Specimens of the mussel Perna perna were collected along Moroccan coasts to determine the concentrations of polycyclic aromatic hydrocarbons (PAHs) bioaccumulated in the tissues, and to measure benzo[a]pyrene hydroxylase (BPH) activity. Chemical analysis of PAHs show that the Mediterranean (Nador, Martil, Tanger) and central Atlantic coasts (from Rabat to Jorf Lihoudi) are those most contaminated (351 245 ng.g− 1 dry weight in Tanger). The mussel contaminants were of mixed origin for most of the locations with non negligible inputs of petrogenic origin in many of them. Baseline levels of PAHs were between 6 and 55 ng.g− 1 dry weight. BPH activity showed significant correlation (r s = 0.64, P < 0.05) with total PAH concentrations at the six most contaminated stations. The baseline level of BPH activity can be identified as in the range 0.1 to 13 pmol.min− 1.mg prot− 1 along the Mediterranean and Atlantic coasts.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAH) are widely distributed in the marine environment (Citation1, Citation2). These organic micropollutants are known for their carcinogenic and mutagenic potency (Citation3, Citation4), and have been monitored in a number of marine-environment programmes such as “Mussel Watch” in the USA (Citation5, Citation6) and the French national network (RNO) in France (Citation7).
The Mediterranean and Atlantic coasts of Morocco play an important socio-economical role in fishing and tourism. Urban and industrial sources of pollution are, however, known to exist in a number of areas along these coasts. On the other hand, the few results from chemical monitoring programs that are available to environmental managers relate to contamination with heavy metals (Citation8, Citation9, Citation10). The contamination of marine ecosystems with PAHs, and the effects of this pollution on living organisms, have still not been studied along Moroccan coasts in spite of the considerable fishing activity along the Atlantic shorelines and the heavy maritime traffic in and around the Mediterranean (with 60 000 ships, including 5 000 oil tankers, passing yearly through the Straits of Gibraltar).
The aims of our investigations were to: (i) evaluate PAH levels in mussels along Moroccan coasts, and (ii) determine the activity of the enzyme benzo[a]pyrene hydroxylase (BPH), which is involved in phase 1 of PAH metabolism (Citation11, Citation12).
MATERIALS AND METHODS
Sampling Sites
Two surveys were carried out along coasts of Morocco: in June 2001 and June 2002. This period was chosen according to the reproduction cycle: June marks the end of the spawning period (Citation13). It was retained to avoid any interference caused by the gametes emission. Moreover, since this period, animals enter in the gonad reestablishment and reserve accumulation phase. Sampling were sites selected along both the Atlantic and Mediterranean coasts ( and ). Specimens of the African mussel, Perna perna, measuring 5–7 cm in length were collected at low tide from the mediolittoral zone of rocky coasts. Samples of 30 mussels with the same physiological status were used to measure the PAH concentrations. No differentiation of sex was made when collecting the mussels from each site. They were placed in plastic bags and then transported at 4°C to the laboratory, where the soft masses were stored at −20°C. The mussels were sampled in the field, on a bed of ice and their soft masses were immersed in liquid nitrogen (at −180°C) awaiting BPH assay. The BPH activity was determined using digestive glands from ten individuals at each site.
