?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Norton SA, Amirbahman A, Bacon L, Ewing HA, Novak M, Nurse A, Retelle M, Stager JC, Yates M. 2019. Chemical, biological, and trophic status of temperate lakes can be strongly influenced by the presence of late-glacial marine sediments. Lake Reserv Manage. 36:14–30.
Lake Auburn, Maine, is a water supply for 60,000 people. Unusual silt/clay sediment, >4 m thick, occurs 15 cm below gyttja in deep water cores. We characterized cores taken in 2015 and 2016 (57 and 425 cm long, respectively), from 35 m. We determined 137Cs, 210Pb, and 206P/204Pb ratios to establish chronology for the cores and to link them stratigraphically. At least 1.1 m of sediment has accumulated since European settlement due to watershed erosion from land clearance and disturbance from about 1750 onward. The increased lake level from dams established in 1851 and the 1950–1960s has caused shoreline erosion. Extraction of sediment with HCl, scanning electron microscopy, and energy-dispersive spectroscopy analyses confirmed the presence of apatite (Ca5(PO4)3(OH)) for at least the upper 3.5 m of sediment. The apatite is soluble because of the circumneutral pH and relatively low Ca2+ concentrations. This modern sediment is derived from postglacial marine silt/clay sediment and represents a rarely considered internal source of P that predisposes the lake’s water column to higher total P, rendering it more susceptible to episodic eutrophication from stresses including higher temperatures, more frequent high-intensity weather phenomena, and longer ice-free periods with stronger and longer stratification. This previously unrecognized source of P must be considered in water quality management, including chemical mitigation such as aluminum addition, lake level manipulation, and watershed erosion control. Similar situations likely exist in other coastal lakes in postglacial terrain that was inundated during deglaciation, and in inland lakes receiving sediment directly during deglaciation.
Lake Auburn, Maine, is the primary source of drinking water for 60,000 people in Lewiston and Auburn, ME. Historically, this lake had Secchi disc transparency depths averaging >6 m in fall and spring, and >8 m in early summer. Turbidity rarely exceeded 1 NTU (CDM Smith Citation2013). Hypolimnetic anoxia in late summer and early fall had been short-lived and of limited spatial extent, if it occurred at all. Consequently, this public water source received US Environmental Protection Agency (EPA) exemption from filtration for the removal of particulate matter. However, in 2011 and 2012, summer epilimnetic total phosphorus (TP) concentrations exceeded 14 µg/L, nearly twice the values in 2005. Secchi disk transparency in September–October, prior to overturn, declined to <3 m (CDM Smith Citation2013). The increase in TP and associated growth of phytoplankton resulted in significant increases in the hypolimnetic bottom area exposed to <2 mg/L dissolved oxygen (DO). The anoxic bottom area in Lake Auburn was 3.8 and 4.9 km2 in 2011 and 2012 (both with warm summers), respectively, compared to an average of 0.78 ± 0.75 km2 from 1993 to 2010. The anoxic factor (sensu Nürnberg Citation1995) was 18.1 in late summer 2011 and reached 18.9 in late summer 2012 (CDM Smith Citation2013). Maximum turbidity exceeded 1 NTU on 7 days in 2005, compared to 83 and 108 days in 2011 and 2012, respectively (CDM Smith Citation2013). As the result of the sustained increase in summer lake turbidity, the Auburn Water District (AWD) was faced with the possibility of being required to build a filtration plant. In 2011 and 2012, cyanobacteria capable of producing cyanotoxins (primarily Gloeotrichia, Microcystis, and Dolichospermum (formerly called Anabaena)) were abundant (CDM Smith Citation2013). In September 2012, the combination of low hypolimnetic DO concentrations and high epilimnetic temperatures led to the death of >200 lake trout (Salvelinus namaycush). This uncharacteristic behavior in 2011 and 2012 led to a major synthesis of data (CDM Smith Citation2013), increased concern, and studies of sources, sinks, and processing of P in Lake Auburn.
Doolittle et al. (Citation2018) evaluated aluminum, iron, and phosphorus (Al, Fe, and P) speciation in Lake Auburn sediment using sequential chemical extractions (Psenner et al. Citation1984, Wilson et al. Citation2010) for 11 short cores (<20 cm) from 17–35 m water depth. The surface sediment (0–2 cm) below the hypolimnion had extractable (Al/Fe) molar ratios between 0.2 and 1.7 (), and (Al/Fe-hydroxide-bound P) molar ratios between 2.0 and 14.5. Both ratios indicated high risk for internal P loading during anoxia (Kopáček et al. Citation2005, Lake et al. Citation2007). Longer cores (50–60 cm) at 15–17 m and 35 m depth had a sharp downward transition from normal brown gyttja (∼15 cm thick) to at least 45 cm of gray silt/clay with lower organic content. Other sediment parameters that changed abruptly included cesium-137 activity (137Cs is a radioactive isotope produced during atmospheric thermonuclear bomb testing and above-ground nuclear accidents), lead-210 activity (210Pb is a radioactive isotope produced from the decay of radon), and speciation of P. The HCl-extractable P and Ca suggested the ubiquitous presence of the mineral apatite ((Ca)5(PO4)3OH).
Figure 1. Bathymetry (m), and location of short sediment cores and extractable sediment (Al:Fe) ratio, dam above inlet Basin Stream (northwest) and outlet stream (east) indicated by arrow tips, and Core 2015 and Core 2016. Figure modified from Doolittle et al. (Citation2018).
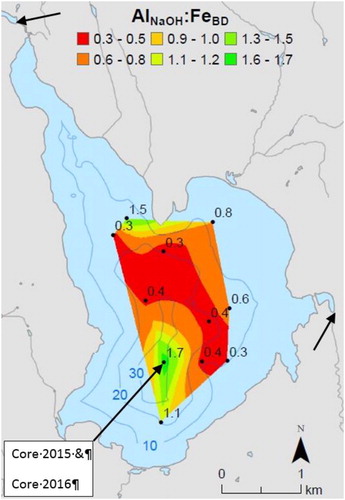
We evaluated whether or not this possibly apatite-bearing sediment, at or close to the modern sediment surface but having atypical characteristics for lakes in Maine at elevations greater than 75 m near the coast, increasing to as high as 120 m, inland, is a possible primary internal source of P to the hypolimnion. The apatite throughout the sediment column was not the direct cause of the 2011/2012 blooms but would explain part of the chronically elevated P in the water column. Knowledge of an additional internal source of P input to this lake, and others in similar geologic settings, would be useful in designing any mitigation strategy against future eutrophication, such as aluminum treatment by the AWD.
Study site
Lake Auburn () is an 897-ha, regulated lake with an average elevation of 85 m above sea level (masl), total watershed area of 4740 ha, direct watershed area of 1,969 ha, maximum depth of 36 m, mean depth of 11 m, and mean hydraulic residence time of 4.1 yr (Dudley Citation2004). The lake is fed from the northwest and northeast by perennial Basin Stream and Townsend Brook, respectively. Lake Auburn has had a summer average Secchi disk transparency of 7.4 m (range 2–11 m), epilimnetic water column TP concentration of 8 μg/L (range 7–14 μg/L), and chlorophyll a concentration of 2.8 μg/L (range 1–17 μg/L) from 1977 to 2009 (Maine Department of Environmental Protection [MEDEP] Citation2010).
The Lake Auburn watershed is largely underlain by highly metamorphosed Silurian-age schists and gneisses with no recognized calcareous rocks (Hussey Citation1983, Osberg et al. Citation1985). Most of the bedrock is overlain by Wisconsinan-age till, 0 to perhaps 10 m thick. Deltaic sand and gravel, up to 20 m thick in the northeast, extend into the northeast and eastern part of the lake to a water depth of ∼10 m, forming a sheet of sand of unknown thickness. Sand and gravel also occur at the south end of the lake. Lake Auburn is likely a kettle lake, formed by a residual ice block that was first covered and surrounded by sand and gravel, and followed by postglacial marine submergence because of slow isostatic rebound of the land, about 15,000 yr ago. During the submergence, Presumpscot Formation (postglacial marine silt/clay) was deposited discontinuously along the present shore of Lake Auburn (Hildreth Citation2008, Thompson Citation2008). Locally, Presumpscot is restricted to low relief areas with elevation <100 masl, or 15 m above average lake level. Basin Stream drains 3 small chain lakes, Mud Pond, Little Wilson Pond, and Basin Pond, from a predominantly till-covered landscape. Basin Pond is a shallow (<1 m) wetland, formed from accumulating sediment and organic matter) behind a 3-m-high dam that was constructed after 1841 (Auburn ME Tax Maps), about 220 m upstream from Lake Auburn (). Townsend Brook, largely groundwater-fed, originates in a sand and gravel esker/delta, with many borrow pits. It drains through a shallow (<1 m deep) wetland area, just before entering Lake Auburn. The wetland formed as a consequence of a causeway constructed after 1841 because of a dam at the outlet (height unknown) installed before 1850. The north Shore Road was relocated to its present location about 1873 (Auburn ME Department of Transportation records). The AWD withdraws pretreatment water in the southeastern part of the lake. Lake Auburn has one surface outlet on the eastern shore, where a dam (2 m high) regulates the water level to balance adequate volume for water withdrawal by the AWD and downstream fishery requirements (). Although poorly documented, the dam was likely raised and reconstructed in the 1950s. Minor southward groundwater flow may occur at the south end of the lake through sand and gravel.
