Abstract
Stager JC, Wiltse B, Cumming BF, Holsen TM, Stetler J, Laxson C, Marcillo CE, Charles DF. 2019. A novel ecological state at Bear Pond (Adirondack Mountains, NY, USA) following acidification and partial recovery. Lake Reserv Manage. 35:208–223.
The pH of precipitation in the northeastern United States has risen as industrial sulfur and nitrogen oxide emissions have declined following amendment of the Clean Air Act in 1990, but the effects of this change on the region's lakes are not yet fully documented. Here we use the siliceous remains of diatoms and chrysophyte algae in a sediment core from Bear Pond (Adirondack State Park, NY), which acidified during the 20th century, to reconstruct pH variability during the last 2 centuries, evaluate the extent to which the lake has recovered from acidification, and determine the timing of that reversal to help identify its causes. Inferred pH declined erratically from a high of 6.4 during the 1860s to 5.7–5.9 during the 1930s, then decreased to the 4.9–5.4 range by 1995–2010. After 2010, inferred pH rose and remained within the 5.4–5.6 range following an abrupt rise in the pH of regional precipitation. Although the acidification trend at Bear Pond has now reversed, the lake ecosystem has not returned to its pre-impact condition. Distinctive members of the pre-acidification diatom community are still absent, chrysophytes have become unusually abundant, and water clarity (Secchi depth) has decreased by about half. Furthermore, the original fish community was lost due to stocking and piscicide treatment in 1958, and high concentrations of toxaphene residues from the piscicide are present in the sediments. Full ecological restoration of formerly acidic lakes such as Bear Pond may be unlikely due to complicating factors such as climate change and fisheries management practices.
Acid deposition or "acid rain" (Likens et al. Citation1979) has been widely recognized as a serious environmental problem in eastern North America since the 1970s, particularly in the Adirondack region of northern New York (Charles Citation1984, Citation1990, Davis et al. Citation1988, Cumming et al. Citation1992, Citation1994, Sullivan et al. Citation2018). Following modifications to the Clean Air Act in 1990, emissions of sulfur and nitrogen oxides (SOx and NOx, respectively) from fossil fuel combustion have declined nationwide and reduced acidic deposition in the Adirondacks (Jenkins et al. Citation2007, Mitchell et al. Citation2013, Rattigan et al. Citation2017, NADP Citation2018a, Citation2018b). As a result, some Adirondack waters have begun to recover from their formerly acidified condition (Jenkins et al. Citation2007, Arseneau et al. Citation2011, Josephson et al. Citation2014, Strock et al. Citation2014, Driscoll et al. Citation2016, Sullivan et al. Citation2018).
Bear Pond, a remote wilderness lake in the 2.4 million ha Adirondack State Park (), was among the sites studied during the 1980s in the PIRLA project (Paleoecological Investigations of Recent Lake Acidification), which employed paleolimnological techniques to determine the timing and origins of lake acidification in the region (Charles and Whitehead Citation1986, Charles et al. Citation1990, Jenkins et al. Citation2007, Smol Citation2008). Fossil diatoms preserved in a 39 cm sediment core collected from Bear Pond by co-author DFC indicated that the lake's inferred surface pH decreased from values close to 5.9 during the mid-19th century to ∼5.5 by the early 1980s (Charles et al. Citation1990). Although the pH of regional precipitation has risen since then (Jenkins et al. Citation2007, NADP Citation2018a), the effects on Bear Pond have yet to be documented.
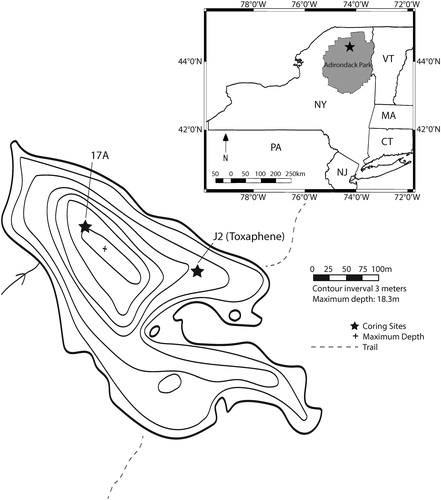
Figure 1. Site map. Depth contours after Adirondack Lakes Survey Corporation data collected in 1985 (ALSC Citation2018). 17A: location of core used for diatom-based pH reconstruction. J2 (Toxaphene): location of core used to test for the presence of residues from piscicide treatment in 1958.
In addition to its scientific value, Bear Pond also developed national symbolic value in regard to acid deposition. US Senator Gary Hart visited the lake during the 1980s to illustrate the harmful effects of acid rain, and in 2018 Senator Charles Schumer visited it to highlight a rise in the lake's pH after modification of the Clean Air Act. In the absence of a continuous long-term monitoring program, however, the precise timing of the pH transition is unknown, and attributing the pH shift to precipitation chemistry alone remains speculative.
In this study, we use siliceous microfossils and geochemical profiles from lake sediments to reconstruct environmental conditions in Bear Pond during the last 2 centuries with greater detail and a longer time frame than were used in the PIRLA project. We focus primarily on changes in the diatom and chrysophyte records in sediment core 17A to address the following questions: (1) How did the surface pH of Bear Pond change during the last 2 centuries? (2) When did the acidification trend reverse? (3) What environmental factors contributed to that reversal? In addition, we examine Bear Pond's ecological history to evaluate the extent to which the lake's chemistry and biotic community have returned to their pre-acidified condition.
Study site
Bear Pond (44°24'06"N; 74°17'11"W) is a seepage lake with a surface area of 22 ha (maximum depth 18 m, mean depth 7 m) surrounded by a mixed forest of northern hardwoods and conifers (ALSC Citation2018). It is situated on glacial till and silt deposits atop anorthositic bedrock within a small watershed (86 ha, 499 m elevation) in the Saint Regis Canoe Area of the Adirondack State Park (). The shoreline of the lake is a mix of public and private land, with the only permanent structures consisting of several rustic camping shelters. Bear Pond lacks major surface inlets and outlets, but a notch in a low ridge on the eastern end of the lake that currently serves as a portage route probably represents a down-cutting channel from a previous high-water stand.
To our knowledge there are no published records of major logging activity or forest fires within the watershed, although logging and fires have affected much of the Adirondacks since the mid-19th century. However, changes in growth ring width in wood borings collected from conifers by DFC in 1978 indicated that an unidentified ecological disturbance might have disrupted local forest growth somewhat during the 1940s.
In the past, Bear Pond has been a popular recreational destination because of its clarity and striking aqua blue color, in spite of a demanding access route. Field notes taken by DFC in 1979 described it as "very clear … like a swimming pool" with a Forel-Ule color of 5–6. During the 1970s and 1980s, the concentrations of dissolved organic carbon (DOC) in surface waters were low (0.7–1.1 mg/L-C), Secchi disk measurements varied between 6.9 and 14.5 m, and surface pH varied between 4.6 and 5.1 (). Since sampling resumed in 2014, DOC concentrations have risen (3.9–4.9 mg/L-C), Secchi disk measurements decreased to the 2.5–5.0 m range, and pH has risen to the 5.5–6.8 range (). Phytoplankton samples collected between May and September during 2016–2018, by co-author JCS have been dominated by dinoflagellates (Peridinium spp.), chrysophytes (Mallomonas spp., Dinobryon spp., and Synura spp.), green algae (Staurastrum spp.), and diatoms (Asterionella ralfsii W. Smith, Tabellaria flocculosa Roth (Kützing), and others). Synura were also common beneath ice cover in February 2018.
