Abstract
De Stasio BT, Acy CN, Frankel KE, Fritz GM, Lawhun SD. Tests of disinfection methods for invasive snails and zooplankton: effects of treatment methods and contaminated material. Lake Reserv Manage. 35:156–166.
The spread of aquatic invasive species (AIS) in lakes and reservoirs is a major issue that will grow in importance due to possible economic, environmental, and human health impacts of AIS as the number of potentially harmful invasive species continues to increase. There is a pressing need for scientific information on which procedures can effectively decontaminate and disinfect sampling gear against multiple AIS. We determined effectiveness of cleaning methods on 4 recent AIS in WI: New Zealand mud snail (NZMS; Potamopyrgus antipodarum), faucet snail (Bithynia tentaculata), spiny water flea (Bythotrephes longimanus), and bloody-red mysid shrimp (Hemimysis anomala). We tested mortality in the laboratory following total immersion and spraying procedures, as well as the effects of 5 types of materials likely to be contaminated. Freezing was effective, but not practical for large sampling equipment and boats. Desiccation did not result in 100% mortality for NZMS or faucet snail. Bleach (500 ppm) was effective on spiny water flea and bloody-red mysid, but not on either snail. Immersion in Virkon (2%) was effective on all species within 20 min. Spraying was less effective than total immersion, especially on some materials. Our results provide rigorous data for establishing practical guidelines on disinfection methods for managers, researchers, and citizen scientists battling the spread of AIS in lakes and reservoirs.
The spread of aquatic invasive species (AIS) is a major concern in lakes and reservoirs (Rothlisberger and Lodge Citation2013, Havel et al. Citation2015). Managers and researchers are spending increasing amounts of time and effort to reduce potential new invasions due to contaminated sampling gear like nets, waders, anchor lines, and boats (Drake and Andrew Citation2017). Making this even more challenging is the wide variety of types of AIS now resident in many ecosystems and differences among species in resistance to commonly used disinfection procedures (Anderson et al. Citation2015). As these various AIS expand their ranges and begin to overlap in occurrence, there is a need for additional data on which methods will effectively address contamination from multiple AIS. In addition to differences based on AIS and type of disinfection method (e.g., heating, freezing, desiccation, chemical exposure), there may be differences in decontamination effectiveness depending on method of application of the treatment (e.g., immersion, spraying, exposure time), as well as the materials contaminated (Hosea and Finlayson Citation2005, Stockton and Moffit 2013). We worked with staff of the Wisconsin Department of Natural Resources (WIDNR) to select specific combinations of disinfection conditions that are likely to occur in practice and that are hypothesized to be useful for managers and researchers. Our study focused on filling gaps in the scientific literature on effectiveness of disinfection methods when applied to various materials commonly contaminated by invertebrate AIS. The tests were not intended to address all possible scenarios, but the results will help managers decide on the most effective comprehensive protocols to decontaminate and disinfect sampling gear in realistic situations.
Many managers and researchers often move between aquatic locations quickly, and this aspect needs to be considered when deciding on best management practices (BMPs; WIDNR Citation2017). Many snail species can survive desiccation for more than 30 days (Havel Citation2011, Havel et al. Citation2014), so simply drying gear will not be practical in most situations. Use of hot water immersion (45 C for 15 min) has been recommended and shown effective for a variety of AIS (Beyer et al. Citation2011, Anderson et al. Citation2015). Hot water/high-pressure spray techniques have also been recommended for disinfecting recreational equipment and boats (ANSTF Citation2013). However, effectively disinfecting large sampling equipment, waders, and so on using hot water immersion, spraying, or freezing is logistically challenging. As a result, chemical disinfection methods will likely be more effective in practice where chemical waste can be managed properly.
Recent work has shown that for sexually reproducing AIS it is important to reduce the number of animals introduced at any one time (Sinclair and Arnott Citation2016). This reduction in “propagule size” requires highly effective disinfection procedures that kill as many invaders as possible during each cleaning event. For asexually reproducing AIS, a single individual introduced into a new aquatic environment can start a population; thus, 100% mortality is the desired outcome for any decontamination method (Sinclair and Arnott Citation2017). Our goal in this study was to focus on questions dictated by management guidelines and recommendations. We examined combinations of disinfection methods being considered for general use in many states, as applied to commonly contaminated materials, which will achieve effective mortality of AIS. We tested 4 AIS that have recently established populations in Wisconsin and that have shown the potential to spread widely in lakes and reservoirs, leading to important ecological, economic, and health effects.
