restoration activities, has dated to the early 2000s and beyond. To date, more than 30 HO systems have been deployed in lakes and reservoirs worldwide (Gerling et al. Citation2014, Singleton and Little Citation2006, Wagner Citation2015).
Modern HO systems rely on oxygen stored adjacent to the lake or reservoir in storage tanks or generated on site by pressure swing adsorption (PSA) as the oxygen source. There are various systems used to transfer the oxygen from the storage or generation site into the lake and hypolimnion. HO transfer systems are generally categorized into three types: (1) direct injection via bubble plume diffusers that are either linear or circular (Singleton et al. Citation2007); (2) in situ contact chambers such as the Speece Cone (McGinnis and Little Citation1998); and (3) side-stream saturation (Fast et al. Citation1975, Gerling et al. Citation2014), for which water is withdrawn from the hypolimnion, oxygenated, and then returned (Beutel and Horne Citation1999, Gafsi et al. Citation2009). Direct injection and in situ contact systems are generally deployed in deeper lakes and reservoirs (i.e., >10 m) whereas side-stream saturation systems have been used in both deep and shallow (i.e., <10 m) waterbodies.
In 1999, the first review on HO was compiled, focusing on the first two decades of HO (Beutel and Horne Citation1999). A 2006 review summarized the state of system design for both HA and HO (Singleton and Little Citation2006). A comprehensive review of HO in 2015 identified the successes and shortcomings of HO technology (Wagner Citation2015), and a 2016 review summarized HO applications to reduce cyanobacterial blooms (Bormans et al. Citation2016). The review presented here builds upon the previous works and focuses on the direct impacts of HO on water quality through (1) decreasing nutrient concentrations, (2) decreasing soluble metal concentrations, and (3) improving biological conditions. Although more than 30 HO systems have been deployed, water quality data are readily available for only a subset. One of the most rigorously studied HO systems is that in Camanche Reservoir, California; although briefly mentioned in this article, more details on the effects of HO on Camanche Reservoir are presented elsewhere in this issue.
Nutrients
HO has been employed to address issues of internal nutrient loading in eutrophic lakes throughout the world, but especially in North America. It is well documented that internal P release from lake sediments to the water column occurs when anoxic or hypoxic conditions are present at the SWI, and conversely, that P is sequestered in sediments when DO at the SWI exceeds about 2 mg (Ashley Citation1983, Hupner and Lewandowski Citation2008, Mortimer Citation1941, Citation1942, Prepas and Burke Citation1997).
Decreased internal P load and lake total phosphorus (TP) have formed the primary objectives for the majority of HO projects. However, of the 18 studies reviewed for lowering nutrient concentrations, only 8 indicated HO/aeration (as operated) was effective in maintaining DO at the SWI at 2 mg/L or greater for the duration of the study. Despite this, 17 of the 18 studies reported a decreased TP, soluble reactive phosphorus (SRP), or both (); many reported substantial decreases (i.e., >50%) in P concentrations. While these findings offer promising evidence of HO efficacy, this conclusion is complicated by concurrent application of other restoration tools, such as alum treatment (). In cases where HO has been less effective, there is strong evidence that hydroxide–oxide and sulfur concentrations are critical to P sequestration in lake sediments (Gächter and Wehrli Citation1998, Gächter and Müller Citation2003, Grochowska and Gawrońska Citation2004, Liboriussen et al. Citation2009). A sediment Fe:P ratio of 10–15 is considered sufficient to avoid saturation of P sorption sites, but significant levels of sulfur may interfere with P absorption in the Fe complex, requiring even higher ratios to achieve the same degree of sequestration (Gächter and Wehrli Citation1998, Gächter and Müller Citation2003, Christophoridis and Fytianos Citation2006).
Table 1. A summary of studies investigating the effects of HO on water column nutrient concentrations.
Most available studies have analyzed oxygenation over a relatively short time span (1–5 yr); results may therefore not be indicative of the ability of HO to facilitate long-term P sequestration, especially as legacy organic matter in the sediments is oxidized with ongoing HO. It is likely that HO must be implemented for a decade or more to realize its full effectiveness (Liboriussen et al. Citation2009, Moore et al. Citation2012). HO may decrease the pool of easily degradable legacy organic matter close to the sediment surface to the point that sediment oxygen demand no longer results in anoxic conditions at the SWI. Yet this phenomenon has only been observed after 10–15 yr of HO along with simultaneous reductions in external load (Liboriussen et al. Citation2009, Moore et al. Citation2012). Relatively short-term studies do not offer guidance on how long HO must be continued until oxygen demand is decreased to the point that oxygen supplementation is not required to maintain an oxic hypolimnia and/or a SWI. In other words, the current literature offers limited support for evaluating HO as a long-term solution to internal P loading in eutrophic systems. This limitation is primarily due to the paucity of studies of time frames greater than 10+ yr and may reflect both the difficulty of obtaining long-term funding and of publication pressures on researchers. Nevertheless, there is strong evidence that HO, particularly when combined with other nutrient reduction tools, is effective in achieving lower in-lake P concentrations ().
HO also appears effective in reducing N concentrations in lakes and reservoirs. Specifically, an oxic SWI may increase nitrifying bacteria populations (Beutel Citation2006, Gemza Citation1997, Thomas et al. Citation1994), leading to a subsequent decrease in hypolimnetic ammonium (NH4+) and increases in nitrate (NO3−). HO may decrease the niche distance between zones inhabited by nitrifying and denitrifying bacteria (Höhener and Gächter Citation1994). As the physical distance between these niches decreases, NO3− becomes more readily available to denitrifying bacteria, denitrification rates increase, and hypolimnetic dissolved N is removed from the system (Beutel Citation2006). In all case studies that reported N results, decreases in NH4+ concentrations were shown ().
The reviewed studies suggest HO is effective in decreasing P and NH4+ concentrations in lakes and reservoirs if (1) DO concentrations at the SWI and in the hypolimnion are maintained at 2 mg/L or greater, (2) sediment Fe concentrations are sufficient, and/or (3) external nutrient loading is also addressed. Further research is necessary to clearly define the role of HO alone in internal nutrient sequestration and to determine when HO could effectively cease without a subsequent increase in internal nutrient loading. A rational means of estimating expected lifetime of HO systems for a particular lake/reservoir is especially needed for economic assessments and projected long-term operating and amortization costs.
Metals
HO has been applied in drinking-water reservoirs to decrease soluble metals such as Mn and Fe (Moore Citation2003, Munger et al. Citation2016). These nuisance contaminants often reach high concentrations during summer stratification. Elevated Fe and Mn concentrations can cause issues with water staining, odor, and taste (WHO Citation2004). Chronic exposure to Fe and Mn can also cause adverse human health effects (Wasserman et al. Citation2006). Based on aesthetic concerns, such as taste, odor, and color, the US Environmental Protection Agency (EPA) has established secondary maximum contaminant limits for Fe (0.3 mg/L) and Mn (0.05 mg/L) in drinking water (EPA 2016). Removing Fe and Mn from water extracted from lakes or reservoirs is especially difficult and expensive, causing substantial problems for the drinking water industry (AWWA Citation1987). As such, there is a growing interest in treating the lake or reservoir itself to improve water quality prior to water entering the treatment plant (Gantzer et al. Citation2009).
Maintaining an oxic boundary is key to preventing mobilization of Fe and Mn from the sediment to the overlying water column. Sufficient oxygen delivery to the SWI can decrease hypolimnetic metal concentrations, and a growing number of studies show it is a viable option to improve drinking-water quality (Bryant et al. Citation2011, Chen et al. Citation2016, Gemza Citation1997, Gantzer et al. Citation2009).
Adding oxygen to a lake or reservoir hypolimnion does not eliminate the formation of reduced Fe or Mn. Instead, HO pushes the oxic-anoxic boundary (oxicline) out of the water column and into the sediments (Bryant et al. Citation2011, Gantzer et al. Citation2009). This allows oxidation processes to precede in the sediment, thereby lowering Fe and Mn translocation to, and accumulation in, the water column.
Because oxidization of Fe is a rapid reaction, HO quickly and effectively decreases soluble Fe in the water column by converting Fe+2 to Fe+3. This was exemplified in North Twin Lake, Washington, where soluble Fe concentrations were significantly lowered within 8 h of HO initiation (Dent et al. Citation2014). Similarly, in Carvins Cove Reservoir, Virginia, HO quickly decreased soluble Fe concentrations in the water column (Gerling et al. Citation2014). During treatment in Whittaker and Heart lakes in Ontario, hypolimnetic Fe concentrations were lower in both lakes compared to control years (Gemza Citation1997).