Table 1. Surface pH units (air equilibrated), Secchi disk depth, and DOC measurements from Bear Pond.
The submerged botanical community of Bear Pond has been characterized previously by Jackson and Charles (Citation1988). During the 1970s and 1980s macrophytes including Eriocaulon sp. and Isoëtes sp. were frequently observed in water depths of 3 m or more, and Lobelia sp. and Nuphar sp. were common in the littoral zone (DFC, JCS). In 2017, none of those plants were observed but red-brown, fibrous macrophytes (Fontinalis?) were dredged from depths of up to 15 m in the main basin of the lake (JCS).
Mean annual temperatures in the Adirondack uplands since 1990 averaged 5.5 C and mean annual precipitation was approximately 1 m/yr (USHCN Citation2017). In addition to rising temperatures and decreasing ice cover (Beier et al. Citation2012), regional precipitation, lake levels, and river discharge have increased since the 1960s (Stager et al. Citation2009). As a result, a small island that was present in Bear Pond during the late 19th century (Wallace Citation1880) and a large boulder near the northern shore ("swimming rock"), both observed by DFC and JCS during the 1970s and 1980s, have been submerged for the last decade or more.
A native fish community including brook trout (Salvelinus fontinalis), white suckers (Catostomus commersonii), brown bullheads (Ictalurus nebulosus), pumpkinseeds (Lepomis gibbosus), creek chubs (Semotilus atromaculatus), common shiners (Luxilus cornutus), and an unidentified species of dace was documented in 1930 by the New York State Department of Environmental Conservation (DEC). The lake has been stocked with numerous genetic strains of brook trout almost continuously since 1929 (Horn Lake, Honnedaga, Randolph, Windfall, Assenica, Paradise, Crown Point, and an experimentally produced strain), including at least one possible case in 1963 of accidental aerial stocking of fish that were intended for another lake (DEC unpubl. data). Recreational fishing success was typically low, however, perhaps due in part to the extreme clarity of the water, which made boats and angling equipment more apparent to the fish. Lake trout (Salvelinus namaycush) were present, along with bullheads, suckers, and brook trout, in 1955 and 1958.
Yellow perch (Perca flavescens) were also reported to be abundant for the first time in 1955 (DEC, unpubl. data), and in August 1958, Bear Pond was treated with 10 gallons of rotenone and 45 gallons of toxaphene (ALSC Citation2018) to eliminate them. The amount of toxaphene used per unit of lake volume for the treatment, a procedure referred to as "reclamation" by the DEC (Harig and Bain Citation1995, Demong Citation2001), was substantially higher than in a reclamation of another Adirondack lake (Black Pond) in 1958 (ALSC Citation2018). Unpublished DEC records and interviews with DEC fisheries employees have indicated that several times the normal amount of toxaphene was used inadvertently and that the lake therefore failed to "clear up" (i.e., was unsuitable for fish survival) until 1961, when stocking resumed. In fishery surveys conducted in 1967 by the DEC (unpubl. data) and in 1985 by the Adirondack Lakes Survey Corporation (ALSC Citation2018), only brook trout and bullheads were reported.
Materials and methods
Core J2 (55 cm long), intended for toxaphene analysis, was hand driven into the sediment at 8.5 m depth in Bear Pond by divers in June 2014 (), using a 1 m long section of polyvinyl chloride (PVC) conduit pipe (10 cm diameter). The relatively shallow site was selected because of logistical difficulties in the field with the diving team that prevented sampling at greater depth. The upper 26 cm of sediment in J2 was extruded vertically in 1 cm increments after removal of the outermost 1 cm of sediment from each section, sealed in glass jars, and refrigerated prior to analysis.
Toxaphene content of sediments in core J2 was analyzed using an accelerated solvent extraction (Dionex ASE 350) followed by Florisil cleanup. The concentrations of toxaphene parlars in the concentrate were determined using a gas chromatograph coupled to a PolarisQ (ThermoFinnigan, San Jose, CA, USA) with a 60 m × 0.25 mm × 0.25 μm J&W DB-XLB capillary column (after Xia et al. Citation2012). Electron ionization was conducted at 70 eV with an emission current of 100 μA. Fragment ion m/z 125 was isolated as the parent ion with a default width of 3 μm. The secondary fragment m/z 89 was chosen for quantification. PCB 204, as an internal standard, was quantified in selective ion monitoring mode using m/z 430. Five-point calibration curves were generated using the technical standard and selected congeners before sample analysis. Response factors were calculated relative to the internal standard (PCB 204). Selected individual congeners were identified by comparison of retention times and mass spectra obtained from standard solutions. Method blanks and NIST SRM 1946 were analyzed with every 10 samples and duplicates were processed every 20 samples. The average SRM recoveries ranged between 70% and 140% for parlars 26, 50, and 62 with relative standard deviations of 7–24%. Total toxaphene and individual parlars were below the detection limits in all method blanks.
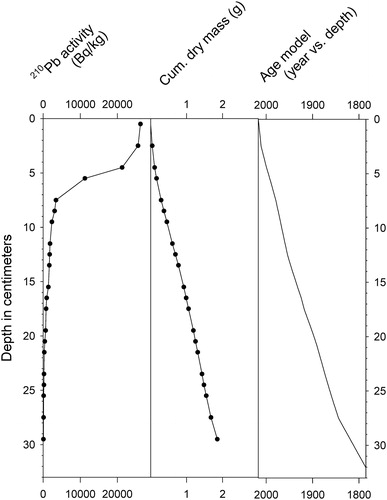
Core 17A (32 cm long) was collected from 18 m depth near the center of the lake () with a Glew gravity corer in June 2017. The mud–water interface appeared to be intact, with vertical chironomid tubes present. The entire core was subsampled vertically in 0.5 cm increments, and subsamples were stored in Whirlpak bags under refrigeration prior to analysis. An age–depth model for the core was constructed by D. Engstrom and colleagues (Science Museum of Minnesota) by measuring 210Pb activity with depth through alpha counting and applying the constant rate of supply model (Appleby and Oldfield Citation1978). Sediment organic content estimates were obtained at 1 cm intervals by determination of percent weight loss on ignition (LOI) at 500 C (Sutherland Citation1998, Heiri et al. Citation2001).
Samples for microfossil analysis were diluted in distilled water to a thin slurry, pipetted and dried on coverslips, and mounted on glass slides with Permount mounting medium. Microscopic examination of the prepared slides showed little clumping or viewing interference from organic detritus that would require removal by chemical oxidation or other common treatments for preparation of diatom samples. The samples were therefore not subjected to chemical processing, centrifuging, and repeated decanting, which can damage or eliminate delicate siliceous microfossils, an approach that has also been used successfully elsewhere (Stager et al. Citation2018).