Materials and methods
Doolittle et al. (Citation2018) retrieved a 63-cm core in March 2015 (henceforth Core 2015) through the ice with a Davis and Doyle (Citation1969) stationary piston corer (10.16 cm internal diameter) from the deepest part of the lake (35 m; ). The sediment–water interface was undisturbed. In June 2016, at the same depth and within 15 to 20 horizontal m of the 2015 coring site (lat. 44.13690°N, long. 70.25155°W), we retrieved a 4.25-m continuous core (henceforth Core 2016) with a stationary piston in a sharpened Al irrigation pipe (7.6 cm internal diameter). Sediment penetration was by human-powered vertical thrusting to refusal, with a weighted hammer.
Core sectioning
Core 2015 was sectioned in 1-cm intervals from 0 to 20 cm, and in 2-cm intervals below 20 cm; samples were placed in Whirl-Pak sampling bags, and kept on ice until frozen the same day. They remained frozen until processing for chemical extractions and other analytical procedures. Core 2016 was transported in a horizontal orientation and opened in the laboratory. Unfortunately, the sediment was supersaturated with methane. During removal of the piston, the combination of slight warming plus depressurization of about 3.5 bars resulted in expansion of methane bubbles and “self-extrusion” of all the gyttja and some gray silt/clay. The sectioned gray silt/clay sediment, therefore, started at >15 cm, the thickness of the gyttja based on Core 2015. The core was split longitudinally and protected from desiccation with polyethylene wrap. The 4.25 m of sediment was sampled in 1-cm intervals from 0 to 20 cm, and then 1-cm subsamples at 5-cm intervals (20–21, 25–26, …40–41 cm), 10-cm intervals (50–51, 60–61, …100–101 cm), and 25-cm intervals (125–126, 150–151, … to 420–421 cm). A color change from brown to gray occurred at ∼15 cm in Core 2015 and at an unknown depth in Core 2016. Laminated gray and black sediment occurred at about 325 cm, where additional samples were taken. During sampling, separate aliquots were placed directly in prewashed porcelain crucibles for determining percent water and percent loss-on-ignition (LOI), a surrogate for organic matter. Samples for Psenner chemical sequential extractions, 137Cs and 210Pb activity, scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), sulfur (S) and Pb isotopes, pollen, and diatoms were placed directly into individual prewashed plastic bottles. All Core 2016 sections were refrigerated at 4 C except for percent water, which was determined as sectioning occurred.
Percent water and percent LOI
Water content for sediment was determined by drying at ∼70 C for 5 h (Core 2015) or 90 C for 12 h (Core 2016). LOI for Core 2015 (%) was determined at 550 C for 5 h. Core 2016 (%) LOI samples were ramped to 225 C over 1 h, to 550 C over the next 1 h, and held at 550 C for 3 h.
Dating of sediment
With only an estimate of how much sediment had been lost during self-extrusion of Core 2016, we chronologically spliced cores 2015 and 2016 by assessing natural and anthropogenically influenced parameters including 137Cs and 210Pb specific activities (becquerels/g), Psenner extraction chemistry, stable S and Pb isotopes, diatoms, and pollen, all of which yielded chronological data. 137Cs is entirely from anthropogenic sources and post 1945. 210Pb in sediment is partly from the atmosphere (unsupported 210Pb) and partly from decay of radium in the sediment (supported 210Pb). All 137Cs and 210Pb data were determined in 2016 at the Department of Physics and Astronomy, University of Maine. 137Cs and 210Pb were determined for 4 samples from Core 2015 (1 above and 3 below the transition in sediment color). Core 2016 samples (n = 19) were distributed from the top of the core to 400 cm, with high resolution in the top 10 cm (every 1 cm, n = 10), and progressively less intensive sampling below 10 cm. Dried sediment was homogenized and sealed in 1 × 4-cm polyethylene vials for ≥2 weeks to establish secular equilibration and then counted for 43,200 to 259,200 sec. Gamma rays from 137Cs and 210Pb were counted using the 662- and 46.52-keV emission, respectively, on a Canberra germanium well detector (1 × 4 cm) with 22.5% efficiency for 60Co. Data were processed using MAESTRO software (ORTEC) and analyzed by Compton continuum subtraction near the activity peaks.
Stable Pb isotopes
Stable Pb occurs naturally as 4 different isotopes: 204Pb, 206Pb, 207Pb, and 208Pb. Only 204Pb is nonradiogenic, that is, not derived from the decay of 238U (uranium), 235U, or 232Th (thorium). When air pollution in a region has Pb isotope ratios that differ from those of the local bedrock and surficial material, changes in the isotopic ratios in the sediment have been interpreted as a mixture of deposited air-pollution and indigenous-watershed contributions (Gobeil et al. Citation2013). The Pb isotopes were determined on oven-dried samples (105 C), extracted with 10% ultrapure HNO3. Digested samples were analyzed for 204Pb, 206Pb, 207Pb, and208Pb using a Thermo Element 2 sector field inductively coupled plasma–mass spectrometer (ICP-MS) at the Sawyer Environmental Chemistry Laboratory, University of Maine. An Elemental Scientific self-aspirating PFA nebulizer with an uptake rate of 100 µL/min and a quartz cyclonic spray chamber were used for sample introduction. Sample concentrations were high enough so that all isotopes were measured in the analog mode. A sample of NIST 981 was run before and after each sediment sample. Samples were standardized to the NIST samples. Both cores were measured the same day. The average standard deviations on the 206Pb/204Pb ratio, reported here, were 0.0231 (Core 2015) and 0.0230 (Core 2016).
Sediment chemical speciation
Sediment P studies using a chemical sequential extraction technique (Psenner et al. Citation1984) distinguish “reactive” P in iron hydroxide (Fe(OH)3-bound P) and “nonreactive” P in aluminum hydroxide (Al(OH)3-bound P). Lake sediments with (NaOH-extractable Al(OH)3)/bicarbonate-dithionite (BD)-extractable (Fe(OH)3) molar ratio >3 and (Al(OH)3)/(Fe(OH)3-bound P) ratio >25 do not release appreciable P and do not contribute to eutrophication even under anoxic conditions (Kopáček et al. Citation2005, Lake et al. Citation2007). The converse, (Al(OH)3)/(Fe(OH)3) < 3 and (Al(OH)3)/(Fe(OH)3-bound P) < 25, results in some lakes, but not all, releasing sediment P during anoxia. Sediments from Core 2015 were chemically speciated for Al, Fe, and P by Doolittle et al. (Citation2018), following a modified procedure of Psenner et al. (Citation1984) and Wilson et al. (Citation2008). We repeated 2 intervals from Core 2015 (31 and 52 cm) and did comparable extractions in Core 2016 at 50.5, 60.5, 90.5, 130.5, 230.5, and 355.5 cm, adjusted depths (see later description). Approximately 2 g of wet sediment were sequentially extracted with (1) 0.1 M NH4Cl to extract the ion-exchangeable fraction; (2) Na bicarbonate-Na dithionite (henceforth BD) solution consisting of 0.11 M NaHCO3 and 0.11 M Na2S2O4 at 40 C for 0.5 h to dissolve loosely adsorbed and reducible species (primarily Fe) and associated P; (3) 0.1 M NaOH, rather than 1.0 M (Ahlgren et al. Citation2005, Wilson et al. Citation2008, Homyak et al. Citation2014), at 20 C for 16 h to dissolve Al(OH)3, organic matter, and associated P; and (4) 0.5 M HCl at 20 C for 16 h to dissolve calcium (Ca)-bound P (apatite) and P associated with any recalcitrant Al(OH)3 and Fe(OH)3. The fifth extraction (85 C, 1 N NaOH) was omitted because of the biological irrelevance of such insoluble Al, Fe, and P in the context of this study. The extracted solutions were analyzed by inductively coupled plasma–optical emission spectrometry (ICP-OES; Thermo Electron iCAP 6300) for Al, Ca, Fe, and P. A blank and a replicate sample were extracted and their solutions were analyzed every 10 samples for quality control. Replicate samples were within 5% of each other.