In contrast to Fe, Mn oxidation kinetics are slower, and Mn persists in the water column longer after initiation of HO (Bryant et al. Citation2011, Debroux et al. Citation2012, Gantzer et al. Citation2009). Mn oxidation and sediment sequestration is strongly dependent on Mn-oxidizing microbial communities (Bryant et al. Citation2012, Nealson et al. Citation1988), hypolimnetic mixing, and sedimentation of organic matter (Gantzer et al. Citation2009). Sediment–water chamber incubations have confirmed oxic conditions at the SWI inhibit sediment release of Mn. For example, Debroux et al. (Citation2012) found oxygenation decreased internal Mn loading, from 2–5 mg/m2/d under anoxic conditions to insignificant or negative release. A number of field studies have also found HO is effective for controlling Mn concentrations as long as sediments consistently remain oxic (Bryant et al. Citation2011, Dent et al. Citation2014, Gantzer et al. Citation2009, Gerling et al. Citation2014). However, a study in Falling Creek Reservoir, Virginia, found maintaining well-oxygenated conditions via HO did not increase the rate of Mn oxidation enough to prevent soluble Mn accumulation in the hypolimnion (Munger et al. Citation2016). The authors suggest maintaining a well-oxygenated hypolimnion alone is not sufficient to remove Mn from the water column. Because the abiotic oxidation rate of Mn is pH dependent, waterbodies with slightly acidic to neutral conditions, such as Falling Creek Reservoir (pH 5.6 to 7.5), are prone to chronic Mn issues. Thus, even under well-oxygenated conditions soluble Mn can still accumulate in the water column if pH is not high enough. Since higher DO may promote the oxidizing activity of Mn-oxidizing organisms, HO may be most effective in removing Mn from the water column in waters with neutral to alkaline pH (Munger et al. Citation2016).
Mercury (Hg) is another metal whose mobility in aquatic systems is potentially regulated by oxygen. Hg in all forms is poisonous, with a variety of toxic effects, ranging among neurological, dermatological, teratogenic, reproductive, and other issues (Ratcliffe et al. Citation1996). Organic MeHg compounds, formed in reducing environments, are especially toxic with very low LD50, and pose particular challenges in lakes with depressed oxygen levels. Inorganic mercury (Hg2+) can be converted to MeHg under anaerobic conditions in the presence of sulfate- or Fe-reducing bacteria, (Eckley and Hintelmann Citation2006, Watras Citation2009). MeHg is soluble and biomagnifies through increasing trophic levels. MeHg concentrations may increase 2–5 times with each step through the trophic cascade (Wood et al. Citation2013). Piscivorous fish, which are apex organisms in many aquatic food webs can have especially high levels, with concentrations magnified over 1 million-fold higher than lake water (Davis et al. Citation2010, Herrin et al. Citation1998, Slotton et al. Citation1995). MeHg concentrations in the tissue of fishes inhabiting many eutrophic water bodies may exceed 300 ppb or more. Indeed, more than 6 million hectares of freshwater lakes in the United States contain fish with tissue MeHg concentrations above the consumption advisory (USEPA Citation2011).
As with Mn and Fe, HO does not eliminate Hg methylation, but it could potentially push methylation deeper into the sediment, necessitating that MeHg compounds diffuse through oxidized sediment strata before reaching overlying waters (Benoit et al. Citation2003, Fleming et al. Citation2006). The combined treatment effect on Mn and Fe could also provide a sorptive layer of oxidized metals at the surface sediments that may further impede MeHg diffusion into the overlying waters (Chadwick et al. Citation2006, Dent et al. Citation2014).
In some cases, HO applications may exacerbate MeHg uptake within an aquatic ecosystem. In a case study at Twin Lakes, Washington, the bubble plume HO system did not deliver oxygen to the sediment surface, allowing MeHg to freely efflux from the lake floor. The bubble plume then provided a conduit for MeHg transport into the oxygenated upper strata, thereby increasing fish MeHg exposure (Beutel et al. Citation2014). In contrast, in Calero Reservoir, California, the bubble plume HO operation caused a substantial decrease in hypolimnetic MeHg; however, a substantial mass of MeHg was still present in the water column and concentrations did not decrease in zooplankton or small fish (McCord et al. Citation2016). MeHg presence in the water column and biota may have been due to (1) HO starting after hypoxic conditions had been established or (2) failure of the HO system to oxygenate the profundal waters under the HO system. Like the Twin Lakes example, the small hypoxic vertical gap between the SWI and the HO system represented an ongoing MeHg source to bottom waters. It is possible that if different HO designs (e.g., Speece Cones) had been utilized in these waterbodies the hypoxic gap would be decreased and MeHg would be better controlled. Thus, in waterbodies where MeHg control is the main objective, incorporation of lateral diffusion into the HO system may minimize hypoxic gaps and better prevent MeHg release from the SWI.
As with internal nutrient control, the success of HO systems in reducing soluble metal concentrations is dependent on adequate oxygen delivery to the hypolimnion (i.e., >2 mg at the SWI) to facilitate oxidation and sediment sequestration (Debroux et al. Citation2012, Gantzer et al. Citation2009, Zaw and Chiswell Citation1999). To effectively repress accumulation of redox-sensitive compounds, HO systems must also start prior to onset of anoxia and operate continuously throughout the stratified season (Debroux et al. Citation2012, Dent et al. Citation2014, Moore et al. Citation1996, Moore et al. Citation2012, Moore Citation2003). Furthermore, maintaining oxic conditions directly at the SWI is likely the best method for preventing metal release into the water column.
Biological
Phytoplankton
Generally, the greatest lake and reservoir water quality concern relative to phytoplankton is nuisance cyanobacteria blooms in which rapid growth and surface scums occur; while slower algal growth can contribute to increased organic matter, the focus of many studies, and this review, is on nuisance blooms. Excessive cyanobacteria populations and their associated ecological effects are persistent and escalating worldwide water quality problems. Cyanobacteria blooms reduce phytoplankton diversity (Bockwoldt et al. Citation2017) and alter community food web dynamics (Karjalainen et al. Citation2007); cyanobacterial toxins pose major health threats to wildlife, livestock, and humans. The science and practice of lake and reservoir management and restoration have been predominately directed at controlling cyanobacteria through nutrient reduction (“bottom-up control”) for well over a half century. While external or watershed nutrient reduction is the first line of defense, many lakes have been subjected to a long history of nutrient inputs, creating eutrophic conditions in which the internal cycling processes related to oxygen depletion as described previously have become well established, and can only be addressed through internal nutrient load controls. Internal nutrient loading can be especially problematic because nutrients released from lake sediment are present in predominantly dissolved forms that are directly available for algae uptake.
There is growing consensus that a dual (N and P) nutrient control strategy is necessary to reduce cyanobacteria blooms (e.g., Nelson et al. Citation2018, Paerl Citation2014), particularly in shallow lakes (Pearl et al. Citation2011). Unlike many mitigation options, HO can reduce internal nutrient loading of both N and P () by precipitating P from the water column and decreasing hypolimnetic NH4+ through increases in nitrifying bacteria populations (Beutel Citation2006). Although N is available in many forms, it is most actively assimilated and recycled as NH4+, and thus is a major nutrient source to cyanobacteria (Blomqvist et al. Citation1994). As such, the ability to reduce SRP and NH4+ and/or manipulation of the N:P ratio (Harris et al. Citation2014) through HO treatment presents a promising tool for reducing nuisance algal blooms.
Examples where HO has successfully managed phytoplankton communities have typically been in lakes or reservoirs with long residence times and where internal nutrient loading drives nuisance blooms. Because HO has generally been implemented in waterbodies where external nutrient loading was also decreased and/or other restoration measures were applied simultaneously, it is difficult to determine whether HO alone is responsible for controlling cyanobacteria blooms. Nonetheless, it is clear that HO, combined with other restoration activities, has successfully decreased overall algae biomass and changed the phytoplankton community composition from domination by cyanobacteria to green algae and/or diatoms in a number of shallow and deep waterbodies around the world (e.g., Moore and Christensen Citation2009, Moore et al. Citation2012, Prepas and Burke Citation1997, Toffolon et al. Citation2013).