At least 500 diatom valves per sample from the 17A core were identified at 1000× magnification under oil immersion to species level or, when possible, to variety level using the PIRLA diatom iconograph (Camburn et al. Citation1986) and other standard references (Patrick and Reimer Citation1966, Citation1975, Krammer and Lange-Bertalot Citation1991, Spaulding et al. Citation2010, Burge and Edlund Citation2017). Diatom assemblages in the J2 core were enumerated at coarser temporal and taxonomic resolution for general comparative stratigraphic purposes only. In both cores, siliceous scales and cysts of chrysophytes were enumerated along with the diatoms. Diatom-inferred pH values were generated for the 17A core using a maximum-likelihood model developed from the northeastern North American (NENA) 494-lake training set following the recommendations of Ginn et al. (Citation2007).
Results
Total 210Pb activity in the 17A core declined steeply with depth below the 5 cm interval, reaching background levels well above the base of the core (). The date of deposition for the 5 cm depth increment based upon the 210Pb activity was about 1995, and that of the basal sample (31.5 cm) was about 1790–1800. No radiometric ages were obtained for core J2 because of the incompleteness of the sediment record indicated by the microfossil stratigraphy (discussed later). Sediment organic content (LOI) in core 17A varied between 43 and 62% with a mean value of 54% (). Low values of 43–47% persisted between 15 and 5 cm depth.
Figure 3. Stratigraphic summary of the most common diatom taxa in the 17A core, along with percent weight loss on ignition (LOI) and ratios of chrysophyte scales to diatoms (S:D ×10). Letters and dotted lines indicate assemblage zones A–D, each with estimated dates indicated.
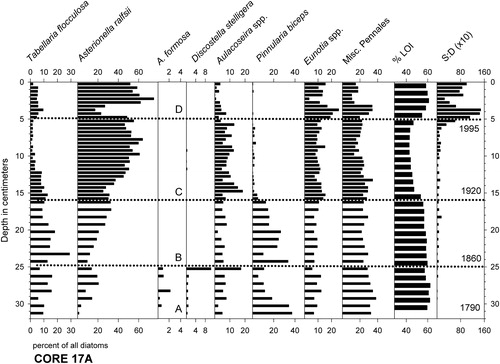
The most numerous planktonic taxa in the oldest, lowermost sediments of core 17A were T. flocculosa strain IIIP and elongated varieties of A. ralfsii (A. ralfsii var. americana), but the latter taxon became much more abundant than T. flocculosa in the upper sediments (). Several planktonic to tychoplanktonic Aulacoseira species were also moderately common, primarily varieties of A. lirata and the A. distans complex sensu lato (Spaulding et al. Citation2010). Percentages of T. flocculosa increased again in the uppermost 5 cm of the core where chrysophyte scales also became abundant. A diverse assortment of pennate diatoms represented approximately one-third of the taxa in the assemblages. Members of the genus Eunotia were particularly numerous, but Cymbella, Frustulia, Navicula, Neidium, Pinnularia, and other genera were present as well. Within the genus Eunotia, E. exigua Brébisson ex Kützing, E. septentrionalis Østrup, and E. bilunaris (Ehrenberg) Schaarschmidt were among the most common, although they rarely exceeded 2% of the assemblages. We visually grouped distinctive microfossil assemblages within core 17A into 4 stratigraphic zones for purposes of discussion here (). Details of that zonation are as follows:
Zone A (31.75 cm–24.75 cm): This zone was unique in that it contained small numbers of Discostella stelligera (Cleve & Grunow) Houk & Klee and A. formosa Hassal. The diatom assemblages were otherwise characterized by percentages of Pinnularia biceps W Gregory that decreased upward in the core from initially high (∼40%) to low values (∼10%), as well as variable percentages of T. flocculosa var. IIIP (∼10–15%) and increasing A. ralfsii (∼5–20%).
Zone B (24.75 cm–16.0 cm):Apart from a rise in the percentages of A. ralfsii and decrease in P. biceps, the diatom assemblages in this zone resembled those of Zone A, but without D. stelligera or A. formosa being present. Percentages of P. biceps were again high at the base of the section but decreased to ∼15%, while A. ralfsii percentages rose from ∼10% to ∼30%.
Zone C (16.0 cm–4.75 cm): Percentages of A. ralfsii reached a sustained peak (∼50–60%), percentages of P. biceps were close to 5–10%, and percentages of T. flocculosa strain IIIP were the lowest of the entire record (∼1–5%).
Zone D (4.75 cm–0.0 cm): Percentages of A. ralfsii fluctuated between low (∼20%) and high values (∼60–70%), and were mirrored by opposing fluctuations in the percentages of pennate diatoms. Percentages of T. flocculosa strain IIIP increased slightly to the 5–10% range, and the 6–3 cm interval contained small (<1%) but persistent numbers of Fragilariforma acidobiontica (Charles) D.M. Williams and Round. The scales of chrysophyte algae became roughly 10 times more abundant than diatom valves in the uppermost 5 cm. They were mostly represented by the genus Mallomonas, particularly taxa resembling M. crassisquama (Asmund) Fott. and M. duerrschmidtiae Siver, Hamer, & Kling. Chrysophyte cysts also became more numerous. (Siver Citation1991)
Mean diatom-inferred pH values in the 17A record ranged between 4.9 and 6.4 (), varying between 5.9 and 6.4 in the lowest 15 cm of the core (mean 6.2) and declining erratically through most of the shallower depth intervals (mean 5.6). Minimum values of 4.9–5.4 were reached in the 5.0–2.5 cm interval, above which pH increased to the 5.4–5.6 range. The overall pattern of variability in the pH reconstruction resembled that displayed by the percentages of P. biceps and was strongly correlated with them (r2 = 0.7, p < 0.0001). Percentages of A. ralfsii had a weaker, negative, but still significant influence on inferred pH values as well (r2 = 0.4, p < 0.0001).
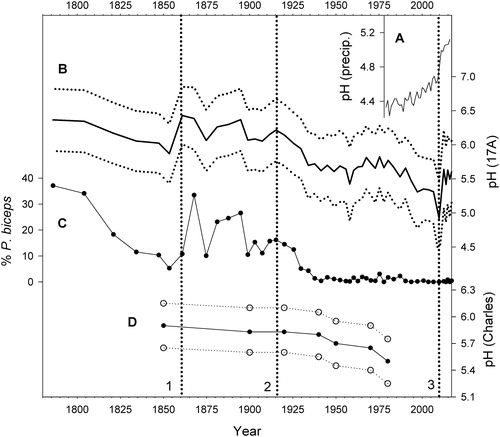
Figure 4. Diatom- and field-based measures of pH history at Bear Pond. (A) Precipitation pH at Huntington Wildlife Forest, Newcomb, NY (NADP Citation2018a). (B) Inferred pH based upon the diatom record of core 17A, with mean and maximum/minimum values indicated by solid and dotted lines, respectively. (C) Percentages of P. biceps in the 17A core, which strongly influenced the inferred pH values. (D) Inferred pH derived from the PIRLA diatom analysis by Charles et al. (Citation1990). Vertical lines indicate (1) 1860 onset of long-term pH decline, (2) 1915 onset of steeper decline, and (3) pH reversal ∼2010.