Imaging and chemical mapping of sediment
Sediment samples were mounted on glass slides with SPI-TAC liquid adhesive. The samples were coated with a 150-Å-thick carbon coating using a high-vacuum carbon coater. Analyses were performed using a Tescan Vega XMU SEM equipped with an EDAX Apollo EDS detector. The SEM and EDS were automated with VegaTC and EDAX Genesis software, respectively. All imaging, elemental mapping, and EDS analyses were performed with a 20-kV, ∼200-pA beam at a working distance of ∼15 mm. SEM images were collected with dwell times of 150 msec/pixel. EDS spectra were collected for 100 sec of live time. Elemental mapping was performed with a dwell time of 5 msec.
SEM and EDS analyses were performed on sediment from intervals 5-6, 15–16, 34–36, 50–51, and 55–57 cm in Core 2015 and the 100–102 cm interval in Core 2016. In each sample, 3 areas, ∼1 mm2, were imaged using backscattered electrons (BSE) and chemically mapped for Ca, Fe, and P using emitted x-rays. Data were collected every 1 µm in the X-Y dimensions, or 106 measurements/1 mm2. Spatial resolution of images was <0.05 µm. High overlapping Ca and P concentrations in the resulting elemental maps led to the discovery of apatite grains. Three apatite grains from each 1 mm2 (9 per sediment interval) were imaged with BSE and chemically characterized with EDS.
Total S (sulfur) and stable S isotopes
Total S from sediment samples was extracted as sulfate (SO42-) by the Eschka procedure (ASTM D3177-02 Citation2007) by thoroughly mixing a sample with the Eschka mixture (2 parts calcined magnesium oxide and 1 part anhydrous sodium carbonate). The mixture was ashed in a muffle furnace at 800 C. The ashed sample was leached with hot water, filtered, and the S was precipitated as barium sulfate with a 10% barium chloride solution. The precipitate was filtered, dried, and combusted at 800 C to constant weight to calculate the amount of precipitated S. The barium sulfate was mixed with silica, vanadium pentoxide, and copper, decomposed in vacuum at 1050 C (Yanagisawa and Sakai Citation1983), and collected as sulfur dioxide for determination of δ34S (Equationequation 1(1)
(1) ). The 34S/32S ratios were determined on a Delta V isotope ratio mass spectrometer (ThermoFisher Scientific).
(1)
(1)
The overall reproducibility of the δ34S measurement was 0.3‰, which includes the uncertainty of measurement (external reproducibility is ∼0.1‰) and the uncertainty of the preparation method (∼0.2‰). The detection limit for total S, and thus determination of δ34S, was 0.01%.
Diatom analysis
Subsamples for diatom analysis, 6 from Core 2015 and 5 from Core 2016, were digested with hydrogen peroxide (H2O2). The siliceous remains were enumerated at 1000× under oil immersion. At least 400 valves were identified to the species level on each slide using standard references (Patrick and Reimer Citation1966, Citation1975, Krammer and Lange-Bertalot Citation1991).
Pollen analysis
Pollen concentration determination techniques were adapted from Faegri et al. (Citation1989) and Vandergoes (Citation2000). Four samples were evaluated from Core 2015 and 12 from Core 2016. One cubic centimeter of wet sediment was dispersed in 10% KOH and 10% HCl, and then sieved through 160-µm mesh. Flotation in a solution of sodium polytungstate (specific gravity 2.3) separated the pollen from denser, inorganic material. Treatment with hydrofluoric acid (HF) removed fine inorganic silt from the supernatant. Acetolysis with a 1/9 mix of sulfuric acid and acetic anhydride removed humic acids and cleared the pollen cells. To allow calculation of pollen concentrations, a 5.0 × 104 (±8%) microsphere marker (National Lacustrine Core Facility, University of Minnesota, Minneapolis, MN) was added to each sample. Samples were rinsed 3 times with deionized water to remove reagent traces. Tertiary butyl alcohol dehydrated the pollen concentrate, and the pollen concentrate was suspended in silicon oil. Pollen, fern and fern-ally spores, and charcoal particles larger than 40 µm were evaluated using a Nikon Labophot-2 microscope at 400× magnification. For each sample, at least 300 arboreal and shrub pollen cells were counted, a statistically significant number for quantification of the relatively abundant taxa (Faegri et al. Citation1989).
Results
Percent water and LOI
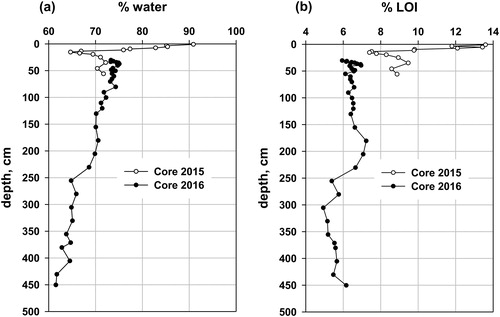
Percent water in Core 2015 declined with increasing depth (r2 = 0.89; ). In detail, percent water declined from 91% at the surface to 66% at 15–16 cm, and then increased to 72% by 35 cm, remaining relatively constant to the bottom of Core 2015. Percent LOI declined from 13.6% at the surface to 7.4% at 15 cm (the abrupt transition from brown gyttja to gray silt/clay) and then increased gradually to 8.9% at 56 cm. In Core 2016, percent water declined gradually from 75% at the top sample to 62% at the bottom of the core, whereas percent LOI increased irregularly from 6% at the surface to about 7% at 200 cm, then declined to ∼5.5% by 250 cm, with relatively constant values from there to the bottom of the core. The percent water and depth in Core 2016 positively correlated (r2 = 0.96), but the relationship between percent water and percent LOI (Supplementary material, Figure S2) indicates 2 different sediment populations, suggesting 2 different sources or depositional processes for the lower and upper parts of Core 2016, with a gradual transition between 200 and 250 cm. The slightly lower percent water (∼2%, ) in Core 2015 at the proposed overlap depth for Core 2016 may be caused by the lower dehydration temperature (70 C) for Core 2015, compared to 90 C for Core 2016. Water not driven off in Core 2015 would become part of the LOI and inflate the percent LOI.
137Cs and 210Pb
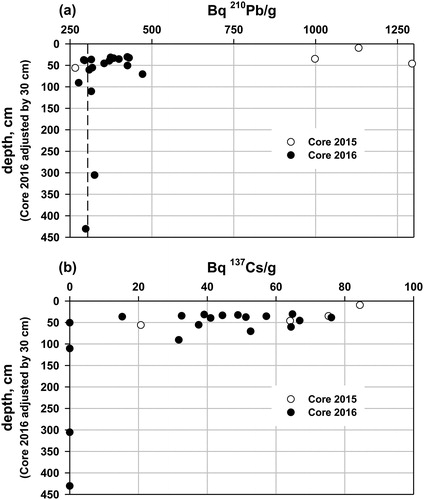
The goal of these determinations, and stable Pb isotopes, was to chronologically link the 2 cores and establish the possible range of age in the silt/clay. The 137Cs and 210Pb analyses of Core 2015 included 1 sample (9–10 cm) above the sediment type transition and 3 (34–36, 45–47, and 55–57 cm) below the transition. The upper 2 depth intervals had high unsupported 210Pb specific activity (. The loss of the gyttja from Core 2016 during extrusion made continuous 210Pb dating of that core impossible. We estimated the sediment loss, on the basis of the 210Pb activity, to be ∼30 cm, an amount that is consistent with field and laboratory observations, and with the percent water and LOI curves (. We adjusted all depths for Core 2016 downward by 30 cm (. The same adjustment for 137Cs data produced a reasonable decline in 137Cs from the surface (. It is not possible to date either core individually, but the combined specific 137Cs activity profiles for the combined cores suggest that at least the top 40 cm, and possibly the top 60 cm of the gray silt/clay, are post 1945, and likely post 1963/1964, the period of highest atmospheric deposition of 137Cs from atmospheric thermonuclear bomb testing by the United States, the United Kingdom, and the Soviet Union prior to the Nuclear Test Ban Treaty (Citation1963). The reasonability of this chronological splicing of the 2 cores was tested with several other parameters, described in the following.