The direct link between P release from the SWI and algal bioavailability is particularly prevalent in shallower lakes (<20 m), where stratification is typically unstable and internal seiches and wind-induced turbulence can enhance the entrainment of nutrient-rich water from the hypolimnion into the epilimnion (Blanton Citation1973, MacIntyre et al. Citation2006). In such lakes, cyanobacteria often dominate phytoplankton communities because of high P, good light availability throughout the water column, and warm summer water temperatures that favor their growth. Newman Lake, Washington (zmax = 9 m), is a prime example in which restoration efforts, including the first application of a downflow contact bubble oxygenation system (Speece Cone technology) and whole-lake and microfloc alum treatments, have reduced total peak phytoplankton biovolume more than 100-fold and decreased cyanobacteria percentage in the phytoplankton community to less than about 10% (Moore and Christensen Citation2009, Moore et al. Citation2012). In recent years, problems with oxygen generators have reduced oxygen inputs and have shut down the system for long periods during the summer for repairs (P. Gantzer and B. Moore, personal communication). Cyanobacteria populations have again increased, but are still much lower than in prerestoration years (B. Moore, unpublished data).
Similarly, in Lake Serraia, Italy (zmax = 18 m, zmean = 7 m), implementation of HO curtailed nutrient release from the sediments, resulting in an inverse trend in the summer phytoplankton community, with a decrease in cyanobacteria and a shift in dominance to chlorophyta (Toffolon et al. Citation2013). A side stream supersaturation oxygenation system in Lake Thunderbird, Oklahoma (zmax = 17.6 m, zmean = 4.7 m), also resulted in decreased algal biomass and average Chl-a. However, even with implementation of the HO system, Chl-a remains above the state water quality standard, likely because cultural eutrophication and external nutrient loading continue to stress Lake Thunderbird (OWRB Citation2015, Citation2016).
HO projects in other shallow lakes have produced variable results. For example, in 2 southern Ontario lakes treated with HO, phytoplankton response was better in the lake with lower posttreatment hypolimnetic DO. This was likely due to external nutrient loading, not the operation of the HO systems. In Heart Lake, Ontario (zmax = 10.9 m, zmean= 2.7 m), HO levels were maintained at 2.0 ppm. Here, lower cyanobacteria biovolumes, increases in Ceratium sp. biovolumes, and a change in the cyanobacteria assemblage from domination by Aphanizomenon sp. and Microcystis sp. to Dolichospermum sp. was attributed to HO (Gemza Citation1997). Cyanobacteria remained the dominant taxa in Whittaker Lake, Ontario (zmax = 11.0 m, zmean = 2.5 m), even though HO maintained hypolimnetic DO between 4.0 and 5.0 ppm (Gemza Citation1997). Although surface blooms appeared to decrease, it is thought that continued external nutrient loading prevented the system from responding as well as in Heart Lake.
Variable water quality responses to HO were also reported in 3 shallow, dimictic, eutrophic Danish lakes (zmax = 9.5–12.0 m, zmean = 3.9–7.0 m) (Liboriussen et al. Citation2009). In Lake Vedsted, Chl-a decreased in the first few years following initiation of HO, but has varied greatly after the initial response. Overall, Chl-a concentrations have been lower compared to pretreatment. Improvements have also been observed in Lake Torup with a decreased total algal biomass and elimination of Planktothrix prolifera blooms since HO treatment. However, in Lake Viborg Nørresø Chl-a levels have remained constant and little changed following HO. Like the lakes in southern Ontario, external nutrient loading appears to have been the cause for the varied response to HO.
Multiple mechanisms exist for transfer of SRP accumulated in the summer hypolimnetic to epilimnetic phytoplankton in deeper lakes. Like shallow lakes, entrainment mechanisms also operate in deeper lakes, as seiche-induced vertical mixing during storms or high wind events can inject internally derived nutrients accumulated in the summer hypolimnion into the littoral zone (Blanton Citation1973). With high light availability and additional nutrients, littoral areas can become the foci of algae blooms. Upwelling and internal seiches can enhance nutrient availability in the metalimnion through energy dissipation between stratified layers within lake pelagic zones (MacIntyre and Jellison Citation2001). In addition, cyanobacteria have multiple mechanisms to control or effect buoyancy (Horne and Goldman Citation1994), and during quiescent periods can essentially migrate vertically. Using buoyancy control, cyanobacteria may sink to access relatively high P within the metalimnion or upper hypolimnion, then rise into the high light environment of the upper epilimnion where P supplies may be depleted due to high algae productivity. Many cyanobacteria can take up P as “luxury uptake” beyond their immediate metabolic needs. These mechanisms make cyanobacteria more competitive than other algae during hot summers when high light availability can drive intense blooms. Essentially the cyanobacteria themselves become a vehicle in deep lakes for P translocation from the hypolimnion to the epilimnion (Huisman et al. Citation1999, Xie Citation2006). During fall turnover, SRP accumulated in the hypolimnion is redistributed throughout the entire lake. In this period, warm autumn water temperatures and nutrient availability can drive cyanobacteria blooms. HO potentially can target all of these internal load mechanisms if it is effective in sequestering P in the sediments.
There are a number of examples in which HO has been effective at reducing cyanobacteria or shifting the phytoplankton community in deep lakes (i.e., >20 m). Perhaps the most intensively studied HO system in a deep lake was the pure oxygen injection in Amisk Lake, Alberta (zmax = 34 m). Following HO, DO increased in the hypolimnion, Chl-a biomass was reduced, and the phytoplankton community composition shifted from cyanobacteria to a more mixed community that also included diatoms (Prepas and Burke Citation1997, Webb et al. Citation1997). HO also delayed stratification in the spring, allowing the established diatom populations to persist longer into the spring and summer (Webb et al. Citation1997). Despite the successes of HO in Amisk Lake, a lack of funding forced the discontinuation of the treatment after 5 yr. Another successful HO project involves the Speece Cone in Camanche Reservoir, California (zmax = 31 m, zmean = 17m). Long-term oxygenation of this system (i.e., 25 yr) has reduced Chl-a. Before restoration, large blooms of Aphanizomenon and Dolichospermum were frequent; these declined by 93% in the first 5 yr of treatment and by 99% thereafter (see multiple articles by Horne and colleagues, this issue). HO, in combination with hypolimnetic withdrawal, also reduced the frequency of nuisance algal blooms in Lake Varese, Italy, and average algal density has declined by a factor of 4 (zmax = 26.0 m, zmean = 10.7 m) (Premazzi et al. Citation2005).
In other deep lakes, response of the algal community to HO treatment has been difficult to interpret. In highly eutrophic Lake Sempach, Switzerland (zmax = 87 m), oxic conditions were maintained for over a decade with HO, and then pure oxygen was replaced with fine bubble aeration (Bürgi and Stadelmann Citation2002). During the latter years of treatment, a decrease in P release led to decreased dominance by N-fixing cyanobacteria. Phytoplankton biodiversity measures, such as increased species evenness and richness, improved and the number of algae species increased, from about 20 to 60 taxa. It is important to note that the researchers in this study only were able to make these conclusions after implementation of a long-term monitoring program. In the short term, phytoplankton responses to HO were more variable. Changing physical and chemical factors throughout restoration, including variable N/P ratios, caused several algae blooms to form. A follow-up study by Gächter and Müller (Citation2003) suggest improvements in Lake Sempach were not due to HO, but instead were due to reductions in external nutrient loading. This highlights the difficulty in determining what water quality results can be attributed to HO compared to other restoration measures. Similarly, improved summer Chl-a and less frequent algal blooms in Lake Hald, Denmark (zmax = 31 m, zmean = 13.1 m), could not be specifically attributed to HO; instead, the authors suggest improved water quality may have been due to reductions in external nutrient loading (Liboriussen et al. Citation2009).
Overall, in waterbodies with HO, cyanobacteria biomass has decreased, and the frequency and magnitude of cyanobacteria blooms have decreased in both shallow and deep lakes. While specific mechanisms facilitating these changes vary with lake depth, the overall factor driving this dynamic appears to be the ability of HO to control and lower internal nutrient loading. Lower internal nutrient release has been attributed to increases in phytoplankton species richness and diversity, which may help restore the phytoplankton community (Tian et al. Citation2017). Simultaneous reductions in external nutrient inputs are also essential; otherwise, total P load may only be held steady. Although P reductions have been attributed to much of the success in decreasing cyanobacteria, decreases in NH4+ have also correlated with reduced algae blooms (Bürgi and Stadelmann Citation2002, Liboriussen et al. Citation2009). The ability of HO to significantly lower nutrients derived from internal loading suggests that it can be an effective tool for reducing cyanobacteria, especially in systems where external nutrient loading is also decreased.