TABLE 1 Description of the Sampling Locations Along the Coasts of Morocco
PAH Analysis
Mussel tissues were freeze-dried, and one-gram samples were then subjected to microwave-assisted extraction using a Maxidigest 350 apparatus (Prolabo, France) after addition of perdeuterated PAHs as internal standards, as reported elsewhere (Citation14). The conditions of the extraction were 10 min at 30 W, using dichloromethane as the extraction solvent. The organic extract was filtered, and the total volume reduced using a rotary evaporator, with subsequent purification and division into aliphatic and aromatic fractions on alumina and silica micro-columns (Citation15). The aromatic fraction was then purified using high-pressure liquid chromatography with an aminosilane column as reported by Baumard et al. (Citation16). Sieving the pooled freeze-dried mussel tissues produces a homogeneous matrix (Citation16), so only one analysis of this matrix was required per sample. The aromatic fraction was analyzed by gas chromatography coupled to mass spectrometry, as reported by Baumard et al. (Citation17). The PAHs were quantified, by relation to the perdeuterated PAHs added to the matrix prior to extraction (Citation18). One deuterated internal standard was used for each group of aromatic compounds among the PAHs studied. The responses of the various compounds were measured by injecting a standard reference material, SRM 2260 (24 aromatic hydrocarbons in toluene; NIST, Gaithersburg, MD): a solution spiked with the solution containing the same perdeuterated PAHs as used for spiking mussels.
The following abbreviations are used for the PAHs: phenanthrene, Phe; anthracene, A; fluoranthene, Fluo; pyrene, Pyr; benz[a]anthracene, BaA; chrysene, Chrys; triphenylene, Triph; Ch = Chrys + Triph; benzo-[b]fluoranthene, BbF; benzo[k]fluoranthene, BkF; benzo[j]fluoranthene, BjF; BF = BbF + BjF + BkF; benzo[e]pyrene, BeP; benzo[a]pyrene, BaP; perylene, Per; indeno[1,2,3-cd]pyrene, IP; benzo[ghi]perylene, BP; dibenz[a,h]anthracene, DahA; dibenz[a,c]anthracene, DacA; DA = DahA + DacA. The methylated PAHs (MPs) are: 1-, 2-, 3-, 4- and 9-methyl-phenanthrene. Total PAHs are the sum of the 17 separate PAHs. Individual concentrations of the separate PAHs, MPs and total PAHs (sum of the concentrations of the separate compounds) in mussel tissues are given on a dry weight basis in .
TABLE 2 Concentrations of Individual Polycyclic Aromatic Hydrocarbons, and of Methyl-Phenanthrene, in Mussels (ng.g−1 of Mussel Dry Weight). nd = Concentration not Determined. Other Abbreviations are Given in the Text
The origin of PAHs (whether petrogenic or pyrolytic) was determined by using the following two ratios: phenanthrene/anthracene, or Phe/A, and fluoranthene/pyrene or Fluo/Pyr (Citation19, Citation20). These ratios were calculated only for the most polluted locations (PAH > 45 ng.g−1 of dry weight). The PAHs are of clear marked petrogenic origin if Phe/A ≫ 20 and Fluo/Pyr ≪ 1; they are of a major pyrolytic origin if Phe/A is in the range 1–10 and if Fluo/Pyr > 1, and of mixed origin otherwise.
BPH Activity Determination
This activity was assessed in microsomes of the digestive gland, using the fluorometric microplate method described by Akcha et al. (Citation12). Ten individuals were analyzed separately at each station. The microsomal fraction of each mussel sample was incubated with BaP at 25°C for 10 minutes; any reactions were stopped by adding 10% Triton X-100, and BPH activity was then assayed using a microplate reader. For each mussel sample, the difference in fluorescence levels between the respective emission and excitation wavelengths of 492/430 nm and 510/430 nm were determined. The compound 3-OH-BaP was used as an internal standard in each sample, to control for quenching. BPH activity is expressed in picomoles.minutes−1.mg of proteins−1. The concentrations of protein in the digestive-gland microsomes were determined using the method described in Bradford (Citation21).
Statistics
PAH concentrations were calculated for 30 mussels at each location. Means ± standard deviations (SD) for BPH activities were calculated from 4 replicates each for 10 mussels per station. The values recorded for BPH activity in the mussels from the various sites were compared using two-way analysis of variance. As samples included no more than 30 individuals and frequencies were low, an angular transformation (arcsine ✓p) was used to approximate normality.