Materials and methods
Species tested
Four invasive, nonnative aquatic invertebrate species currently spreading in Wisconsin were tested: New Zealand mud snail (NZMS; Potamopyrgus antipodarum), faucet snail (Bithynia tentaculata), spiny water flea (Bythotrephes longimanus), and bloody-red mysid shrimp (Hemimysis anomala). Specimens were collected from sites with established populations, following consultation with appropriate WIDNR staff (). Specimens were collected and placed in plastic totes containing water from each collection site for transport (at least 60 L of water in each tote). Rocks covered with NZMS and specimens gently removed from concrete bridge supports were obtained. Faucet snails were collected with dip nets and transported with aquatic vegetation to which they were attached. Spiny water fleas were collected with oblique plankton tows (250 μm mesh, 0.5 m diameter). Bloody-red mysid were collected at night with oblique plankton tows (250 μm mesh, 0.5 m diameter). Collections were transported to the laboratory at Lawrence University, Appleton, WI, within 3 h of collection and then maintained in aged tap water (i.e., dechlorinated with aeration). Snails and bloody-red mysid were held for at least 24 h at 20 C before being used in experiments, to reduce artifacts due to stress of collection and transfer. Experiments on spiny water fleas were run on the same day as collection following a short holding period (typically 4–8 h) at 20 C to reduce mortality due to handling stress. New Zealand mud snails were tested during summer 2014 and 2015, spiny water fleas were tested in summer 2015, and faucet snails and bloody-red mysid were tested during summer 2016. Collections contained juvenile as well as adults of each species: NZMS size range was 1.7–4.5 mm (median = 3.6 mm), faucet snails ranged from 3.2 to 11.0 mm (median = 8.03 mm), spiny water flea body size range was 1.2–3.6 mm (median = 2.4 mm), and bloody-red mysid were from 1.1 to 10.2 mm (median = 2.6 mm).
Table 1. Collection sites for aquatic invasive species tested in disinfection experiments.
Experimental design
Experiments were conducted as separate, replicated studies for each species following standard protocols we have used previously (Beyer et al. Citation2011, Acy Citation2015). Once established in the laboratory, batches of animals were transferred to glass bowls and conditioned for 1 h, exposed for varying durations to treatment conditions, and then transferred to a recovery bath of aged tap water at 20 C to mimic reimmersion in a new location following transport (). Exposure by total immersion and spraying to saturation were tested separately. For spray applications we employed procedures that are currently recommended by WIDNR and other states. Test materials with animals attached were moved to new glass bowls and sprayed to saturation (mean = 5.4 mL, SD = 0.3 mL). Test material and animals remained in test bowls for the specified duration of exposure in each treatment (mean temperature = 20.1 C [SE = 0.3]; relative humidity = 65% [SE = 3.0]) and then immersed in recovery bowls. Following 20 min in the recovery bath, animals were tested for survivorship using standard procedures (Ellis and MacIsaac Citation2009, Anderson et al. Citation2015). For snails this entailed observing whether animals extended from their shell and were active or responded to gentle probing. Spiny water fleas and bloody-red mysid were examined under the microscope for movement to determine if they were alive.
Figure 1. Example of experimental design for disinfection tests of aquatic invasive species, including three exposure durations. Holding period refers to the time between collecting animals from the field and the start of experiments. Two control treatments were employed for each experiment to assess possible influence of materials on survivorship.
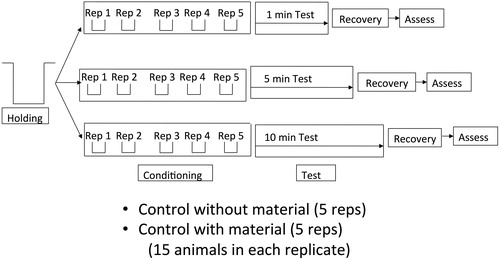
Groups of 15 individuals were tested in each replicate run of the experiment to determine percent survivorship (). Five replicates of each treatment were performed for all combinations tested. Each test employed between 375 and 525 animals, depending on the number of time durations included. Given the number of test animals per replicate, the minimum detection limit for survivorship is 1.33% (i.e., only a single animal survives in a single replicate). For chemical treatments, at least 3 immersion or contact times were employed lasting for 20–25 min total exposure. These durations were chosen to focus on the 20 min exposure recommended by the BMPs in Wisconsin, and are typical times recommended for other regions as well (MIDEQ Citation2018, NYDEC Citation2018, WGFD Citation2018). Multiple control treatments were included to isolate potential effects of aged tap water or chemicals released into the water by the various materials tested compared to the intended disinfection treatment. Controls in spray treatments were sprayed with aged tap water, from the same source used in holding, conditioning, and recovery phases of tests.
Experimental treatments
We employed combinations of treatments currently used or being considered by the WIDNR for decontamination and disinfection of sampling equipment (). Selection of treatments focused primarily on combinations for which information was lacking based on a review of the scientific literature and that are appropriate considering the main risks of contamination for each species (WIDNR Citation2017). We did not use full-factorial experimental designs, given the focus on filling gaps in existing knowledge related to practical application of disinfection methods. Our results therefore do not attempt to draw broad conclusions about effectiveness of procedures under all possible conditions, but rather target specific combinations of highest concern for management purposes.
