Abstract
Concentrations of parent polycyclic aromatic hydrocarbons (PAHs) in the bivalves Macoma balthica and Astarte borealis were studied as an indication of the state of the Baltic Sea. Samples were collected between 1999 and 2001 from the Gulf of Finland, the Eastern Gotland Basin, and the Southern Baltic Proper. PAHs were quantified by liquid chromatography with fluorescence detection. The sum of 12 PAHs varied between 44 and 298 ng/g (ww), with the most contaminated bivalves found in Hanö Bight (the Bornholm basin). High-molecular-weight PAHs predominated among the PAHs. The PAH profiles of M. balthica differed from those determined in sediment from the same area. Both pyrolytic and petrogenic origins were indicated. Toxic equivalency factors evaluated as benzo[a]pyrene equivalents (BaPEs) were used to assess the PAH contamination. BaPEs were higher than values for M. balthica found in the southern part of the Gulf of Finland in 1995 and much lower than values measured in 1987 after the most serious oil spill in the Gulf of Finland in the past 25 years.
In the marine environment, polycyclic aromatic hydrocarbons (PAHs) in the water phase tend to bind to particulate matter in amounts depending on the properties of the PAHs, and thereafter settle down to the bottom sediment (Citation1). Accordingly, sediments are considered to be an important source of PAHs especially for deposit-feeding bottom fauna.
The Baltic Sea is connected to the world's oceans solely by the narrow and shallow waters of the sound and the Belt Sea. The shallowness and the limited exchange of water with adjacent sea areas (30 years for water renewal) render the brackish water environment of the Baltic Sea highly vulnerable. With its many sources of pollution, the Baltic Sea is considered one of the worst polluted marine areas in the world (Citation2). Increased oil transportation along the Baltic Sea heightens the risk for oil spills. There also are many illegal oil discharges, which are estimated to be responsible for about 10% of all the oil hydrocarbons in the Baltic Sea (Citation2,Citation 3).
The importance of Baltic Sea protection has been recognized since the 1970s in the form of a Convention on the Protection of the Marine Environment of the Baltic Sea (Helsinki Convention) signed by the surrounding countries (Citation4). Today hazardous substances which include some PAHs, are listed in the European Union Water Framework Directive (2000/60) and the Directive on Discharges of Dangerous Substances into the Aquatic Environment (76/464), both aimed at diminishing their use. Seven of the 12 parent PAHs dealt with in this article are included in the above-mentioned EU directives.
Bivalves are appropriate organisms for the study of PAH contamination owing to their ability to accumulate PAHs. Macoma balthica, a deposit feeder that lives in sediment, was selected for PAH study because it is abundant in the area, possesses a large depth range, and can withstand a variety of physical conditions (Citation5). The accumulation of PAHs in bivalves was determined in samples collected from different areas of the Baltic Sea: the Gulf of Finland, Eastern Gotland Basin, and the Southern Baltic Proper. Analyses were made by liquid chromatography with fluorescence detection. Concentrations were compared with those found in sediments. PAH sources (pyrolytic and petrogenic) were evaluated on the basis of molecular indices, the PAH load in biota as accumulation factors (ratio of the concentration of an individual PAH in biota to that in sediment), and the toxicities of PAHs as benzo[a]pyrene equivalents (BaPE values).
MATERIALS AND METHODS
Sampling
Bivalves were collected in May 1999 and 2001 from the coastal area and the open sea in an area extending from the Gulf of Finland to the Southern Baltic Proper (). M. balthica was the species investigated, except for a sampling area between Arkona and Bornholm Basins (see , code AB) where Astarte borealis was the only bivalve species found in sediment during the sampling period. Bivalves were collected during May which is spawning time (Citation6) for M. balthica (between May and July). Spawning time for A. borealis is in February (Citation6).