Figure 3. Lake Auburn, Maine: (a) 210Pb specific activity in Cores 2015 and 2016 (Core 2016 adjusted downward by 30 cm). The vertical dashed line represents background 210Pb from decay of 226Ra (radium) in the sediment. (b) 137Cs specific activity in Cores 2015 and 2016 (Core 2016 adjusted downward by 30 cm). Background 137Cs is zero.
Stable Pb isotopes
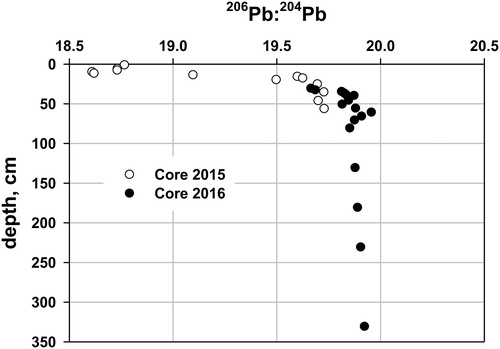
In Core 2015, the 206Pb/204Pb ratio decreased from 18.76 (0–2 cm) down-core, reaching its lowest value, 18.61, at 9–10 cm, and below 10 cm increased down-core, reaching a long-term value of ∼19.9 in Core 2016 at an adjusted depth of about 50 cm (). The surface ratio in Core 2015 was similar to that of sediment dated to about 2007 from Sargent, Mountain Pond (SMP) (18.5, Norton, University of Maine, unpublished), Acadia National Park, coastal Maine, 160 km to the east. The enrichment factor and accumulation rates for total Pb in Maine lakes reached their maxima about 1980, more than 10 times the prepollution Pb accumulation rates in ∼1900. The prepollution SMP sediment 206Pb/204Pb ratio (18.8–19.0) is slightly less radiogenic than at Lake Auburn (19.9) because of differing bedrock and soil; however, sediment 206Pb/204Pb at both sites converges on the same value, dominated by air pollution by the late 20th century. The dominant source of Pb in sediment deposited during the late 20th century at both sites, separated by 160 km, was from atmospheric pollution. The upper part of Core 2016 is clearly influenced by modern Pb pollution. We assign an age of ∼1980 to the 9–10 cm depth in Core 2015 on the basis of the minimum 206Pb/204Pb ratio, corresponding to the time of maximum atmospheric Pb deposition. Stable Pb isotopes yielded the most convincing evidence of the reasonableness of the chronological splice. The occurrence of unsupported 210Pb, 137Cs, and anthropogenic Pb pollution in at least the upper 20 cm of Core 2016, plus an estimated additional 30 cm lost during sampling, suggest that a minimum of 50 cm of the gray silt/clay has been deposited in the last 120 yr. Less compelling but consistent with this conclusion is the evidence from the sequential chemical extractions, imaging and chemical mapping, total S and S isotopes, and pollen and diatom analyses.
Figure 4. 206Pb:204Pb for Cores 2015 and 2016, from 35 m, from Lake Auburn, Maine. Depth for Core 2016 has been adjusted downward by 30 cm based on the specific activities of 210Pb and 137Cs. One standard deviation for the 206Pb:204Pb ratio is 0.0232 (Core 2015) and 0.0230 (Core 2016), based on multiple analyses of the same sample.
Psenner speciation
Doolittle et al. (Citation2018) omitted the first extraction step for Core 2015 (NH4Cl, which extracts the ion-exchangeable species), which is normally low in Al, Fe, and P under the oxic condition (Supplementary material, Figure S3). Fe was predominantly in the BD extraction (reducible) and was particularly enriched in the upper few centimeters of sediment (, Supplementary material, Figure S3). Lower concentrations of Fe occurred in the NaOH extraction (third) than in the HCl extraction (fourth). These last 2 fractions have relatively constant concentrations down core through the gyttja (0–15 cm) and the gray silt/clay (15–57 cm). The PBD fraction is the largest P fraction in upper sediment, in parallel with the FeBD fraction. Lower in the core, P fractions are variable, but the PHCl fraction becomes relatively more important and nearly constant (about 12 µmol P/g dry weight). Virtually all extractable Al is from the NaOH and HCl fractions, nearly equally, with stable proportions and concentrations from 20 cm downward.
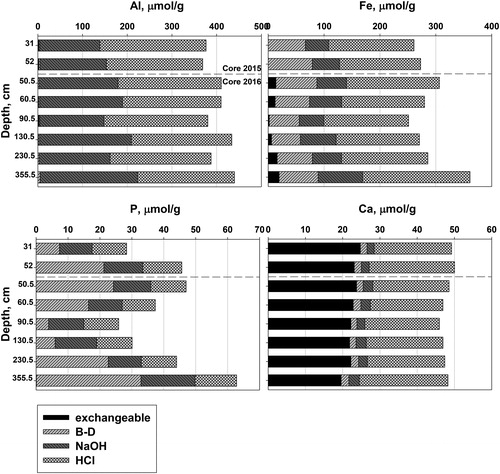
We analyzed Al, Ca, Fe, and P in the extracts for Cores 2015 (n = 2) and 2016 (n = 6) (). Sample 2 from Core 2015 (52 cm) and sample 3 from Core 2016 (50.5 cm, adjusted) are from nearly the same depth below the sediment–water interface, based on the 206Pb/204Pb ratio matching of the 2 cores. The AlNaOH/FeBD and AlNaOH/PBD molar ratios for near-surface intervals are below the thresholds of 3/1 and 25/1 ( and Supplementary material, Figure S3). These relationships prevail down to 60.5 cm (adjusted) in Core 2016. Thus, all sediment above 60 cm was susceptible to reduction and release of P during anoxia (Kopáček et al. Citation2005) when first deposited. PBD was enriched in the top 5 cm, parallel to FeBD (Supplementary material, Figure S3), and varied irregularly below that (). The concentration of PHCl in Core 2015 is higher below 15 cm than above (Supplementary material, Figure S3), and is relatively constant in Core 2016 (, samples 3–8). Extracted Ca is dominantly in the ion-exchangeable and HCl extractions (), with <10% of the total Ca in the BD and NaOH fractions. The ion-exchangeable fraction is due to loosely sorbed Ca on organic matter and solid inorganic phases; CaHCl corresponds to Ca liberated from the mineral apatite or calcite (CaCO3), or small amounts from HCl leaching of Ca-silicate minerals. However, no calcite was seen in the EDS analysis of any sediment. No calcite was expected because of the relatively low pH, Ca 2+, and Gran alkalinity in the hypolimnion (6.3–6.8, <6 mg/L, and 250–300 µeq/L, respectively) and the lack of any calcite-bearing meta-sediments in the watershed. Hupfer et al. (20009) suggested that during the Psenner extractions in calcite-bearing sediment, the NaOH removal of Al(OH)3 with its adsorbed P results in precipitation of the P as apatite in Ca-rich environments. The molar CaHCl/PHCl ratio ranged from 1.83 to 1.92, slightly higher than stoichiometric apatite, 1.67. More importantly, the absolute values of CaHCl and PHCl were low.
Figure 5. Extractable Al, Fe, P, and Ca (all in µmol/g dry sediment) in 4 Psenner extractions for Lake Auburn, Maine, sediment: 31 and 52 cm from Core 2015, and sediment from 50.5, 60.5, 90.5, 130.5, 230.5, and 355.5 cm from Core 2016 (depths adjusted downward by 30 cm). Dashed line is the demarcation between Core 2015 (above) and Core 2016 (below).