Table 2. Summary table of combinations of materials tested, decontamination treatments (Virkon, bleach, freezing, hot water, and drying), and application method (I = immersion, S = spray) for species used in the experiments.
We examined the use of chemical treatments (Virkon Aquatic, Formula 409 cleaner, bleach), hot water, freezing, and desiccation. Virkon Aquatic was used as a 2% solution, as stipulated in WIDNR guidelines. Strength of Virkon solutions was monitored using standard test kits (Western Chemical, Ferndale, WA). Tests with Clorox Formula 409 brand cleaner (Clorox Company, Oakland, CA) used full-strength solutions purchased at a local hardware store. Anecdotes from local fishermen indicated that Formula 409 is often used on waders after fishing in streams. Based on this we also tested whether mud would reduce the effectiveness of immersion or spray application of Formula 409. Mud was made fresh for each experiment from 100 g topsoil mixed with 60 mL aged tap water. Approximately 15 mL (1 tablespoon) of mud was used in each container for 15 NZMS. Bleach (Clorox Company, Oakland, CA) for testing NZMS and spiny water flea was employed during 2014 and 2015 at 400 ppm, the concentration recommended by WIDNR BMPs at that time. The recommended concentration for bleach was increased to 500 ppm following 2015, so this concentration was employed for tests on bloody-red mysids. Reports had indicated that age of bleach solution might affect killing effectiveness, perhaps due to volatilization of chlorine (Knight S, WIDNR, Jan 2014, pers. comm.). Consequently, we prepared fresh solutions daily for use in experiments.
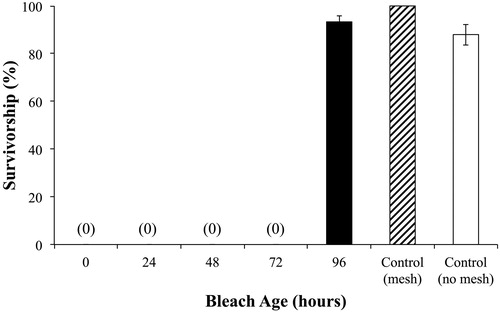
In a separate experiment we also specifically tested aging on the effectiveness of bleach for disinfection. Bleach was prepared in 1 L batches at a concentration of 500 ppm and allowed to sit in open containers for 0, 24, 48, 72, and 96 h before use. The ratio of surface area to liquid height in the containers was 6.5. These batches were maintained at a mean temperature of 21.7 C (SE = 0.1) with a relative humidity of 67.0% (SE = 3.0). All tests with differently aged bleach solutions were run simultaneously with a common set of controls. Bloody-red mysid were sprayed with the aged bleach solutions according to protocols detailed in the preceding.
Effectiveness of freezing was determined for spiny water fleas. Animals were frozen in water for 2, 4, 8, and 24 h prior to thawing and assessment of survivorship of animals and embryos. Survivorship of bloody-red mysid following desiccation for 1, 2, or 3 h was tested at 20.8 C (SE = 0.3) and average relative humidity of 60.0% (SE = 1.0). To test the effectiveness of Wisconsin BMP guidelines for hot water disinfection, bloody-red mysid survival was also determined following immersion in 60 C hot water (140 F). Batches of animals on nylon mesh were transferred to bowls of aged tap water maintained at 60 C in water baths, according to protocols detailed already.
Surfaces and materials tested
We employed the selected treatments on materials found on equipment used by professional staff and researchers. Materials included a synthetic line used for anchors (nylon, polyester, and polypropylene; Lehigh Group, Macaugie, PA), nylon mesh (Nitex) typically used for dip nets and plankton nets, neoprene and rubber from waders and hip boots (Hodgman Model W202GRN, Columbia, SC), canvas used for dip nets (Wildco, Yulee, FL), and felt soles from waders (Caddis Wading Systems, Inc., La Pine, OR). Combinations tested were chosen with guidance from WIDNR staff as those most likely to occur under normal management activities and for which data were lacking. In each experiment animals were gently placed on pieces of the material (5 cm × 5 cm). Many animals quickly attached to materials, while some moved on and off material during tests. All animals in containers were assessed equally for survivorship at the end of the experiment, regardless of whether or not they remained on materials.
Statistical analysis
We examined data for heteroscedasticity and normality and transformed with the arcsine procedure if required. Analysis of variance (ANOVA), followed by Tukey’s HSD post hoc tests, determined the significance of differences in survivorship among treatments within the same experiment. Significance levels of tests are reported as P values. For testing effectiveness of treatments at killing all individuals, 95% confidence intervals of final survivorship values were constructed for comparison with 0% survivorship. All statistical analyses were performed using PAST (Paleontological Statistics Package, version 3.1; Hammer et al. 2001).