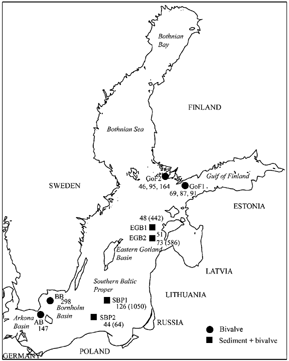
FIG. 1 A map of bivalve sampling areas and sums of 12 PAHs in bivalves (ng/g ww) and sediments (ng/g dw, presented in parenthesis) in the Baltic Sea (GoF, Gulf of Finland; EGB, Eastern Gotland Basin; SBP, Southern Baltic Proper; AB, an area between the Arkona and Bornholm Basins; BB, the Hanö Bight/Bornholm basin). Bivalve sampling was performed in May 1999 (EGB1 (n = 1), EGB2 (n = 1), and SPB1 (n = 1)) and in May 2001 (GoF1 (n = 3), GoF2 (n = 3), EGB2 (n = 1), SBP2 (n = 1), BB (n = 1), AB (n = 1)).
Immediately after collection, bivalves were placed into glass containers filled with brackish water. In order to empty the intestines of bivalves (Citation7), the containers were kept in the dark at about 4°C for 24 h. After the water was removed the bivalves were stored frozen at −20°C (in 1999) or −70°C (in 2001) until prepared for PAH analysis.
PAHs
Altogether 15 parent PAHs could be determined with the method employed. These were naphthalene (Naph), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phen), anthracene (Ant), fluoranthene (Fluoranth), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenz[a,h]anthracene (D(ah)A), benzo[ghi]perylene (B(ghi)P), indeno [1,2,3-cd]pyrene (Ind). Eight of these (Naph, Ant, Fluoranth, BbF, BkF, BaP, B(ghi)P, Ind) are mentioned in the Water Framework Directive (2000/60) of the European Union. Twelve of the 15 PAHs determined in the bivalves are included in the results reported below, presented on a wet weight basis. PAH concentrations of the Baltic Sea sediments referred to in this article are based on unpublished data (Citation8). Sediments were collected in 2001 and 2002 and PAH concentrations were determined by high-performance liquid chromatography (HPLC) with fluorescence detection.
Sample Handling
Because the bivalves were relatively small in size, several (17–37 individuals per location, average 24) were pooled to make up a representative sample of their soft parts. Soft parts of several bivalves from the same sampling area (combined weight from 2.9 g to 11.7 g with an average value of 7 g per location) were saponified (Citation9) in a mixture (9:1, 40 mL) of methanol (Rathburn, HPLC grade) and MQ water (water purified in the laboratory) to which were added NaOH (Merck, p.a., 10% m/v) and, as internal standard, benzo[b]chrysene in acetonitrile (Dr. Ehrenstorfer GmbH, Labor Dr. Ehrenstorfer-Schäfers, Germany). The internal standard was changed from that used by Webster et al. (Citation9), where GC/MS was the method of analysis, to one suitable for the HPLC method of this study. The mixture was refluxed and extracted with hexane as presented by Webster et al. (Citation9). Finally, the hexane phase was dried chemically with sodium sulfate. After most of the solvent had been evaporated with a rotary evaporator, the sample solution was reduced to 0.5 mL under a gentle stream of nitrogen.
The sample was purified by the method of Poutanen et al. (Citation10) somewhat modified. The sample solution was purified by passage through end-to-end alumina N (SepPak Vac 6 cc, 1 g sorbent, Waters) and silica (SepPak Vac 3 cc, 0.5 g, Waters) cartridges (alumina cartridge above, silica cartridge beneath), with the help of a vacuum system to shorten the time. In this step, the cartridges were washed with dichloromethane (Rathburn, HPLC grade) and hexane (Rathburn, HPLC grade), the sample solution was added and hexane:dichloromethane (1:1, 8 mL) was added as eluent. Hexane:dichloromethane was gradually changed to acetonitrile (Rathburn, HPLC grade, 1 mL) under nitrogen stream. The final sample solution was filtered through a 0.2 μm PTFE filter (Waters Acrodisc CR Minispike 13 mm, 0.2 μm) before HPLC analysis. A procedural blank was analyzed during each sample series. The method performance was evaluated by analyzing a quality control sample (Mytilus edulis homogenate from QUASIMEME laboratory performance studies).