Imaging (SEM) and chemical mapping (EDS)
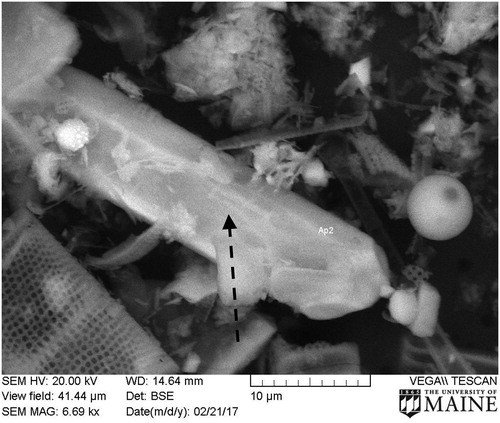
All scanned intervals, 5 from 5.5 to 56 cm from Core 2015 and 1 (101 cm, adjusted) from Core 2016, had particulate apatite, ranging in size from <0.1 µm to >30 µm. All 1 × 1-mm maps of sediment chemistry (n = 3 for each scanned sediment interval, totaling 18 maps) had observable apatite. Although difficult to quantify, grains >5 µm were more common in the lower part of Core 2015 and in the 101-cm depth sample from Core 2016, the deepest scanned interval. Most apatite grains were irregularly shaped with 1 exception, a 33-µm crystal (). Grain size of the matrix ranged from <0.1 µm to about 50 µm, consisting of individual mineral grains and diatoms (predominant). Quantification of the absolute abundance of apatite grains was not done. EDS analysis of the imaged grain () and all other identified grains of apatite had a Ca:P ratio of ∼1.7 (Supplementary material, Figure S4) and an HCl-extractable Ca:P molar ratio of 1.8–2.
Total S and stable S isotopes
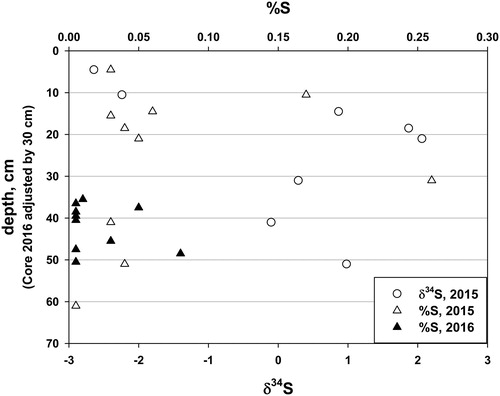
In Core 2015, total S ranged irregularly from 0.03% at 4.5 cm, to a maximum of 0.26% at 31 cm, 15 cm below the sediment type transition (). From 31 cm to near the bottom of Core 2015 (61 cm), total S for most samples was below detection, 0.01%. Total S in Core 2016 was irregular and low, with 7 of 9 samples at or below detection. The δ34S could not be determined in Core 2016 because of either low total S or insufficient sample. Core 2015 had negative values for the upper 2 samples (4.5 and 10.5 cm); below that, δ34S was positive or nearly so, ranging from −0.1 to 2.1.
Diatom assemblages
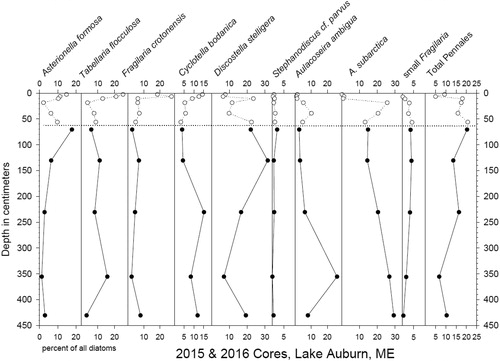
Diatom assemblages in the 2 cores, spliced together by shifting Core 2016 data downward 30 cm, suggest continuity between the 2 cores; percentages of diatom taxa were generally similar or appeared to continue trends from Core 2015 downward (). Because of sparse data and the lack of good chronology, it is not possible to interpret the causes of changes down the core in Core 2016. Although we hypothesized that the parent material of the gray silt/clay from the 2 cores originally was the marine Presumpscot Formation, no marine diatoms were seen in the 2 cores. Marine bivalve fossils, normally in sandier facies within the Presumpscot, were also absent in these fine-grained sediment cores. Increasing abundances of the Asterionella and Fragilaria assemblages above the sediment transition zone in Core 2015 typically represent nutrient enrichment in temperate zone lakes (Saros et al. Citation2005). Concurrently, Tabellaria and Cyclotella increase upward from the sediment type transition zone. The rest of the assemblages indicate a generally mesotrophic plankton community.
Pollen assemblage
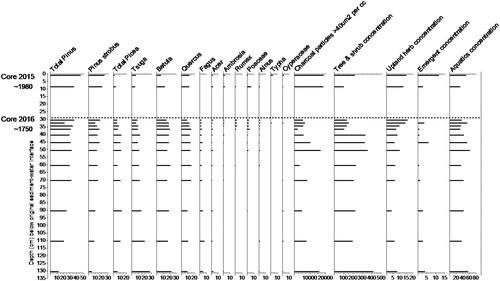
We used the top sediments from Core 2015 to track pollen shifts in the upper 30 cm of sediment due to lost portions of Core 2016 during core recovery. Pollen assemblages throughout both cores indicate forests of mixed conifers—white pine (Pinus), spruce (Picea), fir (Abies), and hemlock (Tsuga)—and hardwoods including oak (Quercus), birch (Betula), ash (Fraxinus), beech (Fagus), and maple (Acer). Beech is notably absent in the upper sediment of Core 2015 () and declines through Core 2016 above a depth of 36 cm, perhaps due to its historic use for wood products and charcoal production. Percentages of herbaceous plant pollen, including grasses (Poaceae), ragweed (Ambrosia, a native species that increases with disturbance), and dock (Rumex, a nonnative plant carried from Europe with hay), increased, while tree/shrub pollen declined above 40 cm in Core 2016 (). Expansion of grasslands, declining forest area, indication of landscape disturbance (increase in Ambrosia), and the appearance of European flora (Rumex) signal the onset of European settlement. Historical records indicate that the Europeans cleared extensive areas of forest in the Lake Auburn watershed starting ∼1750 (at or above 36 cm below the sediment/water interface). The occurrence of Typha and Cyperaceae may relate to expansion of wetlands on the 2 inlet streams due to dam and causeway construction.
Figure 9. Pollen from Cores 2015 (top) and 2016 (bottom) from Lake Auburn, Maine. Charcoal and pollen concentrations are particles or pollen/cm3 of sediment. X-axis values for columns 16–19 are multiples of 1000. X-axis values for columns 1–14 are percentages of total tree and shrub, upland herb, and emergent pollen. Core depths are in cm below the original sediment/water interface.
Discussion
Origin and age of the silt/clay sediment
Several lakes in Maine, including Lake Auburn, have evidence of massive sediment slumping of subaqueous sediment and observable fault scarps (Supplementary material, Figure S1, and Belknap, University of Maine, pers. comm.). All of these lakes are deep, below the postglacial marine limit (∼76 masl at the present coast and rising to ∼125 masl inland, ∼100 masl at Lake Auburn), and have Presumpscot Formation within the drainage basin, and likely beneath Holocene lake sediment. More than 600 lakes in near-coastal areas of Maine have the potential of Presumpscot sediment in their watersheds or as part of the stratigraphic record within the lake basin. Many lakes in Maine have Presumpscot sediment at their shoreline (Thompson and Borns Citation1985). At least 20 Maine lakes below 100 masl are deeper than 30 m (Maine Department of Inland Fish and Wildlife [MEDIFW]).
Different independent lines of evidence suggest that as much as 90 cm of sediment has been deposited since about 1900:
The upper 60 cm of silt/clay in Core 2016 sediment contains unsupported 210Pb, indicating it is <120 yr old. 137Cs, a radioactive Cs isotope produced in limited quantity in the atmosphere between 1945 and 1963, and sporadically after that, exists down to as much as 90 cm. Diffusion on an atomic level to depths greater than the original depth of deposition may exaggerate the amount of sediment deposited since 1963. Atmospheric 137Cs and unsupported 210Pb would have also accumulated in terrestrial soils developed on Presumpscot sediment and till.
The stable Pb isotope record in Core 2016, corrected for the laboratory loss of 30 cm of gyttja, provides a continuous record of Pb pollution that starts in the mid to late 19th century in Maine (Norton et al. Citation2014, Citation2016). The 206Pb/204Pb ratio in the silt/clay decreased upward, starting at about 80 cm depth (adjusted) and extending into the gyttja of Core 2015 to about 8 cm (equated with 1980, the peak of Pb atmospheric pollution), followed by an increase in the ratio over the next 35 yr, as atmospheric Pb pollution declined nearly to zero (). Legacy Pb pollution adsorbed on the suspended detritus from eroding soil would be transported to the lake and incorporated in the sediment record, extending the elevated pollution signal forward in time. Variability in erosion and deposition rates would further obscure direct correspondence between the sediment record and atmospheric deposition of 137Cs, 210Pb, and stable Pb isotopes.