Three toxaphene parlars were identified throughout most of the J2 core (parlars 26, 50, and 62) at concentrations ranges of 0–4 ng/g, 9–45 ng/g, and 4–16 ng/g wet weight, respectively. The total combined concentration of toxaphene parlars ranged between 11 and 59 ng/g, with maximum concentrations of 48–59 ng/g in the 16–18 cm interval ().
Figure 5. Common diatom genera, ratios of chrysophyte scales to diatoms (S:D × 10), and concentrations of toxaphene in core J2.
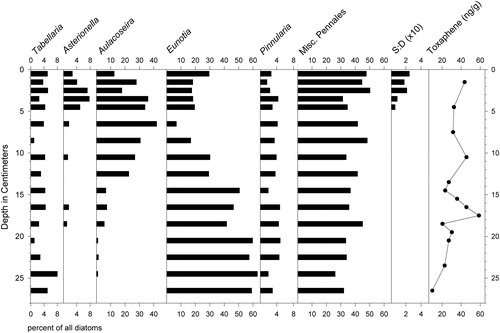
An aquatic macrophyte (Fontinalis?) was abundant in the uppermost and lowest centimeter of the J2 core, suggesting that the stratigraphic integrity of the core might have been compromised by sediment disturbances and/or frictional interference with the core barrel during collection in the field. In addition, a relative scarcity of chrysophyte scales and A. ralfsii, which were abundant in the youngest layers of core 17A (), indicated that the most recent sediments were missing from core J2 (). The divers who collected the core reported dark, turbid working conditions that made it difficult to determine whether benthic macrophytes offered significant resistance to penetration of the core barrel and/or whether the sediment–water interface was retrieved intact. On the basis of these results, radiometric dating of the J2 core was not performed.
Discussion
The microfossil and geochemical records of core 17A, along with published and unpublished historical literature, document major ecological changes in Bear Pond during the last 2 centuries. From ∼1800 to ∼1860, the lake supported a planktonic diatom community that included small but persistent numbers of A. formosa and D. stelligera, which are typically found in temperate zone lakes that have not been severely acidified (Sivarajah et al. Citation2017). Percentages of bottom-dwelling pennate diatoms were generally higher than in younger assemblages, which probably reflects strong light penetration to the benthos due to high clarity of the water and/or low standing crops of phytoplankton.
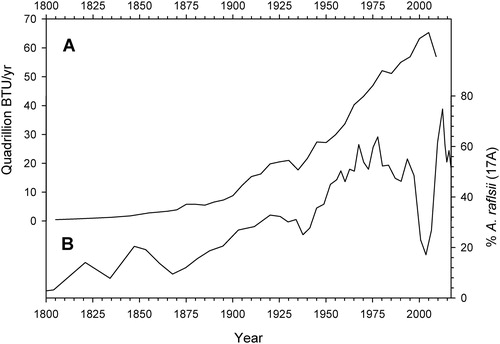
During most of the 19th century, inferred pH values varied mainly within the 6.0–6.4 range, but the composition of the planktonic diatom community began to change markedly during the mid-19th century. Percentages of A. ralfsii rose after A. formosa and D. stelligera disappeared from the lake sometime around 1860 (). Because A. ralfsii is an acidophilic to acidobiontic species (McIntyre and Duthie Citation1993; Dixit et al. Citation2002; Sivarajah et al. Citation2017), and because inferred pH declined erratically by ∼0.3 units during the second half of the 19th century (), the increase in A. ralfsii likely represents an early response to the onset of acidic precipitation due to industrial activities. The rise of A. ralfsii percentages coincided with increasing combustion of wood and fossil fuels in the United States (), which would have increased the deposition of SOx, NOx, and other atmospheric contaminants in the Adirondacks, which lie downwind of the center of the continent. The A. ralfsii trend might also reflect moderate nutrient enrichment through the deposition of airborne soot, nitrogen compounds, and/or agricultural dust from midcontinental sources that could have increased phytoplankton productivity in a small, oligotrophic lake such as Bear Pond. In either case, this lower portion of the 17A record appears to represent some of the earliest known evidence of atmospheric pollution impacts in the Adirondacks. The visually apparent similarities between the emissions and A. ralfsii curves ceased, however, after the 1970s () when additional ecological factors complicated the diatom record, as discussed below.
Figure 6. Percentages of A. ralfsii in core 17A plotted with total energy generation from wood, coal, petroleum, and natural gas in the United States (USEIA Citation2019) since the early 19th century.
Acidification during the 20th century
A sustained reduction in the organic matter content (% LOI) of the sediments deposited at the 17A coring site took place between the 1930s and 1990s (). This change might reflect reduced organic matter flux from surrounding soils and/or inhibition of primary productivity, both of which would be likely to occur as the lake became more acidic (Seekel, Lapierre, and Karlsson Citation2015, Seekel, Lapierre, Ask, et al. Citation2015, Solomon et al. Citation2015, SanClements et al. Citation2018).
The chemical record derived from core 17A indicates that Bear Pond experienced an irregular pH decline from a high of 6.4 during the 1860s to 6.1–6.2 around 1915–1920, followed by a steeper decrease into the 1930s when pH varied between 5.6 and 5.7 (). A more gradual decline followed, with pH values falling to the 4.9–5.4 range by ∼1995 to 2010. The onset of major acidification during the early 20th century has been documented in other Adirondack lakes as well (Cumming et al. Citation1994, Josephson et al. Citation2014), and a period of minimum pH also occurred at Brooktrout Lake, NY, between 2000 and 2010 (Josephson et al. Citation2014). The inferred pH trend at Bear Pond reversed around 2010 and rose by ∼0.5 pH units thereafter.
Our diatom-derived pH values for Bear Pond are generally consistent with observational data since the 1950s with the exception of lower values measured during the 1970s by DFC (). The integration of several years of diatom record within each core subsample might account for some of the differences between inferred pH values and the single-point field observations (). Our pH record is also generally consistent with that obtained by Charles et al. (Citation1990) during the PIRLA study (). In addition, the magnitude of the pH decline during the 20th century indicated by the diatom record of core 17A (roughly 1 pH unit) was similar to that reported from several other Adirondack seepage lakes where the groundwater supply was already somewhat acidic to begin with due to organic acids from forest soils and/or granitic bedrock (Charles et al. Citation1990, Cumming et al. Citation1992, Citation1994, Dixit and Smol Citation1995, Jenkins et al. Citation2007).