A posteriori tests (Newman-Keuls) were performed each time and effect was found to be significant (P < 005). A Spearman correlation test was used to compare the total PAH concentrations with the values for BPH activity. A similar study was performed to compare the concentrations of PAHs (grouped according to the number of aromatic rings) with the values for BPH activity.
RESULTS
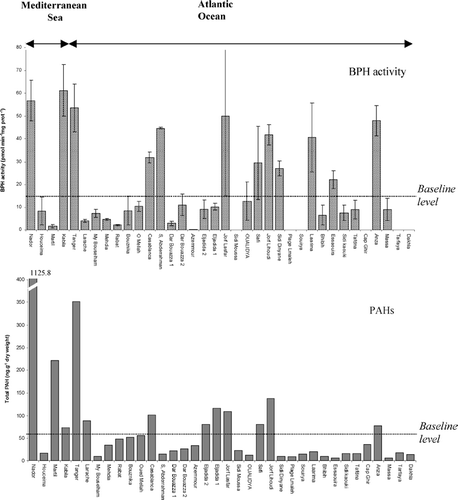
shows the pattern for 14 individual measures of PAH concentrations in the mussels collected along Moroccan coasts. The total PAH concentrations measured at the various sites varied considerably: from 5 to 351 ng.g−1 of dry weight, and even that range excludes the value obtained in Nador harbor (1 126 ng.g−1 of dry weight). The most contaminated sites on the Mediterranean are Nador and Martil, with respectively 1126 and 222 ng.g−1 of dry weight. Tanger is the most contaminated site along the Atlantic coast with 351 ng.g−1 of dry weight. Intermediate values (of 60 to 140 ng.g−1 of dry weight) were recorded for PAHs in the mussels at Kabila (73 ng.g−1 dry weight), Larache (89 ng.g−1 dry weight), El Jadida (80 and 116 ng.g−1 dry weight), Jorf Lasfar (109 ng.g−1 dry weight), Safi (80 ng.g−1 dry weight), Jorf Lihoudi (138 ng.g−1 dry weight), and Anza (78 ng.g−1 dry weight). The less polluted sites are generally on the southern coasts (6.2, 17.7 and 13.7 ng.g−1 dry weight respectively for Massa, Cape Juby and Dakhla). The baseline levels of contamination identified from 24 stations sampled along the Mediterranean and Atlantic coasts were between 6 and 55 ng.g−1 dry weight.
The origin of the PAHs (petrogenic or pyrolytic) was determined for the most polluted sites (PAH > 45 ng.g−1 dry weight) using the ratios applied in Mediterranean sea (Citation2). Two ratios (P/A and Fluo/Pyr) were calculated, and their values are grouped in . These data indicate that, as classically encountered nowadays (Citation22), the origin of PAHs in the aquatic environment is a mixture between petrogenic and pyrolitic inputs. Nevertheless the ratios put in evidence a clear and major petrogenic origin for PAHs in mussels from Nador, Tanger, Casablanca and El Jadida 1 (P/A > 20 and Fluo/Pyr < 1). Locations such as Martil and Rabat also exhibit petrogenic inputs but to a lesser extent with Phe/A ratios > 20 but Fluo/Pyr ratios > 1 associated with quite high amounts of methylated phenanthrenes clearly related to petrogenic inputs. Jorf Lasfar and Jorf Lihoudi (Fluo/Pyr < 0.1) seem also to show non negligible proportion of petrogenic inputs with is corroborated by high methylphenanthrenes for Jorf Lihoudi. The highest contamination level (222 ng.g−1 dry weight, at Martil, with the highest P/A ratio, of 650) was not sufficient to induce BPH activity, in contrast to Anza, a station with a lower contamination (78 ng.g−1 dry weight) but the highest ratio of Fluo/Pyr 1.67 demonstrating important inputs of pyrolytic PAHs mixed with petrogenic ones (Phe/A > 20 and abundant methylphenanthrenes).