HPLC Analysis with Fluorescence Detection
PAH concentrations were determined by HPLC with fluorescence detection. Analyses were performed with a 1100 Series Agilent high-performance liquid chromatograph (Agilent Technologies Deutschland GmbH, Waldbronn, Germany) equipped with thermostated autosampler, binary pump, vacuum degasser, thermostated column compartment, fluorescence detector, and Agilent ChemStation software (Version A.09.01). Temperature set points in the sample chamber and HPLC column oven were 12°C and 25°C, respectively.
Results were calculated in relation to the internal standard (benzo[b]- chrysene). The calibration solution for HPLC analysis with fluorescence detection was a PAH mixture prepared by Dr. Ehrenstorfer, Labor Dr. Ehrenstorfer-Schäfers, Germany (PAH-Mix 9, containing 16 EPA compounds, 100 ng/μL in acetonitrile). Several dilutions were made to prepare six different calibration levels for each PAH. The internal standard was added to each calibration solution. HPLC analysis with gradient elution was made with a Vydac 201TP54 (C18, 5 μm, 4.6 × 250 mm) column with a Vydac guard column RP C18 with particle size of 5 μm. Acetonitrile and MQ water (with L(+) ascorbic acid, p.a., Merck, min. 99.7%, 1 mg/L) were used as eluents during a gradient HPLC run of 62 min: 30% acetonitrile (0–13 min), acetonitrile gradient from 30% to 100% (13–50 min), 100% acetonitrile (10 min), and decrease in the amount of acetonitrile to 30% (2 min). The solvent flow rate was 1 mL/min and the injection volume 20 μL. The following excitation/emission wavelength pairs (nm/nm) were selected on the basis of excitation and emission spectra and the elution times of the PAHs of interest in this study: 270/340 (Naph, Ace, Flu), 250/380 (Phen, Ant), 270/420 (Fluorant, Pyr, BaA, Chr), 290/420 (BbF, BkF, BaP, D(ah)A, B(ghi)P), 302/506 (Ind), and 290/420 (benzo[b]chrysene). The most volatile components (Naph, Ace) were excluded from the final results because of the very low concentration found in bivalves and Flu was excluded because of coelution observed in a blank sample.
Quality Control
Suitable commercial certified reference material was unavailable when the analyses were carried out and instead a mussel sample used in the QUASIMEME laboratory performance studies was used as a quality control (QC) sample. Average BIAS values (n = 3) for each of the 10 studied PAHs (Phen, Ant, Fluoranth, Pyr, BaA, Chr, BbF, BkF, BaP, B(ghi)P) that were detected in the QC sample varied from −0.4% to 56% (average 26%).
RESULTS
PAH Concentrations in Bivalves
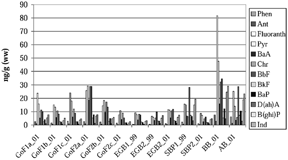
The sum of 12 PAHs in bivalves varied from 44 to 298 ng/g (ww) in the studied area of the Baltic Sea (see ). The lowest PAH level (44 ng/g, ww) was found in the Southern Baltic Proper, close to the Bornholm basin, where the PAH level in sediment (64 ng/g (dw), see ) was exceptionally low (the bottom was very sandy). The highest PAH value (298 ng/, ww) was from Hanö Bight, close to the Swedish mainland. Average PAH levels for M. balthica, calculated on area basis, were the following: 57 ng/g (ww, n = 3) in the most northern part of the Eastern Gotland Basin, 92 ng/g (ww, n = 6) in the Gulf of Finland, 126 ng/g (ww, n = 1) in the Southern Baltic Proper, and 298 ng/g (ww, n = 1) in Hanö Bight, the outer part of the Bornholm basin. One of the samples from the area between the Arkona and Bornholm Basins, was A. borealis with a total PAH content of 147 ng/g, ww.