Fish
In addition to relatively cool temperatures, cold-water fish, such as salmonids, require oxygen concentrations of >4 mg/L, and preferably >6 mg/L; solubility of gasses in liquid is inversely proportional to temperature (Swales Citation2006). Hypolimnetic anoxia forces cold-water fish communities, such as salmonids that rely on oxygenated cool water, from the hypolimnion into the metalimnion of lakes (Aku and Tonn Citation1999, Doke et al. Citation1995, Moore et al. Citation2012, Citation2014, Taggart Citation1984), while high temperatures also exclude them from the epilimnion (Aku and Tonn Citation1999, Coutant Citation1985, Swales Citation2006). This oxygen–temperature “habitat squeeze” drastically limits lake volume available for feeding, reduces or eliminates salmonid access to food sources in the benthos, and may provide hypoxia-tolerant zooplankton with refuge against planktivorous salmonids (Doke et al. Citation1995, Taggart Citation1984, Wang et al. Citation1996).
Restoring adequate hypolimnetic oxygen has been shown to expand cold-water fish habitat, increase forage area and access to food resources, improve benthic habitat, and reduce crowding-related stressors (Aku and Tonn Citation1999, Beutel and Horne Citation1999, Dinsmore and Prepas Citation1997, Liboriussen et al. Citation2009). Yet due to the limited research on HO and fisheries, the long-term effects of HO on fish ecology have yet to be established. Nonetheless, increased oxygen in the hypolimnion may be particularly useful for fisheries in multi-use reservoirs where increased oxygen can improve both fish habitat availability and water quality.
The application of HO to increase fish habitat was first examined by Fast et al. (Citation1975, Citation1977) in 1973 in the Ottoville Quarry pond, which employed a side-stream pumping HO system. Researchers reported increased catch rates of trout in hypolimnetic study nets and by deep-fishing anglers, suggesting increased fish use of the improved oxic habitats in deep strata (Fast et al. Citation1975, Citation1977). Expansion of cold-water fish habitat has been documented in a number of lakes where HO was implemented. Examples include Amisk Lake, Alberta, where a 5-yr HO program provided a year-round oxygenated hypolimnion for cisco (Coregonus artedi) (Aku et al. Citation1997). Although depth distributions of cisco were similar in Amisk Lake and the reference basin in early summer, by late summer the median and maximum depth distributions of cisco were greater in Amisk compared to the untreated reference, Baptiste Lake. Expansion of fish habitat was also documented using active tracking, net captures, and hydroacoustic analyses in North Twin Lake, Washington, after HO was initiated in 2009 (Moore et al. Citation2014). Rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) populations in North Twin quickly expanded into increased hypolimnetic habitat in the first few years of HO treatment in North Twin Lake compared to the reference basin of South Twin Lake. Similarly, depth distributions of fish increased as a result of HO in Lake Fure, Denmark, and in lakes Baldegg, Sempach, and Hallwil, Switzerland (Liboriussen et al. Citation2009, Müller and Stadelmann Citation2004).
In addition to increasing habitat, there is some evidence that HO may also increase fish density and species richness. In Amisk Lake, Alberta, the density of cisco was twice as great in the oxygenated hypolimnion compared to the nearby reference lake (Aku and Tonn Citation1997). During the HO treatment period in Amisk Lake, whole lake fish density increased almost fivefold and biomass tripled, whereas in Baptiste Lake there was no increase in density or biomass. HO has also led to increased species richness in the Douglas Dam tailwaters in the lower French Broad River (Layzer and Scott Citation2006). Here, the oxygenation of dam discharges was found to restore the presence of 36 native fish species (Layzer and Scott Citation2006). An increase of the tailwater fish index (TFI; Scott Citation1999), a modified Index of Biotic Integrity, was also observed after oxygenated discharges began (Layzer and Scott Citation2006).
HO has also been shown to improve the food base for cold-water fish species in some systems (Aku and Tonn Citation1999, Enz et al. Citation2002, Müller and Stadelmann Citation2004). Effects of HO on feeding ecology have been most intensively studies in Twin Lakes, Washington. Here, diet and food-web analyses indicated significant changes in feeding ecology of the principal fish species in North Twin Lake, compared to preoxygenation and to unoxygenated South Twin (Skinner et al. Citation2014). Rainbow trout in North Twin Lake consumed significantly more large-bodied Daphnia than those in South Twin Lake, where rainbow trout fed primarily on littoral amphipods. Additionally, relative gut weight for brook trout was significantly higher in North Twin Lake, compared to South Twin Lake, apparently due to increased access to hypolimnetic zooplankton. However, stable isotope analysis (SIA), which is more representative of assimilated prey sources over time, showed no significant difference in prey sources for trout between oxygenated North Twin and unoxygenated South Twin lakes (Skinner et al. Citation2017). This work suggests that although HO may provide temporary access to ideal habitat for cold-water fish, HO may not increase profundal food sources that would otherwise be restricted due to hypolimnetic anoxia. This may explain why with increased suitable rainbow trout habitat in North Twin Lake, HO did not improve rainbow trout condition or growth relative to unoxygenated South Twin Lake (Cross et al. Citation2017). However, recent observations at the Twin lakes suggest that interlake migration and out-migration of trout may be greater than previously estimated (B. Cross, personal communication), complicating recruitment, survival, and mortality estimates and complicating the ecological assessments for these species.
In contrast to the trout finding, golden shiner (Notemigonus crysoleucas) diets included more zooplankton in oxygenated North Twin Lake compared to unoxygenated South Twin Lake (Skinner et al. Citation2014). SIA indicated that interspecific competition with trout species, rather than a lack of access to prey in an anoxic hypolimnion, may be a limiting factor to the cold-water trout fishery in Twin Lakes, Washington (Skinner et al. Citation2017). This finding is similar to results from Amisk Lake that found decreased growth rate in young (≤ 3 yr) cisco, even as density of the species increased with more access to oxic habitats (Aku and Tonn Citation1999). The authors suggest greater fish densities, coupled with a strong recruitment, may have led to intraspecific food competition and ultimately depressed growth rates (Aku and Tonn Citation1999).
Collectively, these studies show that fish habitat quality can be improved with HO if hypoxic conditions are the primary cause of fish mortality or stress. In terms of feeding ecology, HO may enhance a fishery if prey in the hypolimnion can be utilized in the summer months. The vertical expansion of foraging habitat and addition of food resources in oxygenated lakes provide fish better access to prey items distributed throughout the water column (Aku and Tonn Citation1999, Skinner et al. Citation2014), though this may not ultimately result in an improvement in fish condition (Aku and Tonn Citation1999, Cross et al. Citation2017). Ultimately, the effects of HO on fish density, growth, species condition, and feeding ecology require further research.
Zooplankton
Increasing large bodied zooplankton grazers can be used as a management tool (“top-down control”) to achieve water clarity improvement in eutrophic lakes by increasing predation pressures on phytoplankton. Thus, slightly improved oxygen conditions in eutrophic lakes can provide low-oxygen refuges for large-bodied zooplankton to escape predation by planktivorous fish. In Irondequoit Bay, Lake Ontario, the HO system was operated specifically to provide a zooplankton refuge for water clarity improvements (August metalimnetic DO 1.8 ± 0.5 mg/L; Klumb et al. Citation2004). Despite high planktivore abundances, researchers demonstrated that it was possible to create a low-oxygen refuge from fish predation in an open Great Lakes embayment. Zooplankton size and densities were greater in the low-oxygen refuge created in the metalimnion, compared to the epilimnion, where planktivore abundance was high (Klumb et al. Citation2004).
Although most HO studies have not been focused on creation of zooplankton refuges, zooplankton have been found to migrate to deeper layers after implementation of HO. This was observed in Lake Sempach, Switzerland, where improved access to the hypolimnion allowed zooplankton to become more evenly distributed over the whole water column during the daytime (Bürgi and Stadelmann Citation2002). HO also changed the zooplankton community composition to more carnivorous and less herbivorous species. Similarly, Chironomidae larvae densities were higher in the hypolimnion of treated North Twin Lake, Washington, compared to the unoxygenated reference lake (Cross et al. Citation2017).
Other studies have noted changes in zooplankton community composition and increases in zooplankton biomass related to HO. For example, in both Heart and Whittaker lakes, Ontario, zooplankton biomass significantly increased during HO. Specifically, there were extended periods in the midsummer when large-bodied daphnids dominated the zooplankton community (Gemza Citation1997). Similarly, in Amisk Lake, crustaceous zooplankton increased in deep-water strata (Webb et al. Citation1997).