TABLE 3 Values of Two Ratios for Determining the Origin of PAHs in the Mussels from the Different Moroccan Coasts. P/A = (Phenanthrene)/ (Anthracene), Fluo/Pyr = (Fluoranthene)/(Pyrene). nd: Not Determined
BPH activity measured in the mussels from the various sites sampled, is shown in (with total PAH concentrations). Significant differences (P < 0.01) in BPH activity were recorded for these sites. Significant correlations were obtained between BPH activities and PAH concentrations levels above the baseline level in six stations (Nador, Tanger, Casablanca, Jorf Lasfar, Jorf Lihoudi and Anza). For the most contaminated stations (90 to 351 ng.g−1 dry weight), BPH activity varied from 30 to 60 pmol.min−1.mg prot−1. A significant correlation (rs = 0.64, P < 0.05) was found between the total PAH concentrations and the values for BPH activity. However, some sites with low PAH concentrations show high levels of BPH activity (Kabila 61.2 ± 11.3 pmol.min−1.mg prot−1, Sidi Abderrahman 44.6 ± 0.3 pmol.min−1.mg prot−1, Laarima 40.6 ± 15 pmol.min−1.mg prot−1), while some with high BPH activity have contamination levels that are fairly low or included in the “background noise” of PAH contamination (< 60 ng.g−1 of dry weight). The baseline level of BPH activity can be identified as in the range 0.1 to 13 pmol.min−1.mg prot−1 at 18 stations along the Mediterranean and Atlantic coasts. In the latter cases the PAH pollution is not sufficient to explain the level of BPH activity, which is probably due to interaction of another contaminant and some environmental factors. The majority of stations showing the lowest pollution levels are characterized by similar concentrations of tri- and tetra-cyclic PAHs, while those with the highest levels of pollution are dominated by tetracyclics. BPH activity was also significantly correlated with the PAH groups, such as tri-, tetra- and pentacyclic (respectively, rs = 0.64, 0.64, 0.62, P < 0.05).
DISCUSSION
Levels of PAH contamination in Perna perna along the Moroccan coasts (5 to 350 ng.g−1 dry weight) appeared similar to those determined in Mytilus galloprovincialis living along the coasts of the Ligurian Sea (Italy) where Piccardo et al. (Citation23) reported values that ranged between 2 and 300 ng.g−1 of dry weight. However, Baumard et al. (Citation24) recorded lower levels of PAHs (4 to 87 ng.g−1 of dry weight) in Mytilus galloprovincialis and Mytilus edulis living along the Baltic Sea.
TABLE 4 Concentrations of Parent Polycyclic Aromatic Hydrocarbons tri, quadra, penta and hexa rings (ng.g−1 dry weight), and BPH Activity (pmol.min−1mg prot−1) in Mussels Collected from Different Sites along Moroccan Coasts. Tri (Tri aromatics), quadra (Quadra aromatics), pent (pent aromatics) and hex (hex aromatics). (nd): Activities not Determined
Our results show that Morocco's Mediterranean coasts are more polluted than the Atlantic ones. This difference may be due to the characteristics of the Mediterranean Sea (limited exchanges with others seas and oceans, and limited dynamic tidal effects) and to human activities in the Mediterranean (industrial activities and maritime traffic, with 60 000 ships, including 5 000 oil tankers, passing annually through the Gibraltar Straits). The difference in bioaccumulation could be due to the physiology of the mussels in the Atlantic Ocean and the Mediterranean. Growth and reproduction are the main factors influencing the bioconcentration process in mussel (Citation25). The spawning period of Perna perna was preponderant from March to June (Citation13). June marks the end of the spawning period. As described with Mytillus galloprovincialis along the French Mediterranean coasts, the post spawning period is not a preponderant period for the bioaccumulation of PAH (Citation26). The tidal cycle differs between these two marine ecosystems in the Atlantic Ocean and Mediterranean sea and the bioaccumulation of the chemical contaminants could be influenced (Citation27). The period of immersion is longer in the Atlantic than in the Mediterranean, and this could be at least part of the reason. While immersed, closure of the mussel shell limits exchanges with the external environment and consequently the uptake of pollutants (Citation28). The combined effect of the tidal cycle rhythm and exposure to the chemical pollutants is to increase accumulation of cadmium in the mussel Mytilus edulis (Citation29). The same observation was determined with PAHs in the crab Hemigrapsus nudus subjected to the tidal rhythm (Citation30). The joint effect of immersion and exposure to pollution also limits the resistance of Mytilus edulis to anoxia (Citation31). The reduced resistance is also characterized by a diminution in energy reserves (of glycogen and phosphoarginine) induced by increased metabolic activity during the anoxia.