Concentrations of individual PAH components found in bivalves are presented in . Ranges (ng/g, ww) for the seven PAHs included in the European Union Water Framework Directive (2000/60) and found in bivalves within the studied area were as follows: 0.3–2.8 for anthracene, 6.5–82 for fluoranthene, 5.4–35 for benzo[b]fluoranthene, 1.5–7.3 for benzo[k]fluoranthene, 1.8–12 for benzo[a]pyrene, 1.5–25 for benzo[ghi]perylene, and 2.6–29 for indeno[1,2,3-cd]pyrene. Benzo[a]- pyrene is considered one of the most carcinogenic PAHs (Citation11). The highest values of BaP, 12 ng/g, 11 ng/g, and 7.8 ng/g (ww), were found in samples from the Hanö Bight (Bornholm Basin) and the area between Arkona and Bornholm Basins and in the coastal waters of the Gulf of Finland, respectively. The lowest value, 1.8 ng/g (ww), was found in samples from the above-mentioned sandy bottom of the Southern Baltic Proper and from the Gulf of Finland where the BaP range was 1.8–7.8 ng/g (ww). The highest levels of the other carcinogenic compounds, dibenz[a,h]anthracene (4.8 ng/g, ww), benzo[b]fluoranthene (35 ng/g, ww), and indeno[1,2,3-cd]pyrene (29 ng/g, ww), were found in the Hanö Bight and the lowest levels in the Gulf of Finland (0.4 ng/g, 5.4 ng/g, and 2.6 ng/g, ww, respectively). In the case of benz[a]anthracene, the highest value, 19 ng/g (ww), was found in bivalves from the Gulf of Finland; the second highest value, 16 ng/g ww, in individuals from the Hanö Bight; and the lowest value, 2.5 ng/g ww, in individuals from the Southern Baltic Proper where the bottom was very sandy. The concentration of chrysene varied between 4.7 and 32 ng/g ww, with the lowest value in the Southern Baltic Proper (the sandy bottom area) and the highest value in the Hanö Bight. Concentration ranges for phenanthrene and pyrene were 0.6–7.6 ng/g ww and 5.8–48 ng/g ww, respectively, with the highest values found in the Hanö Bight and the lowest in the most northerly part of the Eastern Gotland basin.
Molecular Indexes as Source Indicators
Sources (pyrolytic or petrogenic) of PAHs evaluated on the basis of molecular indexes (Citation12–15). According to the concentration ratio of phenanthrene/anthracene (calculated range 2.0–4.8 < 10), pollution was of pyrolytic origin in all bivalve samples. The fluoranthene/pyrene ratio indicated a pyrolytic source (1.0–1.8, > 1) except in the Gulf of Finland (0.7–0.9, < 1). The chrysene/benz[a]anthracene ratio (1.5–2.1, > 1) indicated a petrogenic source except in the Southern Baltic Proper (see , SBP1, 0.9, < 1). Concentration ratios of Ind/(Ind+BghiP) ranged from 0.54 to 0.64. The range for Fluoranth/(Pyr+Fluoranth) was 0.42–0.64.
PAH Profiles
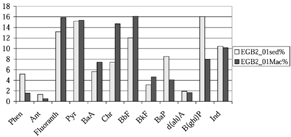
The relative distributions of PAHs in bivalves and sediment were compared using data from the most northern part of the Eastern Gotland Basin (). This was the only area where M. balthica and sediment were collected on the same day (in 2001). Sums of 12 PAHs were 73 ng/g ww and 586 ng/g dw for M. balthica and sediment, respectively. PAH profiles for that area indicated that PAHs with three to four aromatic rings were more abundant in M. balthica than in sediment. The low-molecular-weight PAHs phenanthrene and anthracene as well as high-molecular-weight PAHs BaP and B(ghi)P were more abundantly present in sediment.