While most HO systems are implemented to improve water quality or increase fish habitat, the evidence presented herein suggests that zooplankton communities can also benefit from HO treatment. Yet with the lack of HO research on zooplankton it is difficult to make any definitive conclusions on how HO may benefit or alter zooplankton communities.
Conclusions
HO was largely successful in improving water quality in the studies reviewed, although the conclusions must be tempered with the fact that other in-lake and watershed nutrient restoration efforts have most often been implemented in conjunction with HO. Further, for HO systems to truly decrease legacy organic matter accumulation and associated oxygen demand, oxygen in excess of current bacterial demand must be met (Beutel and Horne Citation1999, Moore et al. Citation1996, Citation2012). Overall, HO has been an effective tool in managing eutrophication and anoxic conditions in lakes worldwide. A summary of the literature results shows that:
Many lakes and reservoirs in the studies reviewed have undergone multiple restoration actions in conjunction with HO, so that attribution of positive outcomes exclusively to HO is not possible. In such waterbodies, it is not possible to fully attribute positive water quality results to enhanced oxygen. However, it often is reasonable to differentiate effects of HO from those of other restoration activities by timing and/or nature of the responses.
HO is an effective tool for reducing P and NH4+ concentrations in lakes and reservoirs when DO concentrations at the SWI and in the hypolimnion are maintained at ≥2 mg/L and sufficient concentrations of sediment hydroxide–oxide compounds, such as ferric iron, are present. High levels of sulfur may counter HO effectiveness by reducing available Fe for sequestering P.
HO most effectively improves water quality when external nutrient loading has been resolved.
Mn reduction can be achieved with HO, but it is critical that HO be implemented prior to onset of anoxia and continued for the duration of stratification. HO is likely to be most effective in reducing Mn in lakes where Mn-oxidizing organisms are present and in systems with neutral to alkaline pH.
Similar to Mn, MeHg reductions are most effective when HO is implemented prior to the onset of anoxia and operates continuously. If a vertical gap exists between the HO system and the sediment, then mercury methylation will continue to occur. That is, oxygenation of the SWI is necessary to prevent methylation.
HO is most effective in preventing cyanobacteria blooms when internal nutrient loading is the primary nutrient source. When this is the case, HO has often been effective at reducing overall phytoplankton biomass, improving evenness and biodiversity of the phytoplankton community, reducing noxious cyanobacteria blooms, and decreasing Chl-a concentrations. HO is less successful in reducing cyanobacteria blooms if external nutrient loading continues during treatment.
HO was largely successful in increasing cold-water fish habitat by maintaining oxygenation at >4 mg/L in the cold hypolimnion, though population-level effects were often ambiguous. When anoxia is the key stressor or cause of fish mortality, HO is most likely to be successful in restoring fisheries.
Zooplankton diversity and density tend to increase with oxic conditions in the sediments, creating additional food sources for cold-water fish such as trout; however, little research is available on HO effects on zooplankton community dynamics and subsequent top-down ecological effects such as reduction in algae biomass.
In conclusion, HO can be an effective restoration tool to decrease internal load and in-lake concentrations of TP and NH4+ concentrations, remove nuisance metals and associated water treatment costs, improve food-web dynamics in eutrophic lakes via increases in deep-water habitat, reduce cyanobacteria blooms, and restore fish prey. HO is most likely to be effective for nutrient reduction when implemented as a comprehensive plan that includes external nutrient load reduction efforts. In cases where external nutrient loading is likely to overwhelm the annual reduction in internal loading, HO may become a maintenance activity, and as such, may not be the most appropriate tool unless the community or regulatory will exists to concurrently reduce watershed sources.
HO success is most likely when sufficient lake-specific design data are available to properly size units to fully satisfy postoxygenation oxygen demand levels, including demand induced by water movements and increases in oxygen at the SWI. Emerging technology, such as hydrodynamic models that incorporate interfacial flux models, shows a promising method for predicting oxygenation-induced oxygen flux and ultimately improving oxygenation design methods for specific waterbodies (Bierlein et al. Citation2017). Use of these tools and other methods to develop lake-specific design is essential to maintain the SWI as truly oxic throughout stratification so that the underlying chemical, physical, and biological mechanisms of HO are achieved (Moore et al. Citation2015). The literature also suggests that lake-specific sediment data on Fe and S levels should be obtained to ensure that Fe/S ratios are sufficiently high for HO to be effective.
Much more research is needed to estimate how long HO will be needed in a particular lake basin. HO in general can be an expensive technology, although the capital costs are reasonable if amortization over long time frames is considered. It is important that maintenance and operational costs be incorporated in economic planning for HO, as these may be the highest long-term investment. Estimates of longevity requirements would be most helpful for decision makers to fully appreciate total investment requirements. From our own experience, we caution that it is essential to assume that HO systems may need to be operational for multiple decades. In almost all cases where HO was only implemented for short periods, water quality improvements usually dissipated rapidly after removal of oxygen. Adequate funds for maintenance, repair, and replacement should be included in the economic analysis, and data from existing systems are emerging to provide experience to guide new installations.
Some have questioned whether HO systems are truly sustainable. The literature does indicate that HO can be effective and, in many cases, may be the best tool available for reducing internal load. For deeper and larger lakes and reservoirs, line diffuser systems offer very economic options for HO. The question of sustainability comes down to balancing the value of clean water and the myriad of aesthetic, recreational, supply, economic, and ecologic values with the costs of restoration and maintenance. Coupling of more sustainable power generation technologies, such as wind and solar, or more creative delivery means may vastly improve HO systems in this respect (A. Horne, personal communication). As climate change and increasing human populations will likely accelerate human-mediated eutrophication, increasing the number, severity, and extent of eutrophic lakes worldwide with oxygen deficits, it is reasonable to expect that more communities will have to confront those very questions. We believe that the number of lakes in which HO is the most appropriate technology will continue to increase concurrently.
Acknowledgments
We thank Cam Irvine for his perceptive comments on the article, as well as the three anonymous reviewers.
References
- OWRB (Oklahoma Water Resources Board). 2015. Lake Thunderbird water quality 2014. OWRB, Oklahoma City, OK.
- Aku PMK, Rudstam LG, Tonn WM. 1997. Impact of hypolimnetic oxygenation on the vertical distribution of cisco (Coregonus artedi) in Amisk Lake, Alberta. Can J Fish Aquat Sci. 54:2182–2195.
- Aku PMK, Tonn WM. 1999. Effects of hypolimnetic oxygenation on the food resources and feeding ecology of cisco in Amisk Lake, Alberta. T Am Fish Soc. 128(1):17–30. doi:10.1577/1548-8659(1999)128<0017:EOHOOT>2.0.CO;2.
- American Water Works Association (AWWA). 1987. Research needs for the treatment of iron and manganese. Report of the AWWA Trace Inorganic Substances Committee. J AWWA. 79:119–122.
- Ashley KI. 1985. Hypolimnetic aeration: practical design and application. Water Res. 19(6):735–740. doi:10.1016/0043-1354(85)90120-4.
- Ashley KL. 1983. Hypolimnetic aeration of a naturally eutrophic lakes: physical and chemical effects. Can J Fish Aquat Sci. 40(9):1343–1359. doi:10.1139/f83-157.
- Ashley KI, Hay S, Scholten GH. 1987. Hypolimnetic aeration: a field test of the empirical sizing method. Water Res. 21(2):223–227. doi:10.1016/0043-1354(87)90053-4.
- Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL. 2003. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. Pp. 262–297. In: Cai, Y, Braids, OC (eds). Biogeochemistry of environmentally important trace elements. Washington, D.C.: American Chemical Society.
- Bernhardt H. 1967. Aeration of Wahnback Reservoir without changing the temperature profile. Amer. Water Works Assoc. 9:624–647.
- Beutel MW. 2001. Oxygen consumption and ammonia accumulation in the hypolimnion of Walker Lake, Nevada. Hydrobiologia. 466(1/3):107–117.
- Beutel MW. 2006. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol Eng. 28(3):271–279. doi:10.1016/j.ecoleng.2006.05.009.
- Beutel MW, Horne AJ, Taylor WD, Losee RF, Whitney RD. 2008. Effects of oxygen and nitrate on nutrient release from profundal sediments of a large, oligo-mesotrophic reservoir, Lake Matthews, California. Lake Reserv Manage. 24(1):18–29. doi:10.1080/07438140809354047.
- Beutel MW, Horne AJ. 1999. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv Manage. 15(4):285–297. doi:10.1080/07438149909354124.