Results
Virkon Aquatic
Immersion in Virkon Aquatic killed 100% of all four species within 20 min (). Consequently, there was no difference in effectiveness based on type of material when animals were completely immersed. Survivorship for both immersion and spray treatments in both control treatments was generally greater than 85%, with a low of 64% in one treatment. When animals were sprayed with Virkon Aquatic, none of the species survived after 20 min exposure, except for faucet snails. Using spray on nylon mesh, an average of 8.0% of faucet snails survived for 20 min. The 95% confidence interval for this treatment did not include zero (1.80–14.19%), meaning that spraying with Virkon-Aquatic on mesh was not completely effective for killing faucet snails. The time course of this treatment showed that about 80% of animals survived for 5 min, with less than 10% surviving to 10 min (). On average, 1.3% of faucet snails survived on synthetic line with a 95% confidence interval from –0.23% to 2.99%. No faucet snails were alive in the canvas test after 20 min (). Overall, there was a significant effect of materials tested on survivorship of faucet snails for spray tests (ANOVA, F2,12 = 6.89, P = 0.01).
Figure 2. Survivorship (mean ± 1 SE percent) of faucet snail (Bithynia tentaculata) following spray exposure on nylon mesh for various time periods to Virkon Aquatic (2%). For clarity purposes, the symbol for Control (mesh) results at 20 min is offset by 1 min on the graph.
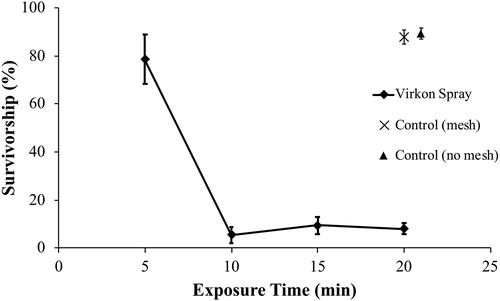
Table 3. Mean survivorship (percent) for animals exposed to Virkon Aquatic (2%) at longest time period tested. Results shown for immersion tests (I) and spray (S) applications. Standard error of mean shown in parentheses. Asterisk indicates that 95% confidence interval does not include 0% survivorship. Dash indicates treatment combinations that were not tested.
Bleach solution
Bleach treatment was not effective against NZMS but did kill spiny water flea and bloody-red mysid (). Spray application of bleach to NZMS was significantly less effective than immersion (2-way ANOVA; F1,40 = 17.32, P = 0.0001), and did not kill all animals on any of the materials tested (). Between 8% and 17.3% of animals survived spray exposure. There was no significant difference in survivorship among material treatments when sprayed with bleach (ANOVA, F4,20 = 0.95, P = 0.456). Extended duration of exposure to 25 min on synthetic line, rubber, and canvas did not improve the effectiveness of sprayed bleach. Immersing NZMS for 20 min in bleach was effective overall and survivorship was not significantly different from 0% except on canvas, where it averaged 12% and was significantly greater than on all other materials (all Tukey HSD comparisons; Q = 4.619, P = 0.028). Exposure to bleach effectively killed spiny water flea and bloody-red mysid in all treatments (). Interestingly, for spiny water flea some live embryos were observed in the brood sac of dead adults at all time periods and for both spray and immersion techniques, suggesting that bleach may not readily penetrate the brood chamber of these animals. We did not observe release of embryos from the brood sac when mothers were exposed to bleach.
Table 4. Mean survivorship (percent) for animals exposed to bleach for 20 min. Results shown for immersion (I) and spray (S) applications. Standard error of mean shown in parentheses. Bleach concentration was 400 ppm, except for bloody-red mysid tests, which employed 500 ppm. Asterisk indicates that 95% confidence interval does not include 0% survivorship. Dash indicates treatment combinations that were not tested.
Aging of bleach solution did not reduce killing effectiveness for bloody-red mysid sprayed on mesh until solution age was between 72 h and 96 h (). There was no significant difference in survivorship compared to controls after bleach solution was sitting in an open container for 96 h at 21.7 C (SE = 0.10) with an average relative humidity of 67% (SE = 3.0).
Formula 409 and mud effects
Formula 409 was completely effective for killing NZMS but the presence of mud reduced effectiveness (). For both immersion and spray application no animals survived 20 min exposure. Survivorship after spraying with Formula 409 when mud was present was significantly greater than 0% for all materials tested (). It was also greater than 0% when animals in mud were immersed on felt sole material.
Table 5. Mean survivorship (percent) for New Zealand mud snail exposed to Formula 409 for 20 min. Results shown for immersion and spray applications, and without mud or with mud present. Standard error of mean shown in parentheses. Asterisk indicates that 95% confidence interval does not include 0% survivorship.