Bioaccumulation Factors (BAFs)
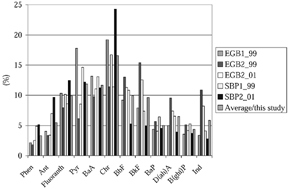
Bioaccumulation of PAHs was estimated for M. balthica collected at five sites in the most northern part of the Eastern Gotland Basin and the Southern Baltic Proper. Bioaccumulation factors for each PAH (n = 12) were calculated by dividing the concentration found in M. balthica (ww) by the concentration found in sediment (dw). Ranges of 12 PAHs were 44–126 ng/g ww and 64–1050 ng/g dw for M. balthica and sediments, respectively (see ). The bioaccumulation factors are presented as relative values (%) in together with the average value for the five sampling sites (n = 5). Accumulation was greatest for high-molecular-weight compounds: chrysene, pyrene, benzo[a]anthracene, fluoranthene, and benzo[b]fluoranthene.
Toxic Equivalency Factors (BaPEs)
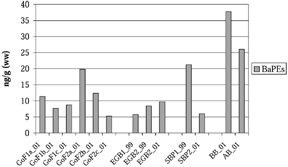
BaPE values were calculated according to the principles presented by Bolger et al. (Citation16) in an assessment made after the Exxon Valdez oil spill in 1989. Ten PAHs, from fluoranthene to indeno[1,2,3-cd]pyrene, were included in the calculation. The sum of BaPEs varied between 5.3 and 38 (ng/g, ww), the highest value being for samples from the Hanö Bight and the lowest for a sample from the site in the Gulf of Finland ().
DISCUSSION
Although M. balthica is broadly distributed in the Baltic Sea, variations in abiotic factors, such as oxygen content in the bottom sediment surface water, affect the distribution. Acute and long-term impact studies on the benthos were made after the Tsesis oil spill (October 1977, Baltic Sea archipelago) (Citation17) and, according to the results, relatively low sensitivity of M. balthica to oil pollution, coupled with its ability to take up hydrocarbons from the sediment, makes it an excellent indicator of the level of oil pollution to which a soft bottom has been subjected. In a comparison of two bivalves, suspension feeder M. edulis (L.) and deposit feeder M. balthica (L.), for their uptake and release of petroleum hydrocarbons, Broman and Ganning (Citation18) concluded that, especially in brackish water systems, the physiology, behavior, and distribution of M. balthica recommend it as an alternative monitoring organism to the more common M. edulis.
In the present study, where less-volatile and larger PAHs were studied in the years 1999–2001, the sum of PAHs (n = 12) found in bivalves varied between 44 and 298 ng/g ww (n = 12). The highest value was measured in the Hanö Bight in 2001 2 months following an oil spill (2500 m3) from a ship (the Baltic Carrier) in the southern part of the Baltic Sea (Citation3). In the Gulf of Finland, also in 2001, the sum of 12 PAHs in M. balthica was found to range from 46 to 164 ng/g ww. These values were one-tenth of those measured in M. balthica in autumn 1987 (454–1762 ng/g ww, sum of 11 PAHs), about 6–7 months after the Antonio Gramsci oil spill (570 tonne of crude oil in the Gulf of Finland) (Citation19). In 1995 8 years after this accident, the sum of PAHs in M. balthica collected from the southern part of the Gulf of Finland close to the Estonian coast was on a lower level, ranging from 4 to 30 ng/g, ww (n = 6) with an average of about 17 ng/g ww (Citation20).
Total PAH concentrations of bivalves were higher in our study than in Alaska (Citation21) and closer to values reported near urbanized areas (Citation22). The sum of 12 selected PAHs in mussels found at five Alaskan sites was about 3.7 ng/g calculated on wet weight basis (Citation21), whereas a range of 92.5–361 ng/g as a sum of 18 parent PAHs on a dry weight basis was reported for M. edulis samples collected in the Southern Baltic Sea (harbor areas and offshore coastal area, August 1996). A still higher value of 2420 ng/g (dw) was reported for Mytilus galloprovincialis collected in Arcachon Bay (France), which is described as a highly urbanized vacation area (Citation22). If a dry/wet factor of 14% is assumed (Citation23) for these last mussels, PAH ranges are about 13–51 ng/g (M. edulis) and 339 ng/g (M. galloprovincialis) on a wet weight basis.