Reversal of acidification: Timing and causes
The 17A diatom record indicated an increase of roughly 0.5 pH units to the present day, beginning around 2010 (). The acidification trend ended in Bear Pond several years later than in most other Adirondack lakes for which long-term chemical records are available (Josephson et al. Citation2014, Driscoll et al. Citation2016, ALSC Citation2018, Sullivan et al. Citation2018), and it did not happen until nearly 2 decades after the 1990 amendment of the Clean Air Act. A rise in the relative abundances of benthic pennate diatoms around 1995–2010 was accompanied by a pronounced decrease in percentages of planktonic A. ralfsii () that ended the formerly close similarities between the A. ralfsii and fuel combustion records (). Because the 17A diatom data represented percentages of various taxa rather than absolute abundances, they cannot readily reveal whether the relative increase in the benthic fraction was due to expansion of the benthic community or a decline in A. ralfsii. However, the latter option is more likely because planktonic chrysophytes also became unusually abundant then () and would have competed with A. ralfsii for light and nutrients. In contrast, the presence of diagnostic pennate taxa indicative of low pH, such as F. acidobiontica, suggests that the low inferred pH values for that time period were not merely statistical artifacts of the replacement of A. ralfsii with nondiatom phytoplankton but instead represented a true pH minimum.
Figure 7. Trends in Adirondack climate, fossil fuel emissions, and major components of the phytoplankton community in Bear Pond during the last century. (A, B) Temperature and precipitation at Lake Placid, NY (USHCN Citation2017). (C, D) Sulfur and nitrogen oxide emissions, respectively, from the electrical power sector of the United States (USEPA Citation2019). (E) Ratio of chrysophyte scales to diatoms in core 17A (S:D × 10). (F) Percentages of A. ralfsii in the 17A core.
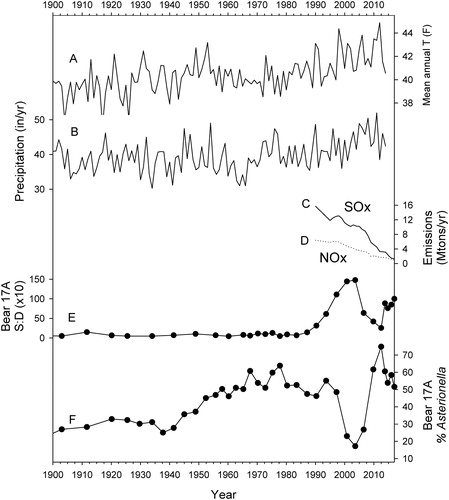
We know of no direct pH measurements from Bear Pond between 1985 and 2014 with which to confirm the exact timing of the pH shift (). However, the pH reversal around 2010 inferred from core 17A followed an abrupt decrease in SOx emissions () and approximately coincided with an accelerated rise in the pH of precipitation () (Strock et al. Citation2014, Driscoll et al. Citation2016, Rattigan et al. Citation2017, NADP Citation2018a, Citation2018b, Sullivan et al. Citation2018), which offers a plausible explanation for the change in lake pH. Other factors might also have been involved to some extent because the pH of regional precipitation had already been rising through the mid 2000s, albeit less rapidly, when the inferred surface pH of Bear Pond continued to decline (). For example, airborne nitrogen and sulfur compounds that had accumulated in local soils over the years could have delayed pH recovery in a seepage lake such as Bear Pond through lingering base cation depletion and acid contamination of groundwater (Watmough et al. Citation2016). Furthermore, some other Adirondack lakes experienced more or less steady or rising pH trends during that time period (Driscoll et al. Citation2016; ALSC Citation2018), so the close temporal association of the sudden changes in precipitation and Bear Pond's chemistry might have been partly coincidental.
Recent changes in phytoplankton, DOC, and climate further complicate attribution of the reversal in lake acidity to precipitation chemistry alone. Bear Pond was undergoing an ecological shift during the 1990s as indicated by increased chrysophyte abundances (), decreased water clarity, and rising concentrations of light-absorbing DOC (). The change in DOC could have been caused by decreasing SOx deposition, warming, or increased precipitation, all of which can enhance terrestrial DOC fluxes for lakes () (Seekel, Lapierre, and Karlsson Citation2015, Seekel, Lapierre, Ask, et al. Citation2015, Solomon et al. Citation2015, SanClements et al. Citation2018). The increase of chrysophytes, in turn, could reflect greater bioavailability of carbon as DOC concentrations rose (Wolfe and Siver Citation2013). Higher epilimnetic temperatures associated with regional warming and enhanced light absorption by DOC or plankton () (Beier et al. Citation2012, Solomon et al. Citation2015) would strengthen or prolong thermal stratification, which can further stimulate phytoplankton growth through internal loading of nutrients from bottom sediments under low-oxygen conditions. If overall primary productivity rose for any of these reasons, then photosynthetic consumption of dissolved CO2 might have contributed to the recent rise in lake pH as well.
In spite of these potential complicating factors, our pH reconstruction suggests that the chemical reversal postdated the major shifts in phytoplankton composition and water clarity. We therefore believe that the weight of available evidence favors the sharp increase in the pH of precipitation around 2010 as the most likely trigger for the reversal of acidification in Bear Pond.
Legacies of fisheries management
New York State fisheries managers have stocked brook trout in Bear Pond almost continuously since the 1920s, and yellow perch became abundant during the 1950s. It is therefore worth considering whether trophic interactions among the fish, zooplankton, and phytoplankton communities influenced the diatom record of Bear Pond. Stocking of fish, for example, can alter food web dynamics and release nutrients into lakes through excretion and/or decomposition (Carpenter et al. Citation1985; Lyons et al. Citation2016). The simultaneous decay of large numbers of fish killed by the reclamation of Bear Pond, if it caused dissolved oxygen levels in the hypolimnion to decline sufficiently, could presumably have initiated a persistent cycle of internal nutrient loading from bottom sediments (Stager Citation2018). However, there was no clear evidence of major phytoplankton responses to the stocking or reclamation in the microfossil record of core 17A.
Although fisheries management practices had little or no discernable impact on the fossil algal assemblages of Bear Pond, a more definitive legacy of the 1958 piscicide treatment was apparent in core J2 (), in which concentrations of toxaphene were as high as 59 ng/g, 2 orders of magnitude higher than sediment quality guidelines recommended by the Canadian Council of Ministers of the Environment (Citation2002). In the North American Great Lakes, which receive both direct and airborne inputs of toxaphene, Swackhamer et al. (Citation1998) found surface sediment concentrations averaging ∼15 ng/g, and cores from those lakes have contained toxaphene concentrations within the 10–40 ng/g range, while cores from lakes in that region with only atmospheric inputs of toxaphene typically contained even lower amounts (<10 ng/g; Pearson et al. Citation1997). We are therefore confident that most or all of the toxaphene in core J2 is a legacy of the reclamation of Bear Pond in 1958.
If the age model of core 17A is applied to J2, then the maximum concentrations of toxaphene in the deeper sediments seemingly predated the reclamation by several decades (). However, the microfossil stratigraphy of the J2 core indicated that 5–10 cm or more of the sediment column was missing, because ratios of chrysophyte scales to diatoms were much smaller than in 17A, and A. ralfsii did not reach comparably high percentages in the upper strata of J2 (). Because the stratigraphic integrity of the J2 core is in question, and because toxaphene itself can be highly mobile in lake sediments, we do not believe that variability in the concentrations of toxaphene in J2 accurately reflects the timing of deposition. Rather, we simply conclude that toxaphene from the reclamation is present in unusually high concentrations within the sediments of Bear Pond. It could therefore have contaminated the lake's food web, as has been documented elsewhere (Kallman et al. Citation1962, Hunt and Keith Citation1963, Terriere et al. Citation1966), not only in the past but today, as well. To our knowledge, no fish from Bear Pond have been tested for toxaphene residues, and no health advisories regarding possible toxaphene contamination have been issued by the DEC despite toxaphene’s toxicity to a wide range of animal species and its tendency to bioaccumulate in both animals and plants (Dykstra and Lennon Citation1966).