Freezing
Freezing of spiny water flea was completely effective. No animals survived being sprayed with water and frozen for 2 h on synthetic line, nylon mesh, or neoprene. No animals survived freezing on the same materials for 4, 8 or 24 h. No embryos in brood sacs survived any of the freezing treatments.
Hot water
Immersion in hot water (60 C) was tested only for bloody-red mysid on nylon mesh (). Hot water killed 100% of animals in all replicates after 5, 10, and 15 min immersion. Most animals stopped moving after less than 2 min, and all turned an opaque white color by 5 min.
Desiccation
Desiccation on mesh effectively killed all bloody-red mysid after 1 h (mean survivorship = 2.7%; SE = 2.4%). All animals in all replicates were dead following drying for 2 h and 3 h.
Discussion
Our tests of the effectiveness of various combinations of disinfection treatments, applied either by immersion or as initial spraying, on materials most likely to be contaminated demonstrated differing efficacy among the four species examined (). Of the treatment combinations tested, only immersion in 2% Virkon Aquatic reliably killed all species tested on all materials. Not all treatments were applied to all AIS or to all materials, so this finding does not indicate that 2% Virkon Aquatic is the only effective treatment for decontamination. Treatment with bleach, the most widely recommended method, was not reliable against NZMS and did not kill all spiny water flea embryos in brood sacs. There were some differences in effectiveness of treatments depending on type of material tested, with canvas resulting in higher survivorship at times. One possibility is that there are differences in exposure due to differing absorbance of chemicals by the various materials. However, these differences were overshadowed by those between spraying and immersion, or choice of treatment employed (i.e., chemical, freezing, hot water, desiccation).
Table 6. Results summary of experimental combinations of materials tested and decontamination treatments (Virkon, bleach, freezing, hot water, and drying) for species used in the experiments. Treatments were considered either effective (E) or not effective (NE) for practical decontamination of sampling gear.
New Zealand mud snail
Previous research on treatment of gear contaminated by NZMS has shown that immersion in Virkon Aquatic (2%) for 20 min killed all animals, and that spraying significantly reduced effectiveness (Stockton and Moffit Citation2013). Stockton and Moffit (Citation2013) also showed that there are differences in rates of contamination of different materials (especially felt soles), but immersion in Virkon Aquatic killed all animals regardless of material tested. Our results are consistent with their findings showing immersion in Virkon Aquatic to be 100% effective against NZMS ( and ) and spraying to be less effective than immersion (). However, our results show that type of material can affect disinfection as well, with canvas reducing ability to kill animals (). New Zealand mud snail also is highly sensitive to Formula 409 treatment, whether sprayed or fully immersed for 20 min (), consistent with previous studies using products containing quaternary ammonium (Hosea and Finlayson Citation2005, Schisler et al. Citation2011, Stout et al. Citation2016).
Mud has been shown to reduce effectiveness of disinfection, and our results with the Formula 409 treatment are consistent with this conclusion (). Reasons for increased survival in mud may include reduction of oxidizing activity of active ingredients as has been found for Virkon Aquatic (Stockton and Moffit 2013), or perhaps prevention of animal contact with disinfecting chemicals. Alonso et al. (Citation2016) found that mud extended survival of NZMS during desiccation, indicating a protective microenvironment around animals, which could also reduce exposure to chemical disinfectants. Given these findings, it would be prudent to remove attached mud, sediment, or clay prior to chemical disinfection to maintain the effectiveness of disinfection protocols (e.g., Stockton and Moffit Citation2013, Alonso et al. Citation2016).
Faucet snail
Much less information is available about faucet snail survival during disinfection. Mitchell and Cole (Citation2008) concluded Virkon Aquatic was not effective against faucet snails. However, they immersed animals in more dilute concentrations than current guidelines recommend and for longer periods of time (i.e., 0.2% for 1 h and 0.16% for 24 h). As we show, immersion in 2% Virkon Aquatic is highly effective for both faucet snails and NZMS after 20 min immersion (). Due to the efficacy of Virkon Aquatic for faucet snails, we decided, in consultation with the WIDNR, not to conduct further tests on faucet snails and to focus additional testing on obtaining data for spiny water flea and bloody-red mysid disinfection.