Sources (pyrolytic or petrogenic) of PAHs in bivalves were evaluated on the basis of molecular indexes. Alkylated PAHs are also used to indicate petrogenic sources. However, this approach was beyond the scope in this study. Ratios of phenanthrene/anthracene below 10, fluoranthene/pyrene above 1, and chrysene/benz[a]anthracene below 1 are considered to indicate a pyrolytic source, whereas ratios of phenanthrene/anthracene above 15, fluoranthene/pyrene below 1, and chrysene/ benzo[a]anthracene above 1 indicate petrogenic pollution (Citation12,Citation 13). Considering the calculated molecular indexes together and taking into account the possible BIAS values, it can be concluded that the source of PAHs was pyrolytic except in the Gulf of Finland where two of the three molecular indexes indicated a petrogenic source. In view of the heavy maritime traffic and the number of illegal oil discharges (Citation3), one would in fact expect the petrogenic origin to dominate. Unfortunately there is no reliable information on the airborne deposition of PAHs in the Baltic Sea.
Two concentration ratios are considered as indicators of diesel engine sources: the ratio of fluoranthene to (pyrene+fluoranthene) within the range 0.60–0.70 (Citation14,Citation 15) and the ratio of indeno[1,2,3-cd]pyrene to (indeno[1,2,3-cd]pyrene+benzo[ghi]perylene) within the range 0.35–0.70 (Citation14,Citation 15). From information in the literature (Citation14,Citation 15) and concentration ratios of Ind/(Ind+BghiP) calculated in this work (0.54–0.64), it could be concluded that PAHs from diesel engines were present in all bivalves. The range of the Fluoranth/(Pyr+Fluoranth) ratio also indicated a contribution from diesel engines (0.42–0.64), though it was somewhat lower than the range (0.60–0.70) given in the literature (Citation14,Citation 15). On the basis of this ratio a contribution from diesel engines was indicated in three of the areas studied, two of them situated in the southern part of the Baltic Sea (see , codes BB and AB) and one in the Gulf of Finland (see , code GoF1).
A useful way to estimate the PAH load in biota is to use accumulation factors—ratios of the concentration of a selected PAH in biota to the concentration in sediment. Bioaccumulation calculations were made for bivalves collected from the Eastern Gotland Basin and Southern Baltic Proper and they indicated accumulation to be greatest for high-molecular-weight compounds. In a comparison of BAFs of deposit feeding M. balthica calculated in this study with BAFs of suspension feeding M. edulis from the southern part of the Baltic Sea calculated by Baumard et al. (Citation22), differences in bioaccumulation were found to be greatest for phenanthrene and pyrene. The accumulation of phenanthrene was at a much lower level and the accumulation of pyrene at a higher level in the present study. When Broman and Ganning (Citation18) compared uptake and release patterns of petroleum hydrocarbons for the same two species, they found M. edulis to have a short period of fast uptake and a fast initial release followed by a longer period of slow release whereas M. balthica had a slower and more extended uptake and release pattern. The different pattern for M. balthica was a reflection of its feeding and release pattern.