A novel ecological state in Bear Pond
The pH of surface waters in Bear Pond now appears to be approaching what it was during the 19th century (), primarily due to a rise in the pH of regional precipitation, and as long as reductions of SOx and NOx emissions are maintained the recently increased lake pH is likely to persist or rise further. However, Bear Pond is no longer the lake it was before it acidified. Wetter conditions have elevated the surface by nearly 1 m, submerged former landmarks, and eroded the shoreline, changes that are also reflected in other Adirondack lakes and rivers due to recent increases in precipitation (Stager et al. Citation2009; Stager and Thill Citation2010). Asterionella formosa and D. stelligera have not (yet) returned to the diatom community, the submerged macrophyte population has changed dramatically, and the phytoplankton has been enriched in chrysophytes, as has been observed in other Adirondack lakes (Arseneau et al. Citation2016). Unpublished sediment records from Bear Pond suggest that the phytoplankton community has changed to a greater extent than at any time within the last 2 centuries, and probably much longer. Two piston cores ∼1 m long that were collected at the same time as core 17A yielded anomalously old radiocarbon ages (3–4 kyr) for reasons that are as yet undetermined and were therefore not analyzed in great detail. Nevertheless, the microfossil assemblages in the lower sections of those longer cores more closely resembled those in zones A and B than in the recent sediments, with few chrysophyte scales and abundant P. biceps. This suggests that the phytoplankton community of Bear Pond may have changed more drastically during the 20th century than at any other time in recent millennia. The lake is not nearly as clear as it was during its acidified phase () or, judging from the high percentages of benthic diatoms in zone A (), during the early 19th century, and the water's former aqua blue color has now shifted to variable shades of brown and green. Few historical plankton data from Bear Pond are available, but surface collections show that dinoflagellates have been abundant during the last decade. Their pigments likely contribute to the new coloration, as do those of the expanded chrysophyte populations.
The recent increase of DOC concentrations might be responsible for some degree of "browning" in Bear Pond (; Monteith et al. Citation2007, Solomon et al. Citation2015) as well, although the water's appearance tends to be more strongly influenced by "greening" from phytoplankton at present. The lake may nonetheless be poised for an ecological transition in which rising DOC concentrations begin to decrease total productivity through light attenuation rather than stimulating it through nutrient inputs, because the DOC concentrations have risen into the critical range of ∼5 mg/L that has been associated with such transitions in many boreal lakes () (Seekel, Lapierre, and Karlsson Citation2015, Seekel, Lapierre, Ask, et al. Citation2015). Although the effects of future DOC variability are difficult to predict due to the complexity of environmental factors involved, further increases of light-attenuating DOC concentrations could begin to reduce whole lake productivity in Bear Pond as acidification once did, while reducing water clarity, changing the thermal structure of the water column (Solomon et al. Citation2015), and possibly reducing fish productivity (Finstad et al. Citation2014).
In addition to regional and global atmospheric pollution, local fisheries management practices have also altered the Bear Pond ecosystem profoundly. Stocking and piscicide treatment have likely delivered allochthonous nutrients to the sediments with the potential to increase future biotic productivity. Any unique genetic diversity that might have evolved among resident fishes was lost to the reclamation and decades of stocking with numerous strains of brook trout. Despite some implications to the contrary (Harig and Bain Citation1995, Demong Citation2001), such practices do not actually restore lake ecosystems but simply remove perceived nuisances or otherwise modify certain parts of the ecosystems in ways that reflect narrowly focused perceptions of lakes as recreational or commercial resources (NRC Citation1992, Carpenter and Cottingham Citation1997). Contamination of the sediments with toxaphene, which is readily biomagnified in fish and can cause nerve and organ damage in mammals (DOHHS Citation2016), indicates that such management practices might also pose unintentional risks to human health.
Summary and conclusions
The diatom record of Bear Pond shows that the lake first began to acidify during the mid 1800s and reached a minimum pH range roughly 1 unit lower than the pre-acidified condition by 1995–2010. Since 2010, pH values thus far have risen by ∼0.5 units, most likely due to the increasing pH of regional precipitation. However, full re-establishment of the lake's pre-acidification ecosystem may not be possible. Changes in precipitation chemistry and climate have increased DOC concentrations, altered the phytoplankton community, and reduced the clarity of the lake. In addition, fisheries management activities have altered the fish community and left toxic residues in the sediments. Microfossil assemblages suggest that the recent environmental changes in Bear Pond exceed the natural range of variability over several centuries, and possibly much longer. We conclude that although certain species, ecological niches, or chemical conditions may return to an impacted lake following efforts to restore or "reclaim" it (NRC Citation1992, Harig and Bain Citation1995, Gunn and Mills Citation1998, NOAA Citation2018; Huang et al. Citation2019), complete recovery of all aspects of such altered systems may be impossible in this Anthropocene epoch, when humans have become a powerful global force of nature. In that context, even remote lakes such as Bear Pond are likely to evolve into novel ecosystems under the influence of changing atmospheric chemistry, anthropogenic climate change, and aggressive fisheries management practices (Morse et al. Citation2014, Stager Citation2018).
Acknowledgments
Assistance in the field was provided by Ken Alton, Rory Fraser, Ed and Karen Hixson, Kary Johnson, Elliott Lewis, Josh Paradis, and Sean Regalado. Karyn Rogers and Kevin Rose of RPI provided use of analytical equipment for LOI analyses, and Jason Lynch provided emissions data from the USEPA. This article is dedicated to the memory of John Glew (1942–2019), who designed and provided much of the paleolimnological equipment.
Additional information
Funding
References
- Adirondack Lakes Survey Corporation (ALSC). 2018. [accessed 2018 Oct 9]. www.adirondacklakessurvey.org.
- Appleby PG, Oldfield F. 1978. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA. 5(1):1–8.
- Arseneau KMA, Driscoll CT, Cummings CM, Pope G, Cumming BF. 2016. Adirondack (NY, USA) reference lakes show a pronounced shift in chrysophyte species composition since ca. 1990. J Paleolimnol. 56(4):349–364.
- Arseneau KMA, Driscoll CT, Brager LM, Ross KA, Cumming BF. 2011. Recent evidence of biological recovery in the Adirondacks (New York, USA): a multiproxy paleolimnological investigation of Big Moose Lake. Can J Fish Aquat Sci. 68(4):575–592.
- Beier C, Stella JC, Dovčiak M, McNulty SA. 2012. Local climatic drivers of changes in phenology at a boreal-temperate ecotone in eastern North America. Clim Change. 115(2):399–417. doi:10.1007/s10584-012-0455-z.
- Burge D, Edlund M. 2017. Diatoms of the United States [accessed 2018 Jan 2]. http://westerndiatoms.colorado.edu/taxa/species/lindavia_bodanica.