Spiny water flea and bloody-red mysid
There was no difference in the effectiveness of 2% Virkon Aquatic between the types of materials on which spiny water flea and bloody-red mysid might reasonably be trapped, indicating that Virkon Aquatic is a highly effective method for both species (). In addition, no juveniles in spiny water flea brood chambers survived these treatments, in contrast to results for bleach. Bleach also killed both species effectively during total immersion and spray application, but juveniles in spiny water flea brood chambers often survived these treatments ( and see the preceding). Consequently, disinfection with bleach will be less effective than Virkon Aquatic because it will not kill all NZMS and some juvenile spiny water flea in brood chambers. One study has shown that hatching of spiny water flea resting eggs is not affected by immersion in 3.4% bleach for 5 min (Branstrator et al. Citation2013), adding support to the conclusion that bleach may not effectively stop the spread of spiny water flea. Further studies on the effects of Virkon Aquatic on spiny water flea resting egg survival would be helpful to determine if this treatment will be effective. Spiny water fleas in nearshore waters of Green Bay, Lake Michigan, have been shown to make resting eggs from June to October (Merkle and De Stasio Citation2018), so finding an effective combination of treatments will be important for slowing the spread of this AIS into inland waters.
There are few data available on tolerance to freezing of bloody-red mysid or spiny water flea, but Branstrator et al. (Citation2013) show that resting eggs of spiny water flea can survive freezing for at least 1 day if not immersed in water at time of freezing. When frozen in water, resting eggs do not hatch following 1 day of freezing. Our results add to this information by showing that freezing can effectively kill spiny water flea when frozen for as little as 2 h.
In a previous study, desiccation of bloody-red mysid on polyester mesh resulted in 50% mortality (i.e., LT50) after 0.15 day (3.6 h) and a predicted 90% mortality (LT90) after 0.95 day (22.8 h; Anderson et al. Citation2015). These lower mortality rates compared to our results could be due to lower temperatures employed (14 C), and possibly different humidity conditions (not reported). No data on desiccation of spiny water flea are available, except for effects on hatching of resting eggs. Resting eggs desiccated for 6 h at 17 C and 45% relative humidity failed to hatch, while some eggs did hatch after 4 h drying (Branstrator et al. Citation2013). Active animals would presumably have higher mortality rates than resting eggs, so desiccation effects could be similar to those we observed for bloody-red mysid. Based on these findings, drying and freezing gear for more than 1 d should kill all bloody-red mysid and spiny water flea adults and resting eggs.
Effectiveness of methods for multiple species
A large number of studies tested the use of hot water to decontaminate sampling gear, showing that immersion at 45 C for 15 min can be highly effective against a variety of AIS (Anderson et al. Citation2015). Our choice of testing 60 C was based on the recommended procedures from the WIDNR aimed at providing a single temperature that can be used for many AIS contaminations (WIDNR Citation2017). Immersion for 5 min at this temperature killed all bloody-red mysid. The only other data available for hot water treatment of this species resulted in 100% mortality after 15 min at 45 C (Anderson et al. Citation2015). Spiny water flea adults show 100% mortality after only 5 min at 43 C (Beyer et al. Citation2011), while resting eggs require 10 min at 50 C to prevent hatching (Branstrator et al. Citation2013). Hot water is also effective against NZMS, which has a critical thermal maximum near 30 C (Koopman et al. Citation2016). Faucet snails have only been tested using 50 C water, but this resulted in 100% mortality in less than 5 min (Mitchell and Cole Citation2008). Exposure to higher temperatures using steam has the potential to be effective for a wider array of situations, and this approach should be tested more carefully to determine conditions required to assure efficacy (i.e., effective distance between steam source and materials or animals, time of exposure, etc.).
Desiccation alone will not be effective or practical against multiple species, especially snails. Prior studies have demonstrated a wide range of tolerance by mollusks to desiccation, depending on temperature and relative humidity (e.g., Ricciardi et al. Citation1995). Faucet snails can survive desiccation for at least 2 weeks and for more than a month depending on conditions (Mitchell and Cole Citation2008, Wood et al. Citation2011, Havel et al. Citation2014). Survival times during desiccation are shorter for NZMS, with drying for 2–3 d typically sufficient to kill all animals (Richards et al. Citation2004, Alonso and Castro-Diez Citation2012).
Freezing could potentially be effective against multiple AIS, but conditions during treatment can affect success. For NZMS, freezing for 1 h during desiccation may be sufficient to produce 100% mortality (Richards et al. Citation2004), but studies with animals immersed in water and exposed to freezing temperatures show they can survive between 4 and 9 d (Cheng and LeClair Citation2011, Moffit and James 2012). Tolerance of faucet snails to freezing has not been published to date, so it may be best to assume they are similar to NZMS. Spiny water flea did not survive freezing for 2 h after being sprayed and allowed to dry while freezing, but resting eggs survive better when dried and frozen (Branstrator et al. Citation2013). This indicates that freezing may not effectively work against NZMS and spiny water fleas if resting eggs are present.