- Beutel M, Dent S, Reed B, Marshall P, Gebremariam S, Moore B, Cross B, Gantzer P, Shallenberger E. 2014. Effects of hypolimnetic oxygen addition on mercury bioaccumulation in Twin Lakes, Washington, USA. Sci Total Environ. 496:688–700. doi:10.1016/j.scitotenv.2014.06.117.
- Bierlein KA, Rezvani M, Socolofsky SA, Bryant LD, Wüest A, Little JC. 2017. Increased sediment oxygen flux in lakes and reservoirs: the impacts of hypolimnetic oxygenation. Water Resour Res. 53(6):4876–4890. doi:10.1002/2016WR019850.
- Blanton JO. 1973. Vertical entrainment into the epilimnia of stratified lakes. Limnol Oceanogr. 18(5):697–704. doi:10.4319/lo.1973.18.5.0697.
- Blomqvist P, Pettersson A, Hyenstrand P. 1994. Ammonium-nitrogen: a key regulatory factor causing dominance of non-nitrogen-fixing cyanobacteria in aquatic systems. Arch. Hydrobiol. 132:141–164.
- Bockwoldt KA, Nodine ER, Mihuc TB, Shambaugh AD, Stockwell JD. 2017. Reduced phytoplankton and zooplankton diversity associated with increased cyanobacteria in Lake Champlain, USA. J Contemporary Water Res. 160(1):100–118. doi:10.1111/j.1936-704X.2017.03243.x.
- Bormans M, Maršálek B, Jančula D. 2016. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review. Aquat Ecol. 50(3):407–422. doi:10.1007/s10452-015-9564-x.
- Bryant LD, Hsu-Kim H, Gantzer PA, Little JC. 2011. Solving the problem at the source: controlling Mn release at the sediment-water interface via hypolimnetic oxygenation. Water Res. 45(19):6381–6392. doi:10.1016/j.watres.2011.09.030.
- Bryant LD, Little JC, Burgmann H. 2012. Response of sediment microbial community structure in a freshwater reservoir to manipulations in oxygen availability. FEMS Microbiol Ecol. 80(1):248–263. doi:10.1111/j.1574-6941.2011.01290.x.
- Bürgi H, Stadelmann P. 2002. Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration. Hydrobiologia. 469(1/3):33–48.
- Chadwick S, Babiarz C, Hurley J, Armstrong D. 2006. Influences of iron, manganese, and dissolved organic carbon on the hypolimnetic cycling of amended mercury. Sci Total Environ. 368(1):177–188. doi:10.1016/j.scitotenv.2005.09.039.
- Chen S, Lei C, Carey CC, Gantzer PA, Little JC. 2016. A coupled three-dimensional hydrodynamic model for predicting hypolimnetic oxygenation and epilimnetic mixing in a shallow eutrophic reservoir. Water Resources Res. 53(1):470–484. doi:10.1002/2016WR019279.
- Christophoridis C and Fytianos K. 2006. Conditions affecting the release of phosphorus from surface lake sediments. J. Environ. Qual. 35:1181–1192.
- Coutant CC. 1985. Striped bass, temperature, and dissolved oxygen: a speculative hypothesis for environmental risk. T Am Fish Soc. 114(1):31–61. doi:10.1577/1548-8659(1985)114<31:SBTADO>2.0.CO;2.
- Cross BK, Moore BC, Skinner MM. 2017. Hypolimnetic oxygenation effects on trout condition and growth in North Twin Lake, Washington. Lake Reserv Manage. 33(1):74–83. doi:10.1080/10402381.2016.1276654.
- Davis JA, Melwani AR, Bezalel SN, Hunt JA, Ichikawa G, Bonnema A, Heim WA, Crane D, Swenson S, Lamerdin C, Stephenson M. 2010. Contaminants in fish from California lakes and reservoirs, 2007–2008: summary report on a two-year screening survey. A report of the surface water ambient monitoring program, Calif. State Water Resour. Control Board, Sacramento, 127.
- Debroux JF, Beutel MW, Thompson CM, Mulligan S. 2012. Design and testing of a novel hypolimnetic oxygenation system to improve water quality in Lake Bard, California. Lake Reserv Manage. 28(3):245–254. doi:10.1080/07438141.2012.716501.
- Dent S, Beutel M, Gantzer P, Moore B. 2014. Response of iron, manganese and mercury in an anoxic water column to short-term hypolimnetic oxygenation. Lake Reserv Manage. 30(2):119–130. doi:10.1080/10402381.2014.898350.
- Dinsmore WP, Prepas EE. 1997. Impact of hypolimnetic oxygenation on profundal macroinvertebrates in a eutrophic lake in central Alberta. I. Changes in macroinvertebrate abundance and diversity. Can J Fish Aquat Sci. 54(9):2157–2169. doi:10.1139/cjfas-54-9-2157.
- Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, Schloesser JT, Thornbrugh DJ. 2009. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environ Sci Technol. 43(1):12–19. doi:10.1021/es801217q.
- Dodds, W. and Whiles, M. (2010) Freshwater Ecology: Concepts and Environmental Applications of Limnology. 2nd Edition, Elsevier, Amsterdam, 330–333.
- Doke JL, Funk WH, Juul STJ, Moore BC. 1995. Habitat availability and benthic invertebrate population changes following alum treatment and hypolimnetic oxygenation in Newman Lake, WA. J. Fresh. Biol. 10:87–102. doi:10.1080/02705060.1995.9663423.
- Eckley C, Hintelmann H. 2006. Determination of mercury methylation potentials in the water column of lakes across Canada. Sci Total Environ. 368(1):111–125. doi:10.1016/j.scitotenv.2005.09.042.
- Einsele W. 1938. Uber chemische und kolloidchemische Vorgange in Eisen-phosphat-systemen unter hmnochemischen und limnogeologischen Gesichtspunkten. Arch. Hydrobiol. Plarnkt. 33:361–387.
- Enz C, Müller R, Mbwenemo Bia M, Heeb J. 2002. A population dynamics model for evaluating mortality factors in Lake Hallwil whitefish (Coregonus suidteri) larvae. Archiv Für Hydrobiologie. Special Issues, Advances in Limnology. 57:343–358.
- Fast AW, Lorenzen MW. 1976. Synoptic survey of hypolimnetic aeration. J Environ Eng Div. 102:1161–1173.
- Fast AW, Moss B, Wetzel RG. 1973. Effects of artificial aeration on the chemistry and algae of two Michigan lakes. Water Resour Res. 9(3):624–647. doi:10.1029/WR009i003p00624.
- Fast AW, Overholtz WJ, Tubb RA. 1977. Hyperoxygen concentrations in the hypolimnion produced by injection of liquid oxygen. Water Resour Res. 13(2):474–476. doi:10.1029/WR013i002p00474.
- Fast AW, Overholtz WJ, Tubb RA. 1975. Hypolimnetic oxygenation using liquid oxygen. Water Resour Res. 11(2):294–299. doi:10.1029/WR011i002p00294.
- Fleming EJ, Mack EE, Green PG, Nelson DC. 2006. Mercury methylation from unexpected sources: molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Appl Environ Microbiol. 72(1):457–464. doi:10.1128/AEM.72.1.457-464.2006.
- Gächter R. 1987. Lake restoration. Why oxygenation and artificial mixing cannot substitute for a decrease in external P loading. Schweiz Z Hydrol. 49(2):170–185. doi:10.1007/BF02538501.
- Gächter R, Müller B. 2003. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol Oceanogr 48(2):929–933.
- Gächter R, Wehrli B. 1998. Ten years of artificial mixing and oxygenation: no effect on the internal P loading of two eutrophic lakes. Environ Sci Technol. 32(23):3659–3665. doi:10.1021/es980418l.
- Gafsi M, Kettab A, Benmamar S, Benziada S. 2009. Comparative studies of the different mechanical oxygenation systems used in the restoration of lakes and reservoirs. J Food, Ag, Env. 7(2):815–822.
- Gantzer PA, Bryant LD, Little JC. 2009. Controlling soluble Fe and Mn in a water-supply reservoir using hypolimnetic oxygenation. Water Res. 43(5):1285–1294. doi:10.1016/j.watres.2008.12.019.
- Gemza AF. 1997. Water quality improvements during hypolimnetic oxygenation in two Ontario lakes. Water Qual Res J Can. 32:365–390.
- Gerling AB, Browne RG, Gantzer PA, Mobley MH, Little JC, Carey CC. 2014. First report of the successful operation of a side stream supersaturation hypolimnetic oxygenation system in a eutrophic, shallow reservoir. Wat Res. 67:129–143. doi:10.1016/j.watres.2014.09.002.