The high values noted for total PAHs in the mussel populations at Martil (222 ng.g−1 of dry weight) and Tanger (350 ng.g−1) can be easily explained by the proximity of harbors. In the case of Casablanca (245 ng.g−1 dry weight), the pollutants found in the mussels might have come from the oil refinery close to the city.
Except for the Nador, Martil, Casablanca and Tanger stations, the values obtained in the various locations are lower than the recommended limit published by the French Food Agency for PAHs in edible mussels (200 ng.g−1).
With regard to the origin of PAHs along the Moroccan coasts, the mussel contaminants were of mixed origin for most of the locations with non negligible inputs of petrogenic origin in many of them. The origin was principally pyrolytic, however, in the case of Kabila and Bouznika, and principally petrogenic in the case of Nador, Tanger, Eljadida 1 and Casablanca.
The values recorded in this study for BPH activity are similar to those reported in Mytilus galloprovincialis along France's Mediterranean coasts (Citation26). Seasonal variations in BPH activity over two years, at two locations on the Mediterranean coast, were analyzed using the same microplate method. The range of values recorded for BPH activity in Mytilus galloprovincialis along the French coasts was between 1.4 ± 0.2 and 80 ± 7 pmol.min−1.mg prot−1. In the same locations and during the same period, the PAH levels bioaccumulated in tissues of the French mussels varied seasonally from 24 to 915 ng.g−1 of dry weight.
There are reports in the literature that BPH activity in mussels can be correlated with the PAH content (11, 12, 32–36). However, at some other Mediterranean locations no correlation was found (Citation37, Citation38, Citation39). Although molluscs have a limited ability to degrade PAHs, mussels do have a limited but significant capacity to metabolize PAHs (Citation40). Our results confirm the correlation between PAH contamination and BPH activity on six stations (Nador, Tanger, Casablanca, Jorf Lasfar, Jorf Lihoudi and Anza) higher than the baseline level of PAHs. (). In fact, chemical analysis of the PAHs in mussels showed that the locations can be classed into three types, depending on the level of pollution: (i) those most polluted (> 140 ng.g−1 of dry weight) along the Mediterranean coasts and the Atlantic coast; (ii) those with intermediate PAH values (60 to 140 ng.g−1 of dry weight: Larache, Safi, Jorf Lihoudi and Anza); (iii) those less polluted (< 60 ng.g−1 of dry weight), which are particularly located along the southern part of the Atlantic coast (Massa, Cape Juby and Dakhla). The BPH activity values found in the mussels from the various locations sampled show a significant correlation (rs = 0.64, P < 0.05) with total PAH concentrations, and also show the locations can be classed into three levels of activity: > 35 pmol.min−1.mg prot−1, between 13 and 35 pmol.min−1.mg prot−1, and < 13 pmol.min−1.mg prot−1. The results reported are a contribution to assessments of the level of PAH and the BPH activity in Perna perna in June. This first approach is fundamental to define the range of values for enzymatic activities (Citation35) in comparison with the level of the chemical contaminants along the whole of Morocco's coastline. The significant correlation between PAH concentrations and BPH activity in the majority of the most contaminated locations confirms the usefulness of this biomarker for biomonitoring.