Based on our findings, immersion in 2% Virkon Aquatic for at least 20 min is the most highly recommended procedure for decontaminating sampling gear. This method will reliably kill NZMS and faucet snails, as well as spiny water flea and bloody-red mysid. Spray application was also highly effective, allowing it to be used on larger equipment such as boats. Removing mud and other debris from gear before disinfection will likely increase effectiveness, especially when using spraying methods. Virkon Aquatic is 99.9% biodegradable and can be disposed of in sanitary sewers, but a drawback is that protective gear should be worn when mixing solutions (i.e., nitrile gloves and chemical splash goggles/face shields; WIDNR 2017). Rinsing treated materials with clean water is recommended to reduce possible degradation of equipment following repeated exposure to Virkon Aquatic. Both freezing and immersion in hot water could kill all animals, but the logistics of these methods make them less desirable as a general disinfection approach and more easily affected by how procedures are conducted. Given the effectiveness of hot water treatment, further testing of steam for disinfection should be examined, especially if portable steamers can be employed in the field. We hope the information provided by this study can be used by management agencies, such as the WIDNR, to aid in protecting natural resources by reducing the spread and limiting the potential economic, environmental, and human health impacts of AIS.
Acknowledgements
We thank Anna Cohen, Savanna Dahl, Anne Ela, Michaela Giampetroni, Cady Greenslit, Cherise John, Emily Kiehnau, Wayne Krueger, Kathrine Ling, Casey Merkle, Alex Poli, Zoe Psarouthakis, JoAnn Stamm, Jori Warwick, and Rachel Wilson for field and laboratory assistance. We are indebted to Christina Wolbers for the initial impetus for the project. The study benefitted greatly from consultation and advice provided by WIDNR staff (Maureen Ferry, Susan Knight, and Scott Van Egeren). We thank Ken Wagner and four anonymous reviewers for helpful suggestions for improving the article.
Additional information
Funding
References
- Acy CN. 2015. Tolerance of the invasive New Zealand mud snail to various decontamination procedures. [honors thesis]. Appleton (WI): Lawrence University. http://lux.lawrence.edu/luhp/76
- Alonso A, Castro-Díez P. 2012. Tolerance to air exposure of the New Zealand mudsnail Potamopyrgus antipodarum (Hydrobiidae, Mollusca) as a prerequisite to survival in overland translocations. NeoBiota. 14:67–74. doi:10.3897/neobiota.14.3140.
- Alonso A, Valle-Torres G, Castro-Diez P. 2016. Survival of an invasive aquatic snail to overland translocation in non-aquatic media: implications for spreading. Limnologica. 57:60–65. doi:10.1016/j.limno.2016.01.002.
- Anderson LG, Dunn AM, Rosewarne PJ, Stebbing PD. 2015. Invaders in hot water: a simple decontamination method to prevent the accidental spread of aquatic invasive non-native species. Biol Invasions. 17(8):2287–2297. doi:10.1007/s10530-015-0875-6.
- [ANSTF] Aquatic Nuisance Species Task Force. 2013. Voluntary guidelines to prevent the introduction and spread of aquatic invasive species: recreational activities [Accessed 4 December 2018]. https://anstaskforce.gov/default.php.
- Beyer J, Moy P, De Stasio B. 2011. Acute upper thermal limits of three aquatic invasive invertebrates: hot water treatment to prevent upstream transport of invasive species. Environ Manage. 47(1):67–76. doi:10.1007/s00267-010-9573-4.
- Branstrator DK, Shannon LJ, Brown ME, Kitson MT. 2013. Effects of chemical and physical conditions on hatching success of Bythotrephes longimanus resting eggs. Limnol Oceanogr. 58(6):2171–2184. doi:10.4319/lo.2013.58.6.2171.
- Cheng YW, LeClair LL. 2011. A quantitative evaluation of the effect of freezing temperatures on the survival of New Zealand mudsnails (Potamopyrgus antipodarurn Gray, 1843), in Olympia Washington's Capitol Lake. Aquat Invasions. 6(1):47–54. doi:10.3391/ai.2011.6.1.06.
- Drake D, Andrew R. 2017. Overland spread of aquatic invasive species among freshwater ecosystems due to recreational boating in Canada. Can. Sci. Advis. Sec. Res. Doc. 31:1–38.
- Ellis S, MacIsaac HJ. 2009. Salinity tolerance of Great Lakes invaders. Freshw Biol. 54(1):77–89. doi:10.1111/j.1365-2427.2008.02098.x.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol Electronica. 4:1–9.
- Havel JE. 2011. Survival of the exotic Chinese mystery snail (Cipangopaludina chinensis malleata) during air exposure and implications for overland dispersal by boats. Hydrobiologia. 668(1):195–202. doi:10.1007/s10750-010-0566-3.
- Havel JE, Bruckerhoff LA, Funkhouser MA, Gemberling AR. 2014. Resistance to desiccation in aquatic invasive snails and implications for their overland dispersal. Hydrobiologia. 741(1):89–100. doi:10.1007/s10750-014-1839-z.