- Greenop B, Lovatt K, Robb M. 2001. The use of artificial oxygenation to reduce nutrient availability in the Canning River, Western Australia. Water Sci Technol. 43(9):133–144.
- Grochowska J, Gawrońska H. 2004. Restoration effectiveness of a degraded lake using multi-year artificial aeration. Pol J Environ Stud. 13(6):671–681.
- Harris TD, Wilheim F, Graham J, Loftin KA. 2014. Experimental manipulation of TN:TP ratios suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ mesocosms. Lrm. 30(1):72–83. doi:10.1080/10402381.2013.876131.
- Herrin R, Lathrop R, Gorski P, Andren A. 1998. Hypolimnetic methylmercury and its uptake by plankton during fall destratification: a key entry point of mercury into lake food chains? Limnol Oceanogr. 43(7):1476–1486. doi:10.4319/lo.1998.43.7.1476.
- Hewitt LF. 1931. Oxidation-reduction potentials in bacteriology and biochemistry. L.C.C. Bull. No. 2819. London.
- Höhener P, Gächter R. 1994. Nitrogen cycling across the sediment-water interface in an euthrophic, artificially oxygenated lake. Aquatic Science. 56(2):115–132. doi:10.1007/BF00877203.
- Horne AJ, Goldman CR. 1994. Limnology. 2nd ed. San Francisco (CA): McGraw-Hill.
- Huisman J, Van Oostveen P, Weissing FJ. 1999. Species dynamics in phytoplankton blooms: incomplete mixing and competition for light. Am Nat. 154(1):46–68. doi:10.1086/303220.
- Hupner M, Lewandowski J. 2008. Review paper: oxygen controls the phosphorus release from lake sediments - a long-lasting paradigm in limnology. Internat Rev Hydrobiol. 93(4–5):415–432. doi:10.1002/iroh.200711054.
- Hutchinson GE, Deevey ES Jr, Wollack A. 1939. The oxidation-reduction potentials of lake waters and their ecological significance. Proc. Nat. Acad. Sci., Wash. 25(2):87–90. doi:10.1073/pnas.25.2.87.
- Karjalainen M, Engström-Ost J, Korpinen S, Peltonen H, Pääkkönen J-P, Rönkkönen S, Suikkanen S, Viitasalo M. 2007. Ecosystem consequences of cyanobacteria in the northern Baltic Sea. Ambio. 36(2–3):195–202.
- Klumb RA, Bunch KL, Mills EL, Rudstam LG, Brown G, Knauf C, Burton R, Arrhenius F. 2004. Establishment of a metalimnetic oxygen refuge for zooplankton in a productive Lake Ontario embayment. Eco App 14(1):113–131.
- Kusnetzow SI, Kusnetzowa ZI. 1935. Bacteriological and chemical investigations on lake muds in connection with a bottom emission of gases. [Russian with English summary.]
- Lampert W. 1987. Vertical migration of freshwater zooplankton: indirect effects of vertebrate predators on algal communities. Pp. 291–299. In: Kerfoot C, Sih A (eds). Predation: direct and indirect impacts on aquatic communities. Hanover, NH: University Press of New England.
- Layzer JB, Scott EM. 2006. Restoration and colonization of freshwater mussels and fish in a southeastern United States tailwater. River Res Applic. 22(4):475–491. doi:10.1002/rra.919.
- Lean DRS, McQueen DJ, Story VR. 1986. Phosphate transport during hypolimnetic aeration. Arch. Hydrobiol. 108:269–280.
- Liboriussen L, Søndergaard M, Jeppesen E, Thorsgaard I, Grünfeld S, Jakobsen TS, Hansen K. 2009. Effects of hypolimnetic oxygenation on water quality: results from five Danish lakes. Hydrobiologia. 625(1):157–172. doi:10.1007/s10750-009-9705-0.
- MacIntyre S, Jellison R. 2001. Nutrient fluxes from upwelling and enhanced turbulence at the top of the pycnocline in Mono Lake, California. Hydrobiologia. 466(1/3):13–29.
- MacIntyre S, Sickman JO, Goldthwait SA, Kling GW. 2006. Physical pathways of nutrient supply in a small, ultraoligotrophic arctic lake during summer stratification. Limnol Oceanogr. 51(2):1107–1124. doi:10.4319/lo.2006.51.2.1107.
- McCord SA, Beutel MW, Dent SR, Schladow SG. 2016. Evaluation of mercury cycling and hypolimnetic oxygenation in mercury-impacted seasonally stratified reservoirs in the Guadalupe River watershed, California. Water Resour Res. 52(10):7726–7743. doi:10.1002/2016WR019061.
- McGinnis DF, Little JC. 1998. Bubble dynamics and oxygen transfer in a speece cone. Wat Sci Tech 37(2):285–292.
- McQueen DJ, Lean DRS. 1986. Hypolimnetic aeration: an overview. Water Pollution Res. J. Can. 21(2):205–217.
- Mercier P, Perret J. 1949. Aeration station of Lake Bret. Schweiz. Ver. Gas. Wasserfach. Monatbull. 29:25–30.
- Moore BC, Christensen D. 2009. Newman Lake restoration: a case study. Part I. Chemical and biological responses to P control. Lake Reserv Manage. 25(4):337–350. doi:10.1080/07438140903172907.
- Moore BC, Cross BK, Beutel M, Dent S, Preece E, Swanson M. 2012. Newman Lake restoration: a case study. Part III. Hypolimnetic oxygenation. Lake Reserv Manage. 28(4):311–327. doi:10.1080/07438141.2012.738463.
- Moore BC, Cross BK, Clegg EM, Lanouette BP, Skinner M, Preece EP, Child A, Gantzer P, Shallenberger E, Christensen D, Nine B. 2014. Hypolimnetic oxygenation in Twin Lakes, WA. Part I: distribution and movement of trout. Lake Reserv Manage. 30(3):226–239. doi:10.1080/10402381.2014.908437.
- Moore BC, Chen PH, Funk WH, Yonge D. 1996. A model for predicting lake sediment oxygen demand following hypolimnetic aeration. J Am Water Resources Assoc. 32(4):723–731. doi:10.1111/j.1752-1688.1996.tb03469.x.
- Moore BC, Mobley M, Little J, Kortmann B, Gantzer P. 2015. Aeration and oxygenation in lakes and reservoirs. Lakeline. 35(1):17–29.
- Moore BC. 2003. Downflow bubble contact aeration technology (Speece Cone) for sediment oxygenation. Proceedings of the Second International Conference on Remediation of Contaminated Sediments (eds. M. Pellei and A. Porta).
- Mortimer CH. 1941. The exchange of dissolved substances between mud and water in lakes. J Ecol. 29(2):280–329. doi:10.2307/2256395.
- Mortimer CH. 1942. The exchange of dissolved substances between mud and water in lakes. Part III. The relation of seasonal variables in redox conditions in the mud to the distribution of dissolved substances in Esthwaite Water and Windermere, North Basin. Part IV. General discussion. J. Ecology. 30:147–201. doi:10.2307/2256691.
- Müller R, Stadelmann P. 2004. Fish habitat requirements as the basis for rehabilitation of eutrophic lakes by oxygenation. Fisheries Manage. 11:251–260. doi:10.1111/j.1365-2400.2004.00393.x.
- Munger ZW, Carey CC, Gerling AB, Hamre KD, Doubek JP, Klepatzki SD, McClure RP, Schreiber ME. 2016. Effectiveness of hypolimnetic oxygenation for preventing accumulation of Fe and Mn in a drinking water reservoir. Wat Res. 106:1–14. doi:10.1016/j.watres.2016.09.038.
- Nealson KH, Tebo BM, Rosson RA. 1988. Occurrence and mechanisms of microbial oxidation of Mn. Adv Appl Microbiol. 33:279–319.
- Nelson NG, Muñoz-Carpena R, Phlips EJ, Kaplan D, Sucsy P, Hendrickson J. 2018. Revealing biotic and abiotic controls of harmful algal blooms in a shallow subtropical lake through statistical machine learning. Environ Sci Technol. 52(6):3527–3535. doi:10.1021/acs.est.7b05884.
- Noll MR. 2011. P cycling in a managed lake ecosystem: seasonal and longer-term trends. Appl Geochem. 26:S234–S237. doi:10.1016/j.apgeochem.2011.03.112.