Toxic equivalency factors are used to evaluate contamination (Citation23). In the case of PAHs, the carcinogenic potency of individual PAHs is expressed relative to that of benzo[a]pyrene (BaPE). The equivalency values of individual PAHs are summed to yield the total BaPEs. A number of sets of toxicity/potency equivalency factors can be found in the literature, as presented by Law (Citation23), though all are based on a unit value for benzo[a]pyrene because the toxicology data for this compound are much better than the data for other PAHs (Citation23). These various sets of factors differ in the data used in the calculation: Data are selected according to the matrices of interest, so that the factors applied for particular compounds may be different. According to Law et al. (Citation23), contamination from diffuse sources generally results in BaPE values up to about 20 ng/g wet weight, while serious contamination events (oil spills, PAH leaching from a former gasworks site) yield much higher values. In the present study for the years 1999 to 2001, the range of BaPEs in the studied areas of the Baltic Sea was 5.3–38 ng/g (ww). The BaPE range of 5.3–20 ng/g ww for M. balthica calculated for the Gulf of Finland is much higher than the range of 0.4–2.5 ng/g ww, n = 8, calculated for M. balthica (1995) in the Southern Gulf of Finland on the basis of data presented by Talvari (Citation20). Toxic equivalency values for M. balthica calculated as BaPEs (n = 10) on the basis of data presented by Hirvi et al. (Citation19) for M. balthica after the crude oil spill in the Gulf of Finland in 1987 were very high (646 ng/g, ww).
CONCLUSIONS
M. balthica is a good bioindicator of environmental levels of PAHs because it dwells in sediment and accumulates PAHs in its tissues. Levels of different, indicator PAHs in M. balthica in the Baltic Sea areas of the present study pointed to various sources for the pollution load of PAHs. Highest concentrations of total PAHs were almost sevenfold the lowest concentrations. Values of benzo[a]pyrene equivalents showed the present level of carcinogenic compounds to vary with the area. Values were highest in the Bornholm basin, and values there were sixfold the lowest levels. PAH concentrations and BaPEs of M. balthica were both on a lower level than levels found after a large oil spill in 1987.
Acknowledgments
The author thanks colleagues Ann-Britt Andersin, Sari Leiniö, Jenni Nieminen, and Eeva-Liisa Poutanen. Anne Talvari kindly provided Estonian data for comparison. Freek Ariese gave constructive comments on the manuscript. Funding was provided by the Maj and Tor Nessling Foundation.
REFERENCES
- Neff , J. M. 1979 . Polycyclic Aromatic Hydrocarbons in the Aquatic Environment, Sources, Fates and Biological Effects , London : Applied Science Publishers .
- HELCOM . 2003 . “ The Baltic Marine Environment 1999–2002 ” . In Baltic Sea Environment Proceedings , Helsinki : Helsinki Commission . no. 87
- HELCOM . Accessed 15 September 2003 http://www.helcom.fi/pollution/oil.html
- Kohonen , J. T. 2003 . Finnish Strategies for Reduction and Control of Effluents to the Marine Environment—Examples from Agriculture, Municipalities and Industry . Marine Pollution Bulletin , 47 : 162 – 168 .
- Bonsdorff , E. , Norkko , A. and Boström , C. 1995 . “ Recruitment and Population Maintenance of the Bivalve Macoma balthica (L.)—Factors Affecting Settling Success and Early Survival on Shallow Sandy Bottoms ” . In Biology and Ecology of Shallow Coastal Waters, Proceedings of the 28th European Marine Biology Symposium 1993 , 253 – 260 . Denmark : Olsen and Olsen, Fredensborg . in
- Jagnow , B. and Gosselck , F. 1987 . Bestimmungsschlüssel für die Gehäuseschnecken und Muscheln der Ostsee (Identification Key for the Sea Shells of the Baltic) . Mitteilungen aus dem Zoologischen Museum in Berlin , 63 : 191 – 268 .
- Sandler , H. 1986 . Heavy Metals in Benthic Crustaceans and Mysids in the Bothnian Sea . Publications of the Water Research Institute, National Board of Waters , 68 : 205 – 210 .
- Pikkarainen , A.-L. Polycyclic Aromatic Hydrocarbons in Baltic Sea Sediments (manuscript submitted for publication)
- Webster , L. , Angus , L. , Topping , G. , Dalgarno , E. J. and Moffat , C. F. 1997 . Long-Term Monitoring of Polycyclic Aromatic Hydrocarbons in Mussels (Mytilus edulig) Following the Braer Oil Spill . Analyst , 122 : 1491 – 1495 .