- Havel J E, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB. 2015. Aquatic invasive species: challenges for the future. Hydrobiologia. 750:147–170. doi:10.1007/s10750-014-2166-0.
- Hosea RC, Finlayson B. 2005. Controlling the spread of New Zealand mud snails on wading gear. California Department of Fish and Game, Sacramento, CA.
- Koopman KR, Collas FPL, van der Velde G, Verberk WCEP. 2016. Oxygen can limit heat tolerance in freshwater gastropods: differences between gill and lung breathers. Hydrobiologia. 763(1):301–312. doi:10.1007/s10750-015-2386-y.
- Merkle CA, De Stasio BT. 2018. Bythotrephes longimanus in shallow, nearshore waters: interactions with Leptodora kindtii, impacts on zooplankton, and implications for secondary dispersal from southern Green Bay, Lake Michigan. J. Great Lakes Res. 44(5):934–942.
- [MIDEQ] Michigan Department of Environmental Quality. 2018. Invasive species decontamination for field operations in Michigan [accessed 15 July 2018]. https://www.michigan.gov/documents/deq/qol-wrd-policy-invasive-species-decontamination_476846_7.pdf
- Mitchell AJ, Cole RA. 2008. Survival of the faucet snail after chemical disinfection, pH extremes, and heated water bath treatments. N Am J Fish Manage. 28(5):1597–1600. doi:10.1577/M07-211.1.
- Moffitt CM, James CA. 2012. Response of New Zealand mudsnails Potamopyrgus antipodarum to freezing and near-freezing fluctuating water temperatures. Freshw Sci. 31(4):1035–1041. doi:10.1899/11-160.1.
- [NYDEC] New York Department of Environmental Conservation. 2018. A New York boater’s guide to cleaning, drying and disinfecting boating equipment [accessed 15 July 2018]. http://www.dec.ny.gov/docs/lands_forests_pdf/boatdisinfect.pdf
- Ricciardi A, Serrouya R, Whoriskey FG. 1995. Aerial exposure tolerance of zebra and quagga mussels (Bivalvia, Dreissenidae)—implications for overland dispersal. Can J Fish Aquat Sci. 52(3):470–477. doi:10.1139/f95-048.
- Richards DC, O'Connell P, Shinn DC. 2004. Simple control method to limit the spread of the New Zealand mudsnail Potamopyrgus antipodarum. N Am J Fish Manage. 24(1):114–117. doi:10.1577/M02-133.
- Rothlisberger JD, Lodge DM. 2013. The Laurentian Great Lakes as a beachhead and a gathering place for biological invasions. Aquat Invasions. 8(4):361–374. doi:10.3391/ai.2013.8.4.01.
- Schisler GJ, Vieira NKM, Walker PG. 2011. Application of household disinfectants to control New Zealand mudsnails. N Am J Fish Manage. 28(4):1172–1176. doi:10.1577/M07-028.1.
- Sinclair JS, Arnott SE. 2016. Strength in size not numbers: propagule size more important than number in sexually reproducing populations. Biol Invasions. 18(2):497–505. doi:10.1007/s10530-015-1022-0.
- Sinclair JS, Arnott SE. 2017. Relative importance of colonist quantity, quality, and arrival frequency to the extinction of two zooplankton species. Oecologia. 184(2): 441–452. doi:10.1007/s00442-017-3874-8.
- Stockton KA, Moffitt CM. 2013. Disinfection of three wading boot surfaces infested with New Zealand mudsnails. N Am J Fish Manage. 33(3):529–538. doi:10.1080/02755947.2013.768569.
- Stout JB, Avila BW, Fetherman ER. 2016. Efficacy of commercially available quaternary ammonium compounds for controlling New Zealand mudsnails Potamopyrgus antipodarum. N Am J Fish Manage. 36(2):277–284. doi:10.1080/02755947.2015.1120830.
- [WIDNR] Wisconsin Department of Natural Resources. 2017. Best management practices (BMP) to prevent and minimize the spread of invasives [accessed 20 December 2017]. http://dnr.wi.gov/topic/invasives/bmp.html
- Wood AM, Haro CR, Haro RJ, Sandland GJ. 2011. Effects of desiccation on two life stages of an invasive snail and its native cohabitant. Hydrobiologia. 675(1):167–174. doi:10.1007/s10750-011-0814-1.
- [WGFD] Wyoming Game and Fish Department. 2018. Wyoming aquatic invasive species fire equipment inspection and decontamination manual [accessed 15 July 2018]. https://gacc.nifc.gov/gbcc/dispatch/wy-tdc/documents/logistics-dispatch/aviation/2017_FEID_Manual_FINAL_052917.pdf</bi>