- Nürnberg GK. 1998. Prediction of annual and seasonal phosphorus concentrations in stratified and polymictic lakes. Limnol Oceanogr. 43(7):1544–1552. doi:10.4319/lo.1998.43.7.1544.
- Nürnberg GK. 1984. The prediction of internal phosphorus load in lakes with anoxic hypolimnia. Limnol Oceanogr. 29(1):111–124. doi:10.4319/lo.1984.29.1.0111.
- Nürnberg GK. 1995a. Quantifying anoxia in lakes. Limnol Oceanogr. 40(6):1100–1111. doi:10.4319/lo.1995.40.6.1100.
- Nürnberg GK. 1995b. The anoxic factor, a quantitative measure of anoxia and fish species richness in central Ontario Lakes. Trans. Am. Fish. Soc. 124:677–686.
- Nürnberg GK. 2004. Quantified hypoxia and anoxia in lakes and reservoirs. Scientific World J. 4:42–54. doi:10.1100/tsw.2004.5.
- Nürnberg GK. 2009. Assessing internal phosphorus load-problems to be solved. Lake Reserv. Manage. 25(4):419–432.
- OWRB (Oklahoma Water Resources Board). 2016. Lake thunderbird water quality 2015. OWRB, Oklahoma City, OK.
- Paerl HW. 2014. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life (Basel). 4(4):988–1012. doi:10.3390/life4040988.
- Pearl HW, Hall NS, Calandrino ES. 2011. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic induced change. Sci Tot Environ. 409:1739–1745. doi:10.1016/j.scitotenv.2011.02.001.
- Premazzi G, Cardoso AC, Rodari E, Austoni M, Chiaudani G. 2005. Hypolimnetic withdrawal coupled with oxygenation as lake restoration measures: the successful case of Lake Varese (Italy). Limnetica. 24(1–2):123–132.
- Prepas EE, Burke JM. 1997. Effects of hypolimnetic oxygenation on water quality in Amisk Lake, Alberta, a deep, eutrophic lake with high internal P loading rates. Can J Fish Aquat Sci. 54(9):2111–2120. doi:10.1139/f97-125.
- Prepas FF, Murphy TP, Dinsmore WP, Burke JM, Chamber PA, Reedyk S. 1997. Lake management based on lime application and hypolimnetic oxygenation: the experience in eutrophic hardwater lakes in Alberta. Water Qual Res J Can. 32:273–293.
- Ratcliffe H, Swanson G, Fischer L. 1996. Human exposure to mercury: a critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 49(3):221–270. doi:10.1080/00984108.1996.11667600.
- Rysgaard S, Risgaard-Petersen N, Niels Peter S, Kim J, Lars Peter N. 1994. Oxygen regulation of nitrification and denitrification in sediments. Limnol Oceanogr. 39(7):1643–1652. doi:10.4319/lo.1994.39.7.1643.
- Schuaser I, Chorus I. 2007. Assessment of internal and external lake restoration measures for two Berlin lakes. Lake Reserv Manage. 23:366–376. doi:10.1080/07438140709354024.
- Scott EM. 1999. Tailwater Fish Index (TFI) development for Tennessee River tributary tailwaters. Pp. 507–522. In: Simon T (ed.). Assessing the sustainability and biological integrity of water resources using fish communities, New York, (NY): RC Press.
- Singleton VL, Gantzer P, Little JC. 2007. Linear bubble plume model for hypolimnetic oxygenation: full-scale validation and sensitivity analysis. Water Resour Res. 43:W02405. doi:10.1029/2005WR004836.
- Singleton VL, Little JC. 2006. Designing hypolimnetic aeration and oxygenation systems: a review. Environ Sci Technol. 40(24):7512–7520.
- Skinner MM, Cross BK, Moore BC. 2017. Using stable isotope analysis to assess the effects of hypolimnetic oxygenation on diet in a mixed cold- and warmwater fish community. Environ Biol Fish. 100(8):1007–1017. doi:10.1007/s10641-017-0625-y.
- Skinner MM, Moore BC, Swanson ME. 2014. Hypolimnetic oxygenation in Twin Lakes, WA. Part II: feeding ecology of a mixed cold- and warmwater fish community. Lake Reserv Manage. 30(3):240–249. doi:10.1080/10402381.2014.908438.
- Slotton D, Reuter J, Goldman C. 1995. Mercury uptake patterns of biota in a seasonally anoxic northern California reservoir. Water Air Soil Pollut. 80(1–4):841–850. doi:10.1007/BF01189735.
- Søndergaard M, Jeppesen E, Peder Jensen J, Lauridsen T. 2000. Lake restoration in Denmark. Lakes Reserv Res Manage. 5(3):151–159. doi:10.1046/j.1440-1770.2000.00110.x.
- Stauffer RE. 1986. Cycling of manganese and iron in Lake Mendota, Wisconsin. Environ Sci Technol. 20(5):449–457. doi:10.1021/es00147a002.
- Stirling DG, McQueen DJ, Johannes MRS. 1990. Vertical migration in Daphnia galeta mendotae (Brooks): demographic responses to changes in planktivore abundance. Can J Fish Aquat Sci. 47(2):395–400. doi:10.1139/f90-041.
- Swales S. 2006. A review of factors affecting the distribution and abundance of rainbow trout (Oncorhychus mykiss Walbaum) in lake and reservoir systems. Lake Reserv Manage. 22(2):167–178. doi:10.1080/07438140609353894.
- Taggart CT. 1984. Hypolimnetic aeration and zooplankton distribution: a possible limitation to the restoration of cold-water fish populations. Can J Fish Aquat Sci. 41(1):191–198. doi:10.1139/f84-020.
- Thomas JA, Funk WH, Moore BC, Budd WW. 1994. Short term changes in Newman Lake following hypolimnetic aeration with the speece cone. Lake Reserv Manage. 9(1):111–113. doi:10.1080/07438149409354738.
- Tian W, Zhang H, Zhao L, Zhang F, Huang H. 2017. Phytoplankton diversity effects on community biomass and stability along nutrient gradients in a eutrophic lake. IJERPH. 14(1):95. doi:10.3390/ijerph14010095.
- Toffolon M, Ragazzi M, Righetti M, Teodoru CR, Tubino M, Defrancesco C, Pozzi S. 2013. Effects of artificial hypolimnetic oxygenation in a shallow lake. Part 1: phenomenological description and management. J Env Manage. 114:520–529. doi:10.1016/j.jenvman.2012.10.062.
- USEPA (United States Environmental Protection Agency). 2011. Biennial national listing of fish advisory. EPA-820-F-11-014. Washington, DC.
- Wagner KJ. 2015. Oxygenation and circulation to aid water supply reservoir management. Project #4222c. Denver, CO: Water Research Foundation.
- Wang L, Zimmer K, Diedrich P, Williams S. 1996. The two-story rainbow trout fishery and its effect on the zooplankton community in a Minnesota lake. J Freshw Ecol. 11(1):67–80. doi:10.1080/02705060.1996.9663495.
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. 2006. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 114(1):124–129., doi:10.1289/ehp.8030.
- Watras C. 2009. Mercury pollution in remote freshwater lakes. Pp. 100–109. In: Likens G (ed.). Ecnyclopedia of Inland waters. New York (NY): Elsevier.
- Webb DJ, Robarts RD, Prepas EE. 1997. Influence of extended water column mixing during the first two years of hypolimnetic oxygenation on the phytoplankton community of Amisk Lake, Alberta. Can J Fish Aquat Sci. 54(9):2133–2145. doi:10.1139/f97-120.
- WHO (World Health Organization). 2004. Guidelines for drinking-water quality: recommendations. 3rd ed. Geneva, Switzerland: World Health Organization.
- Wood, M., Austin, C., Louie, S. 2013. Fish Mercury Impairment in California Reservoirs: Historic Mines and Other Factors. EPA Region 9 State-of-the-Science Workshop on Mercury Mremediation in Aquatic Environments. September 2013.
- Xie P. 2006. Biological mechanisms driving the seasonal changes in the internal loading of phosphorus in shallow lakes. Sci China Ser D. 49(S1):14–27. doi:10.1007/s11430-006-8102-z.
- Zaccara S, Canziani A, Roella V, Crosa G. 2007. A northern Italian shallow lake as a case study for eutrophication control. Limnology. 8(2):155–160. doi:10.1007/s10201-007-0209-1.
- Zaw M, Chiswell B. 1999. Fe and Mn dynamics in lake water. Wat Res. 33(8):1900–1910. doi:10.1016/S0043-1354(98)00360-1.