Two other types of data are less strong for establishment of chronology, but supportive of the recent age of the silt/clay sediment. The δ34S values are substantially negative in the uppermost 10 cm of gyttja, and are positive below that (). The shift is ∼3‰. More negative δ34S in recent sediment is related to elevated SO42- concentration in surface waters, a result of atmospheric SO4 pollution (Fry Citation1986). Sulfate concentrations in Maine waters peaked in the early 1980s and declined thereafter, based on National Atmospheric Deposition Program (NADP Citation2018) data for atmospheric deposition, and local runoff chemistry (Fernandez et al. Citation2010, Navrátil et al. Citation2010). Second, pollen data suggest land clearance, indicated by the presence of Ambrosia and Rumex in the gyttja and in the upper part of the silt/clay to a depth of 36 cm, possibly to as much as 60 cm for Rumex. Typha and Cyperaceae pollen, which first appeared at 40 and 110 cm, adjusted depth in Core 2016, may have originated after partial impoundments created by road construction (over Townsend Brook) and a dam installation (Basin Stream) on the 2 major inlets resulted in expanded wetlands favoring the appearance of these species. The deeper occurrence may be too old for this explanation to be correct.
Our dating methods provide individual dates for the first evidence of western European settlement, subsequent disturbances, and air pollution. They all indicate that there is substantial silt/clay, apatite-bearing sediment deposited in the last 120 to 270 yr.
Lake Auburn has a very complex sedimentary history in the recent past. Average sedimentation rates, over the last 100–150 yr, in undisturbed lakes across northern New England are typically ≤1 to 2 mm/yr (Davis et al. Citation1986). In Lake Auburn, the sedimentation rate of at least 6 mm/yr for the last 120 yr (based on 210Pb activity) indicates that substantial erosion has and is taking place in the watershed. Soil or shoreline erosion contributes unweathered material, and P-rich secondary Al(OH)3 and Fe(OH)3 from weathered till and Presumpscot. We conclude that the presence of the Presumpscot Formation, compounded by land use practices for the last 250+ yr, has poised Lake Auburn with higher concentration of dissolved P than it would have without the Presumpscot in the watershed. Percent LOI and percent water in Cores 2015 and 2016 suggest 3 populations of sediment, but of slightly different source. Gyttja extends from 0 to 15 cm depth and is a mixture of normal autochthonous detritus (largely organic) plus eroded material. Gray silt/clay with lower percent water and percent LOI extends from 15 to about 250 cm and is a mixture of abundant eroded material with some autochthonous detritus. Gray/silt clay with even lower percent water and percent LOI, and with laminations indicative of persistent anaerobic conditions, occured below 250 cm. The deepest sediment may be slumped Presumpscot or Holocene sediment (Supplementary material, Figure S1). The top 15 cm of Core 2015 represents post mid-20th-century conditions. The age of the transition from 200 to 250 cm within the silt/clay is unknown.
The role of climate change
In the last few decades, rainfall amounts and extreme rainfall events (>5 cm/h) have increased significantly in Maine (Fernandez et al. Citation2015), causing increased erosion of soil from the watershed. Earlier ice-out at Lake Auburn (3 weeks earlier since 1880; Hodgkins et al. Citation2002) and later ice-on (Fernandez et al. Citation2015) produce longer summers with warmer epilimnia and stronger thermal stratification. Depletion of DO may occur earlier in the summer, promoting release of P from sediment (Einsele Citation1936; Mortimer Citation1941). Higher hypolimnetic temperature enhances the reductive dissolution of sediment Fe(OH)3 (Amirbahman et al. Citation2013) and more rapid decomposition of organic matter, which accelerates the loss of DO and mineralization of biogenic P (Gächter et al. Citation1988, Wentzel et al. Citation1991, Hupfer et al. Citation2004). Collectively, these changes in processes increase the release of P from sediment, whether the P is sourced from the sediment (apatite, organic matter, and adsorbed) or the watershed.
Implications for modern water quality
From 1977 to 2009, dissolved P in Lake Auburn ranged from 7 to 14 µg/L, with an average of 8 µg/L. Some portion of this P is caused by chemical weathering of apatite-bearing silt/clay in the lower elevation of the watershed, and till in the higher elevation soils. Presumpscot is easily eroded at the shoreline from raising of the lake level with a dam. Land clearance and grazing over the last 250+ yr have almost certainly caused erosion of the Presumpscot, and till at higher elevation. Apatite is soluble and, where it is exposed, yields dissolved P. Nieratko (Citation1992) determined that 600 lakes in Maine with Presumpscot Formation in their watershed had epilimnetic P concentrations up to 6 μg P/L greater than morphometrically comparable Maine lakes with no Presumpscot in their watershed. Since isostatic uplift after deglaciation, soil profiles have not developed deeply in Presumpscot silt/clay because of its low permeability; thus, the development of secondary Al(OH)3 and Fe(OH)3 (favored in soils that are well drained) is limited in the soils developed on Presumpscot. Consequently, adsorption of P in soil from apatite weathering likely was and is low, whereas leaching of the P would be relatively high.
Sediment cores dating back to deglaciation (Kopáček et al. 2007, Norton et al. Citation2011, Boyle et al. Citation2013) indicate that P in lake waters initially was higher because of unweathered apatite in the watershed soil. As apatite dissolved and became depleted in the soil, the flux of dissolved P from the watershed declined, leading to lake oligotrophication. The long core from Sargent Mountain Pond in Maine (Norton et al. Citation2011) indicated that within 2000 yr of deglaciation, the dissolved P declined from about 25 µg/L to 6 µg/L, as a consequence of depletion of apatite in the soil. This conclusion is evidenced by the decline of apatite in detritus reaching the sediment (based on Psenner extractions), and diatom species. Thus, the occurrence of apatite in the modern sediment of a soft-water lake such as Lake Auburn is highly unusual. Apatite in Auburn was confirmed with SEM (e.g., ) and EDS analyses (Supplementary material, Figure S4) for the upper 1 m of sediment, and strongly inferred from the relatively constant CaHCl/PHCl molar ratio of 1.83 to 1.92 in sediment from 31 to 356 cm. (Supplementary material, Figure S3 and ).
We hypothesize that detrital apatite distributed throughout the entire upper sediment is a source of dissolved P that diffuses upward in the sediment and may be adsorbed by secondary amorphous Al(OH)3 and, most importantly in Lake Auburn (Doolittle et al. Citation2018), Fe(OH)3 at the sediment–water interface. These Al(OH)3 and Fe(OH)3 phases are from the watershed soil, and are also produced within the water column (Kopáček et al. Citation2006). The Fe(OH)3 is reductively dissolved during anoxia, releasing its adsorbed P to the water column.
Congruent dissolution of apatite in the sediment at near-neutral pH is given by
(2)
(2)
EquationEquation 2
(2)
(2) indicates that as pH declines (H+ increases), the solubility of apatite increases. EquationEquation 2
(2)
(2) yields the solubility product and constant (Stumm and Morgan Citation1996),
(3)
(3)
As reaction 2 proceeds to the right, pH, Ca, and dissolved PO4 increase, as various pH-dependent P species increase. The pH of Lake Auburn (∼7) corresponds approximately to equal concentrations of HPO42− and H2PO4−1 (pKa = 7.2); thus,
(4)
(4)
At the pH of Lake Auburn, PO43− is 10−5 of total dissolved P. At equilibrium, the extent of apatite dissolution at pH 7 is directly proportional to (H+)5.5, and inversely related to (Ca2+)5.