- Poutanen , E.-L. , Pikkarainen , A.-L. and Haahti , H. 1990 . Öljyhiilivetyjen esiintyminen ulkomerialueella (in Finnish), in Suomenlahden öljyvahinko 1987 (Oil Spill in the Gulf of Finland in 1987) . Publications of the Water and Environment Administration—Series , A51 : 63 – 83 .
- Kennish , M. J. 1997 . Practical Handbook of Estuarine and Marine Pollution , Boca Raton, FL : CRC Press .
- Piccardo , M. T. , Coradeghini , R. and Valerio , F. 2001 . Polycyclic Aromatic Hydrocarbon Pollution in Native and Caged Mussels . Marine Pollution Bulletin , 42 : 951 – 956 . http://dx.doi.org/10.1016/S0025-326X%2801%2900057-1
- Baumard , P. , Budzinski , H. , Michon , Q. , Garrigues , P. , Burgeot , T. and Bellocq , J. 1998 . Origin and Bioavailability of PAHs in the Mediterranean Sea from Mussel and Sediment Records . Estuarine Coastal and Shelf Science , 47 : 77 – 90 .
- Kavouras , I. G. , Koutrakis , P. , Tsapakis , M. , Lagoudaki , E. , Stephanou , E. G. , Baer , D. B. and Oyola , P. 2001 . Source Apportionment of Urban Particulate Aliphatic and Polynuclear Aromatic Hydrocarbons (PAHs) Using Multivariate Methods . Environmental Science and Technology , 35 : 2288 – 2294 .
- Swartz , E. , Stockburger , L. and Vallero , D. A. 2003 . Polycyclic Aromatic Hydrocarbons and Other Semivolatile Organic Compounds Collected in New York City in Response to the Events of 9/11 . Environmental Science and Technology , 37 : 3537 – 3546 .
- Bolger , M. , Henry , S. H. and Carrington , C. D. 1996 . American Fisheries Society Symposium , 18 : 837
- Elmgren , R. , Hansson , S. , Larsson , U. , Sundelin , B. and Boehm , P. D. 1983 . The “Tsesis” Oil Spill: Acute and Long-Term Impact on the Benthos . Marine Biology , 73 : 51 – 65 .
- Broman , D. and Ganning , B. 1986 . Uptake and Release of Petroleum Hydrocarbons by Two Brackish Water Bivalves. Mytilus edulis (L.) and Macoma Baltica (L.) . Ophelia , 25 : 49 – 57 .
- Hirvi , J.-P. , Villa , L. and Erkomaa , K. 1990 . Öljyhiilivetyjen esiintyminen rannikkomerialueella (in Finnish) in Suomenlahden öljyvahinko 1987. (Oil Spill in the Gulf of Finland in 1987) . Publications of the Water and Environment Administration—Series , A51 : 85 – 118 .
- Talvari , A. Estonian Environmental Research Centre, Tallinn, personal communication. 9 July 2003
- Science Advisory Panel and Alaska Department of Environmental Conservation . 2003 . Commercial Passenger Vessel Environmental Compliance Program Accessed 14 September http://www.state.ak.us/dec/press/cruise/documents/impactcruise.htm
- Baumard , P. , Budzinski , H. , Carrigues , P. , Narbonne , J. F. , Burgeot , T. , Michel , X. and Bellocq , J. 1999 . Polycyclic Aromatic Hydrocarbon (PAH) Burden of Mussels (Mytilus sp.) in Different Marine Environments in Relation with Sediment PAH Contamination, and Bioavailabilty . Marine Environmental Research , 47 : 415 – 439 .
- Law , R. J. , Kelly , C. , Baler , K. , Jones , J. , McIntosh , A. D. and Moffat , C. F. 2002 . Toxic Equivalency Factors for PAH and Their Applicability in Shellfish Pollution Monitoring Studies . Journal of Environmental Monitoring , 4 : 383 – 388 .