- Camburn KE, Kingston JC, Charles DF. 1986. PIRLA diatom iconograph. Report Number 3, PIRLA Unpublished Report Series. Bloomington (IN): Indiana University.
- Canadian Council of Ministers of the Environment. 2002. [accessed 2019 April 14]. http://ceqg-rcqe.ccme.ca/download/en/246/.
- Carpenter SR, Kitchell JF, Hodgson R. 1985. Cascading trophic interactions and lake productivity. BioScience. 35(10):634–639.
- Carpenter SR, Cottingham KL. 1997. Resilience and restoration of lakes. Cons Ecol. 1(1):2. [accessed 2019 Feb 1]. http://www.consecol.org/vol1/iss1/art2/.
- Charles DF. 1984. Recent pH history Big Moose Lake (Adirondack Mountains, New York, USA) inferred from sediment diatom assemblages. Verh Internat Verein Limnol. 22:559–566.
- Charles DF. 1990. Effects of acidic deposition on North American lakes: paleolimnological evidence from diatoms and chrysophytes. Phil Trans Roy Soc London. B327:403–412.
- Charles DF, Whitehead DR. 1986. The PIRLA Project: paleoecological investigations of recent lake acidification. Hydrobiologia. 143(1):13–20.
- Charles DF, Binford M, Furlong ET, Hites RT, Mitchell M, Norton SA, Oldfield F, Paterson MJ, Smol JP, Uutala AJ, et al. 1990. Paleoecological investigation of recent lake acidification in the Adirondack Mountains, N.Y. J Paleolimnol. 3:195–241.
- Cumming BF, Smol JP, Kingston JC, Charles DF, Birks HJB, Camburn KE, Dixit SS, Uutala A, Selle AR. 1992. How much acidification has occurred in Adirondack region lakes (New York, USA) since preindustrial times? Can J Fish Aquat Sci. 49(1):128–141.
- Cumming BF, Davey KA, Smol JP, Birks H. 1994. When did acid-sensitive Adirondack lakes (New York, USA) begin to acidify and are they still acidifying? Can J Fish Aquat Sci. 51(7):1550–1568.
- Davis RB, Anderson DS, Charles DF, Galloway JN. 1988. Two-hundred-year pH history of Woods, Sagamore, and Panther Lakes in the Adirondack Mountains, New York State. Aquat Tox Haz Assess. 10:89–111.
- Demong L. 2001. The use of rotenone to restore native brook trout in the Adirondack Mountains of New York: an overview. In Cailteux RL, DeMong L, Finlayson BJ, Horton W, McClay W, Schnick RA, Thompson C, editors, Rotenone in fisheries: are the rewards worth the risk? Trends Fish Sci Mgt 1. Bethesda, MD: American Fish Soc; p. 29–35.
- Dixit SS, Smol JP. 1995. Diatom evidence of past water quality changes in Adirondack seepage lakes (New York, U.S.A.). Diatom Res. 10(1):113–129.
- Dixit SS, Dixit AS, Smol JP. 2002. Diatom and chrysophyte transfer functions and inferences of post-industrial acidification and recent recovery trends in Killarney Lakes (Ontario, Canada). J Paleolimnol. 27(1):79–96.
- US Department of Health and Human Services (DOHHS). 2016. Report on carcinogens, 14th ed. [accessed 2019 Feb 1]. https://ntp.niehs.nih.gov/ntp/roc/content/profiles/toxaphene.pdf.
- Driscoll CT, Driscoll KM, Fakhraei H, Civerolo K. 2016. Long-term temporal trends and spatial patters in the acid-base chemistry of lakes in the Adirondack region of New York in response to decreased acid deposition. Atmos Envir. 146:5–14.
- Dykstra WW, Lennon RE. 1966. The role of chemicals for the control of vertebrate pests. In Knipling EF (chair). Pest Control by Chemical, Biological, Genetic, and Physical Means: A Symposium. United States Department of Agriculture, ARS 33-110; p. 29–34.
- Finstad AG, Helland IP, Ugedal O, Hesthagen T, Hessen DO. 2014. Unimodal response of fish yield to dissolved organic carbon. Ecol Lett. 17(1):36–43.
- Ginn BK, Cumming BF, Smol JP. 2007. Diatom-based environmental inferences and model comparisons from 494 northeastern North American lakes. J Paleolimnol. 43:647–661.
- Gunn JM, Mills KH. 1998. The potential for restoration of acid-damaged lake trout lakes. Rest Ecol. 6(4):390–397.
- Harig AL, Bain MB. 1995. Restoring the indigenous fishes and biological integrity of Adirondack mountain lakes. A research and demonstration project in restoration ecology. Ithaca (NY): New York Cooperative Fish and Wildlife Research Unit, Department of Natural Resources, Cornell University.
- Heiri O, Lotter AF, Lemcke G. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol. 25(1):101–110.
- Huang J, Zhang Y, Arhonditsis GB, Gao J, Chen Q, Wu N, Dong F, Shi W. 2019. How successful are the restoration efforts of China's lakes and reservoirs? Environ Int. 123:96–103.
- Hunt EG, Keith JO. 1963. Pesticide-wildlife investigations in California: 1962. Proceedings of the 2nd Annual Conference on Use of Agricultural Chemicals in California; p. 1–29.
- Jackson ST, Charles DF. 1988. Aquatic macrophytes in Adirondack (New York) lakes: patterns of species composition in relation to environment. Can J Bot. 66(7):1449–1460.
- Jenkins J, Roy K, Driscoll C, Buerkett C. 2007. Acid rain in the Adirondacks: an environmental history. Ithaca (NY): Cornell University Press.
- Josephson DC, Robinson JM, Chiotti J, Jirka KJ, Kraft CE. 2014. Chemical and biological recovery from acid deposition within the Honnedaga Lake watershed, New York, USA. Environ Monit Assess. 186(7):4391–4409.
- Kallman BJ, Cope OB, Navarre RJ. 1962. Distribution and detoxification of toxaphene in Clayton Lake, New Mexico. Trans Am Fish Soc. 91(1):14–22.
- Krammer K, Lange-Bertalot H. 1991. Süßwasserflora von Mitteleuropa, 3 Teil: Centrales, Fragilariaceae, Eunotiaceae. Stuttgart (Germany): Gustav Fischer Verlag.
- Likens GE, Wright RF, Galloway JN, Butler TJ. 1979. Acid rain. Sci Am. 241(4):43–51.
- Lyons RA, Johnson LK, McIntyre BM. 2016. Phosphorus loading rates in lakes with development and stocked fish in the Sierra Nevada Mountains, California, USA. Ecosphere. 7(11):e01554.
- McIntyre SH, Duthie HC. 1993. Morphological variation in populations of the diatom Asterionella ralfsii W. Smith from Nova Scotia, Canada. Hydrobiologia. 269–270(1):67–73.
- Mitchell MJ, Driscoll CT, McHale PJ, Roy KM, Dong Z. 2013. Lake/watershed sulfur budgets and their response to decreases in atmospheric sulfur deposition: watershed and climate controls. Hydrol Process. 27(5):710–720.