The feasibility of apatite dissolution has been investigated with empirical studies and experiments. Two end member hypotheses exist. Golterman (Citation1995, Citation1998) recognized that the mobility of PO4 in sediment was linked to adsorption to and desorption from Fe hydroxide, and soluble Ca-bearing minerals such as calcite. In the presence of hard water (high dissolved Ca2+), apatite could precipitate if PO43- concentration were elevated. Adsorption and desorption by Fe and Al hydroxide would then be the controlling mechanisms on mobility The other hypothesis is suggested by studies of sequential sediment extractions in short-term incubations under reducing conditions (Penn et al Citation1995, Ostrofsky Citation2012). They concluded that P from apatite was not mobile. In both of these studies, the candidate sediments were from hard-water lakes and contained abundant CaCO3. Although the authors do not report pore-water chemistry, equilibrium pH and Ca2+ for calcite water are approximately 8.3 and 10−3.4 molar, respectively, higher than the pH and Ca2+ for Lake Auburn, which are ≤7 and 10−4.5 mol/L, respectively. Lake Auburn sediment does not contain calcite. For Lake Auburn and its sediment, the relatively low pH and Ca2+ lead to the dissolution of apatite and mobilization of PO4 (EquationEquation 4(4)
(4) ). Apatite is very insoluble in high pH–high Ca2+ environments, but substantially increases in solubility as pH and Ca2+ decline. Dissolved reactive P in interstitial water in lake sediment is commonly as high as 30 µmol/L (Carignan and Flett Citation1981, Amirbahman et al. Citation2013) and <1 µmol/L in overlying water, indicating that P does migrate along diffusion gradients until it is trapped temporarily by Fe(OH)3. Pore-water chemistry was not determined for Lake Auburn sediment.
Summary
Radioactive isotopes (210Pb and 137Cs), stable isotope ratios (Pb and S), SEM imaging, EDS analysis, and enumeration of pollen all support the conclusion that Core 2015, with its 2 contrasting sediment types, and at least the upper 20 cm of the sampled Core 2016, a total of 50 cm, were deposited since 1963. Evidence of air pollution, which started approximately in the mid-19th century, occurred at 70–80 (adjusted) cm in Core 2016; initiation of land clearance is estimated at 1750, corresponding to pollen evidence found at least as deep as 36 cm, possibly in Core 2016. Doolittle et al. (Citation2018) found that Lake Auburn is currently susceptible to internal recycling of P because of unfavorable extractable Al:Fe and Al:P ratios. Watershed erosion and redistribution of apatite-bearing detritus and adsorbed P in the detritus to the deepest areas of the lake increase the supply of P to the lake in the area most susceptible to release of P during times of hypolimnetic anoxia. Because the silt/clay sediment and recent gyttja contain no calcite, pH is ≤7, and Ca+2 is low, we hypothesize that apatite dissolution provides dissolved P to the sediment pore water. Upward-diffusing P in pore water would be temporarily trapped by sediment rich in secondary Fe(OH)3, the case for Lake Auburn, until anaerobic conditions develop in the hypolimnion and P is released.
Management of Lake Auburn’s water resource, including chemical remediation, should minimize erosion and point-source pollution leading to higher external P loading, and recognize the roles of the Presumpscot Formation and weather variability in lake stratification and destratification. The role of apatite dissolution in the upper sediment should be quantitatively considered for any chemical remediation, especially aluminum treatment.
Authors’ participation
All authors participated in the preparation of the article and data interpretation. Responsibilities for individual research components were: Amirbahman (coring, sediment processing, and Psenner extractions); Bacon (field and laboratory activities, providing staff for the 2015 and 2016 coring, and core sectioning); Ewing (coring and sediment processing); Norton (project coordination, coring, dating, Pb isotopes); Novak (stable S isotope analysis); Nurse (pollen analysis); Retelle (coring leader, sediment processing, seismic studies); Stager (diatom analysis); and Yates (EDS chemical mapping of sediment and SEM imaging).
Supplemental Material
Download MS Word (1,018.7 KB)Acknowledgments
The Auburn Water and Sewer District, Auburn, ME, provided funding. M. J. Dillingham, former Director of the Water Quality Laboratory at Lake Auburn, was a constant source of encouragement, enabled access to water-quality data and water sampling, and provided important logistical support for coring in 2015 and 2016. Sidney Hazelton, Director of the Auburn ME Water District, conducted a records search for human impacts on Lake Auburn water level. Scott Williams, Executive Director of the Lake Stewards of Maine, provided boat support, and assisted in the field. Professor C. T. Hess and J. A. Cummings, Department of Physics and Astronomy, University of Maine, analyzed 210Pb and 137Cs for both cores. G. McDonald, University of Maine, processed the Psenner extractions for both cores. The extractions were analyzed in the Soils Analytical Laboratory, University of Maine, B. Hoskins, Director. Stable Pb isotope determinations were by M. Handley, Sawyer Environmental Chemistry Laboratory, University of Maine. P. Dostie and W. Hilton, Bates College, helped construct the coring platform, and assisted with the coring and sediment processing. Hilton performed the laboratory determinations of percent water and percent LOI. G. L. Jacobson, University of Maine, kindly helped in the reorganization of the article. Three reviewers patiently provided very important and detailed comments and questions, critical to improvement of the article.
References
- Ahlgren J, Tranvik L, Gogoll A, Waldeback M, Markides K, Rydin E. 2005. Sediment depth attenuation of biogenic phosphorus compounds measured by P-31 NMR. Environ Sci Technol. 39(3):867–72. doi:10.1021/es049590h.
- Amirbahman A, Lake B, Norton S. 2013. Seasonal phosphorus dynamics in the surficial sediment of two shallow temperate lakes. A solid-phase and pore-water study. Hydrobiologia. 701(1):65–77. doi:10.1007/s10750-012-1257-z.
- ASTM D3177-02. 2007. Standard test methods for total sulfur in the analysis sample of coal and coke (withdrawn 2012). West Conshohocken (PA): ASTM International.
- Boyle JF, Chiverrell RC, Norton SA, Plater AJ. 2013. A leaky model of long-term soil phosphorus dynamics. Global Biogeochem Cycles. 27(2):450–62. doi:10.1002/gbc.20039.
- Carignan R, Flett RJ. 1981. Postdepositional mobility of phosphorus in lake sediments. Limnol Oceanogr. 26(2):361–6. doi:10.4319/lo.1981.26.2.0361.
- CDM Smith. 2013. Diagnostic study of Lake Auburn and its watershed. Final report to Lake Auburn Watershed Protection Commission. [accessed 2016]. http://lakeauburnwater.org/wp-content/uploads/2014/05/2014-05-20-LAWPC-Final-Report.pdf.
- Davis RB, Doyle RW. 1969. A piston corer for upper sediment in lakes. Limnol Oceanogr. 14(4):643–8. doi:10.4319/lo.1969.14.4.0643.
- Davis RB, Norton SA, Kahl JS, Anderson DS, Bacon LC, Blake GM, Morrison MM, Patterson BP, Whiting MC, Hites RA, et al. 1986. A comparative paleolimnological study of the impacts of air pollution on three northern New England Lakes: preliminary results. 7-1 to 7-60. in Charles DF, Whitehead DR. 1986. Paleoecological Investigation of Recent Lake Adicification (PIRLA): interim report. Palo Alto (CA): Electric Power Research Institute.
- Doolittle HA, Norton SA, Bacon LC, Ewing HA, Amirbahman A. 2018. The internal and watershed controls on hypolimnetic sediment phosphorus release in Lake Auburn, Maine, USA. Lake Reservoir Manage. 34(3):258. doi:10.1080/10402381.2018.1423588.
- Dudley RW. 2004. Water budget for Lake Auburn, Maine, May 1, 2000 through April 30, 2003. U. S. Geological Survey. Scientific Investigation Report 2004-5106. Washington (DC). 23 p.
- Einsele W. 1936. Uber die Beziehungen des Eisenkreislaufs zum Phosphatkreislauf im eutrophen See. Archiv für Hydrobiol. 29:664–86.
- Faegri K, Iversen J, Krzywinski K. 1989. Textbook of pollen analysis. 4th ed. New York (NY): John Wiley & Sons.
- Fernandez IJ, Adams MB, SanClement MD, Norton SA. 2010. Comparing decadal responses of whole-watershed manipulations at the Bear Brook and Fernow experiments. Environ Monit Assess. 171(1-4):149–62. doi:10.1007/s10661-010-1524-2.
- Fernandez IJ, Schmitt CV, Birkel S, Stancioff E, Pershing A, Kelley JT, Runge J, Jacobson GL, Mayewski PA. 2015. Maine’s climate future: 2015 update. Orono (ME). University of Maine.
- Fry B. 1986. Stable sulfur isotopic distributions and sulfate reduction in lake sediments of the Adirondack Mountains, New York. Biogeochemistry. 2(4):329–43. doi:10.1007/BF02180324.
- Gächter R, Meyer JS, Mares A. 1988. Contribution of bacteria to release and fixation of phosphorus in lake sediments. Limnol Oceanogr. 33:1542–58. doi:10.4319/lo.1988.33.6part2.1542.
- Gobeil C, Tessier A, Couture R-M. 2013. Upper Mississippi Pb as a mid-1800s chronostratigraphic marker in sediments from seasonally anoxic lakes in Eastern Canada. Geochim Cosmochim Acta. 113:125–35. doi:10.1016/j.gca.2013.02.023.
- Golterman HL. 1995. The role of the iron hydroxide-phosphate-sulfide system in the phosphate exchange between sediments and overlying water. Hydrobiologia. 297(1):43–54. doi:10.1007/BF00033500.
- Golterman HL. 1998. The distribution of phosphate over iron-bound and calcium-bound phosphate in stratified sediments. Hydrobiologia. 364(1):75–81. doi:10.1007/BF00014722.
- Hildreth CT. 2008. Lake Auburn West Quadrangle, Open File report 08-72. Augusta (ME): Maine Geological Survey.
- Hodgkins GA, James IC, Huntington TG. 2002. Historical changes in lake ice-out dates as indicators of climate change in New England, 1850-2000. Int J Climatol. 22(15):1819–27. doi:10.1002/joc.857.
- Homyak P, Sickman J, Melack J. 2014. Phosphorus in sediments of high-elevation lakes in the Sierra Nevada (California): implications for internal phosphorus loading. Aquat Sci. 76(4):511–25. doi:10.1007/s00027-014-0350-y.
- Hupfer M, Ruübe B, Schmieder P. 2004. Origin and diagenesis of polyphosphate in lake sediments: a 31P NMR study. Limnol Oceanogr. 49(1):1–10. doi:10.4319/lo.2004.49.1.0001.
- Hussey AM. 1983. Bedrock geology of the Lewiston Quadrangle. Augusta (ME): Maine Geological Survey. Open file 83–84.
- Kopáček J, Borovec J, Hejzlar J, Ulrich K-U, Norton SA, Amirbahman A. 2005. Aluminum control of phosphorus sorption by lake sediments. Environ Sci Technol. 39(22):8784–9. doi:10.1021/es050916b.
- Kopáček J, Marešová M, Norton SA, Porcal P, Veselý J. 2006. Photochemical source of metals for sediments. Environ Sci Technol. 40(14):4455–9. doi:10.1021/es0600532.
- Kopáček J, Marešová M, Hejzlar J, Norton SA. 2007. Natural inactivation of phosphorus by aluminum in pre-industrial lake sediments. Limnol Oceanogr. 52:1147–55.
- Krammer K, Lange-Bertalot H. 1991. Süßwasserflora von Mitteleuropa, 3 Teil: Cantrales, Fragilariaceae, Eunotiaceae. Stuttgart (Germany): Gustav Fischer Verlag. p. 576.
- Lake BA, Coolidge KM, Norton SA, Amirbahman A. 2007. Factors contributing to the internal loading of phosphorus from anoxic sediments in six Maine, USA, lakes. Sci Total Environ. 373(2-3):534–41. doi:10.1016/j.scitotenv.2006.12.021.
- MEDEP (Maine Department of Environmental Protection). 2010. Unpublished data files for lake chemistry. Augusta (ME).
- MEDIFW (Maine Department of Inland Fish and Wildlife). Unpublished data files for lake bathymetry. Augusta (ME).
- Mortimer CH. 1941. The exchange of dissolved substances between mud and water in lakes. J Ecology. 29(2):280–329. doi:10.2307/2256395.
- [NADP] National Atmospheric Deposition Program. National Trends Network. [accessed 2018 Jan 2]. http://nadp.slh.wisc.edu/data/sites/list/?net=NTN.
- Navrátil T, Norton SA, Fernandez IJ, Nelson SJ. 2010. Twenty-year inter-annual trends and seasonal variations in precipitation and stream water chemistry at the Bear Brook Watershed in Maine, USA. Environ Monit Assess. 171(1-4):23–46. doi:10.1007/s10661-010-1527-z.
- Nieratko D. 1992. Factors controlling phosphorous loading to Maine lakes: a statistical model [Unpub MSc thesis]. Orono (ME): University of Maine.
- Norton SA, Perry RH, Saros J, Jacobson GL, Jr., Fernandez IJ, Kopáček J, Wilson TA, SanClements M. 2011. The controls on phosphorus availability in a boreal lake ecosystem since deglaciation. J Paleolimnol. 46(1):107–22. doi:10.1007/s10933-011-9526-9.
- Norton SA, Kopáček J, Fernandez IJ. 2014. Acidification and acid rain. In: Holland HD, Turekian KK, editors. Treatise on geochemistry. Vol. 11. London (UK): Elsevier. p. 379–414.
- Norton SA, Kopáček J, Jacobson GL, Navrátil T. 2016. A comparative study of long-term Hg and Pb sediment archives. Environ Chem. 13(3):517–27. doi:10.1071/EN15114.
- Nuclear Test Ban Treaty. 1963. Treaties and other international agreements series #5433; General records of the U.S. Government; Record Group 11. United States National Archives. Washington (DC).
- Nürnberg GK. 1995. Quantifying anoxia in lakes. Limnol Oceanogr. 40(6):1100–11. doi:10.4319/lo.1995.40.6.1100.
- Osberg PH, Hussey AM, Boone GM. 1985. Bedrock geologic map of Maine. Augusta (ME): Maine Geological Survey. 1:500,000.
- Ostrofsky ML. 2012. Differential post-depositional mobility of phosphorus species in lake sediments. J Paleolimnol. 48(3):559–69. doi:10.1007/s10933-012-9631-4.
- Patrick R, Reimer CW. 1966. The diatoms of the United States. Monogr Acad Nat Sci Philadelphia. 1(13):688.
- Patrick R, Reimer CW. 1975. The diatoms of the United States. Monogr Acad Nat Sci Philadelphia. 2(13):213.
- Penn MR, Auer T, Van Orman EL, Korienek JJ. 1995. Phosphorus diagenesis in lake sediments: investigations using fractionation techniques. Mar Freshwater Res. 46(1):89–99. doi:10.1071/MF9950089.
- Psenner R, Pucsko R, Sager M. 1984. Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten—Versuch einer Definition ökologisch wichtiger Frakionen. Archive Für Hydrobiol Suppl. 70:111–55.
- Saros JE, Michel J, Interlandi SJ, Wolfe AP. 2005. Resource requirements of Asterionella Formosa and Fragilaria crotonensis in oligotrophic alpine lakes: implications for recent phytoplankton community reorganizations. Can J Fish Aquat Sci. 62(7):1681–9. doi:10.1139/f05-077.
- Stumm W, Morgan JJ. 1996. Aquatic chemistry. 3rd ed. New York (NY): Wiley Interscience. p. 1022.
- Thompson WB. 2008. Lake Auburn West Quadrangle, Surficial Geology, Open file report 08-69. Augusta (ME): Maine Geological Survey.
- Thompson WB, Borns HW. 1985. Surficial geologic map of Maine, 1:500,000. Augusta (ME): Maine Geological Survey.
- Vandergoes MJ. 2000. High resolution record of late Quaternary vegetation and climate change, south Westland, New Zealand [Unpub PhD thesis]. Dunedin (New Zealand): University of Otago.
- Wentzel MC, Lötter LH, Ekama GA, Loewenthal RE, Marais GR. 1991. Evaluation of biochemical models for biological excess phosphorus removal. Water Sci Technol. 23(4-6):567–74. doi:10.2166/wst.1991.0506.
- Wilson TA, Amirbahman A, Norton SA, Voytek MA. 2010. Sedimentary phosphorus dynamics in oligotrophic lakes. J Paleolimnol. 44(1):279–94. doi:10.1007/s10933-009-9403-y.
- Wilson TA, Norton SA, Lake B, Amirbahman A. 2008. Sediment geochemistry of Al, Fe, and P for two oligotrophic Maine lakes during a period of acidification and recovery. Sci Tot Environ. 404(2-3):269–75. doi:10.1016/j.scitotenv.2008.06.061.
- Yanagisawa F, Sakai H. 1983. Thermal decomposition of barium sulfate-vanadium pentoxide-silica glass mixtures for preparation of sulfur dioxide in sulfur isotope ratio measurements. Anal Chem. 55(6):985–7. doi:10.1021/ac00257a046.