- Monteith DT, Stoddard JL, Evans CD, de Wit HA, Forsius M, Høgåsen T, Wilander A, Skjelkvåle BL, Jeffries DS, Vuorenmaa J, et al. 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature. 450(7169):537–540.
- Morse NB, Pellissier PA, Cianciola EN, Brereton RL, Sullivan MM, Shonka NK, Wheeler TB, McDowell WH. 2014. Novel ecosystems in the Anthropocene: a revision of the novel ecosystem concept for pragmatic applications. Ecol Soc. 19:12.
- National Atmospheric Deposition Program (NADP). 2018a. [accessed 2018 Sept 20]. http://nadp.slh.wisc.edu/data/sites/siteDetails.aspx/net=NTN&id=NY20.
- National Atmospheric Deposition Program (NADP). 2018b. [accessed 2018 Oct 1]. http://nadp.slh.wisc.edu/data/sites/siteDetails.aspx/net=NTN&id=NY98.
- National Oceanic and Atmospheric Administration (NOAA). 2018. 2019 NOAA Great Lakes habitat restoration regional partnership grants. [accessed 2019 Feb 1]. https://www.fisheries.noaa.gov/grant/2019-noaa-great-lakes-habitat-restoration-regional-partnership-grants.
- National Research Council (NRC). 1992. Restoration of aquatic ecosystems: science, technology, and public policy. Washington, DC: The National Academies Press.
- Patrick R, Reimer CW. 1966. The diatoms of the United States. Volume 1, Monographs of the Academy of Natural Sciences of Philadelphia Number 13, Philadelphia (PA).
- Patrick R, Reimer CW. 1975. The diatoms of the United States. Volume 2, Monographs of the Academy of Natural Sciences of Philadelphia Number 13, Philadelphia (PA).
- Pearson RF, Swackhamer DL, Eisenreich SJ, Long DT. 1997. Concentrations, accumulations, and inventories of toxaphene in sediments of the Great Lakes. Environ Sci Technol. 31(12):3523–3529.
- Rattigan OV, Civerolo KL, Felton HD. 2017. Trends in wet precipitation, particulate, and gas-phase species in New York State. Atmos Poll Res. 8(6):1090–1102.
- SanClements MD, Fernandez IJ, Lee RH, Roberti JA, Adams MB, Rue GA, McKnight DM. 2018. Long-term experimental acidification drives watershed scale shift in dissolved organic matter composition and flux. Environ Sci Technol. 52(5):2649–2657.
- Seekel DA, Lapierre J-F, Karlsson J. 2015. Trade-offs between light and nutrient availability cross gradients of dissolved organic carbon concentration in Swedish lakes: implications for patterns in primary production. Can J Fish Aquat Sci. 72:1663–1671.
- Seekel DA, Lapierre J-F, Ask J, Bergström A-K, Deininger A, Rodríguez P, Karlsson J. 2015. The influence of dissolved organic carbon on primary production in northern lakes. Limnol Oceanogr. 60:1276–1285.
- Sivarajah B, Rühland KM, Smol J. 2017. Are diatoms recovering to pre-acidification assemblages in a warming world? Revisiting Killarney Provincial Park lakes (Ontario, Canada). Fund App Lim. 190(1):13–28.
- Siver PA. 1991. The Biology of Mallomonas: Morphology, taxonomy, and ecology. New York (NY): Springer-Science + Business Media, B.V.
- Smol J. 2008. Pollution of lake and rivers: A paleolimnological perspective. Malden (MA): Blackwell.
- Solomon CT, Jones SE, Weidel BC, Buffam I, Fork ML, Karlsson J, Larson S, Lennon JT, Read JS, Sadro S, Saros J. 2015. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems. 18(3):376–389. doi:10.1007/s10021-015-9848-y.
- Spaulding SA, Lubinski DJ, Potapova M. 2010. Diatoms of the United States [accessed 2018 Aug 1]. http://westerndiatoms.colorado.edu.
- Stager JC. 2018. Still waters: the secret world of lakes. New York (NY): W.W. Norton.
- Stager JC, Thill M. 2010. Climate change in the Champlain Basin: what natural resources managers can expect and do. Report for The Nature Conservancy [accessed 2018 Aug 30]. https://www.researchgate.net/publication/280204504_Climate_Change_in_the_Champlain_Basin_What_natural_resource_managers_can_expect_and_do.
- Stager JC, McNulty S, Beier C, Chiarenzelli J. 2009. Historical patterns and effects of changes in Adirondack climates since the early 20th century. Adirondack J Environ Stud. 15:14–24.
- Stager JC, Wiltse B, Hubeny JB, Yankowsky E, Nardelli D, Primack R. 2018. Climate variability and cultural eutrophication at Walden Pond (Massachusetts, USA) during the last 1800 years. PloS One. 13(4):e0191755.
- Strock KE, Nelson SJ, Kahl JS, Saros JE, McDowell WH. 2014. Decadal trends reveal recent acceleration in the rate of recovery from acidification in the northeastern U.S. Environ Sci Technol. 48(9):4681–4689.
- Sullivan TJ, Driscoll CT, Beier CM, Burtraw D, Fernandez IJ, Galloway JN, Gay DA, Goodale CL, Likens GE, Lovett GM, Watmough SA. 2018. Air pollution success stories in the United States: the value of long-term observations. Env Sci Poll. 84:69–73.
- Sutherland RA. 1998. Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia. 389(1/3):153–167.
- Swackhamer DL, Pearson RF, Schottler SP. 1998. Toxaphene in the great lakes. Chemosphere. 37(9–12):2545–2561.
- Terriere LC, Kiigemagi U, Gerlach AR, Borovicka RL. 1966. The persistence of toxaphene in lake water and its uptake by aquatic plants and animals. J Agric Food Chem. 14(1):66–69.
- United States Environmental Information Administration (USEIA). 2019. [accessed 2019 Feb 13]. https://www.eia.gov/todayinenergy/detail.php/id=10.
- United States Environmental Protection Agency (USEPA). 2019. [accessed 2019 Feb 4]. https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data.
- United States Historical Climatology Network (USHCN). 2017. [accessed 2017 Aug 29]. http://cdiac.ess-dive.lbl.gov/epubs/ndp/ushcn/ushcn_map_interface.html.
- Wallace ER. 1880. Descriptive Guide to the Adirondacks and Hand-Book of Travel to Saratoga Springs; Schroon Lake; Lakes Luzerne, George, and Champlain; the Thousand Islands; Massena Springs, and Trenton Falls. Columbian Book Company and Forest and Stream Publishing, Syracuse (NY).
- Watmough SA, Eimers C, Baker S. 2016. Impediments to recovery from acid deposition. Atmos Env. 146:15–27. doi:10.1016/j.atmosenv.2016.03.021.
- Wolfe AP, Siver P. 2013. A hypothesis linking chrysophyte microfossils to lake carbon dynamics on ecological and evolutionary time scales. Glob Plan Change. 111:189–198.
- Xia X, Hopke PK, Crimmins BS, Pagano JJ, Milligan MS, Holsen TM. 2012. Toxaphene trends in the Great Lakes fish. J Great Lakes Res. 38(1):31–38.