This work was carried out as part of the REMER programme of collaboration between France and Morocco.
REFERENCES
- Berset , J. D. and Holzer , R. 1995 . Organic Micropollutants in Swiss Agriculture: Distribution of Polynuclear Aromatic Hydrocarbons (PAH) and Polychlorinated Biphenyls (PCB) in Soil, Liquid Manure, Sewage Sludge and Compost Samples: A Comparative Study . International Journal of Environmental Analytical Chemistry , 59 : 145 – 165 . [CSA]
- Baumard , P. , Budzinski , H. , Michon , Q. , Garrigues , P. , Burgeot , T. and Bellocq , J. 1998 . Origin and Bioavailability of PAHs in the Mediterranean Sea from Mussel and Sediment Records . Estuarine Coastal and Shelf Science , 47 : 77 – 90 . [CSA] [CROSSREF]
- Keith , L. H. and Teillard , W. A. 1979 . Priority Pollutants. A Perspective View . Environmental Science and Technology , 13 : 416 – 423 . [CSA] [CROSSREF]
- Bizub , D. , Wood , A. W. and Skalka , A. M. 1986 . Mutagenesis of the Ha-ras Oncogene in Mouse Skin Tumours Induced by Polycyclic Aromatic Hydrocarbons . Proceedings of the Natural Academy of Science , 83 : 6048 – 6052 . [CSA] [CROSSREF]
- Farrington , J. W. , Goldberg , E. D. , Risegrough , R. W. , Martin , J. H. and Bowen , V. T. 1983 . US ‘Mussel Watch’ 1976–1978: An Overview of the Trace-Metal, DDE, PCB, Hydrocarbon, and Artificial Radionuclide, Data . Environmental Science and Technology , 17 : 490 – 496 . [CSA] [CROSSREF]
- Beliaeff , B. , O'Connor , T. P. , Daskalakis , D. K. and Smith , P. J. 1997 . US Mussel Watch Data From 1986 to 1994: Temporal Trend Detection at Large Spatial Scales . Environmental Science and Technology , 31 : 1411 – 1415 . [CSA] [CROSSREF]
- Claisse , D. , Joanny , M. and Quintin , J. Y. 1992 . Le réseau national d'observation de la qualité du milieu marin (RNO) . Analysis , 20 : 19 – 22 . [CSA]
- Cheggour , M. , Langston , W. J. , Chafik , A. , Texier , H. , Kaimoussi , A. , Idrissi , H. and Boumezzough , A. 1999 . Metals in the bivalve molluscs Scrobicularia plana (Da Costa) and Cerastoderma edule (L.) and associated surface sediment from Oum Er Rbia estuary (Moroccan Atlantic Coast) . Toxicological and Environmental Chemistry , 77 : 49 – 73 . [CSA]
- Chafik , A. , Cheggour , M. , Cossa , D. and Sifeddine , S. B. M. 2001 . Quality of Moroccan Atlantic Coastal Waters: Water Monitoring and Mussel Watching . Aquatic Living Resources , 14 : 239 – 249 . [CSA] [CROSSREF]
- Banaoui , A. , Chiffoleau , J. F. , Moukrim , A. , Burgeot , T. , Kaaya , A. , Auger , D. and Rozuel , E. 2004 . Trace Metal Distribution in the Mussel Perna perna along the Moroccan Coast . Marine Pollution Bulletin , 48 : 385 – 390 . [INFOTRIEVE] [CSA] [CROSSREF]
- Michel , X. , Salaün , J. P. , Galgani , F. and Narbonne , J. F. 1994 . Benzo(a)pyrene Hydroxylase Activity in the Marine Mussel Mytilus galloprovincialis: A Potential Marker of Contamination by Polycyclic Aromatic Hydrocarbon-Type Compounds . Marine Environmental Research , 38 : 257 – 273 . [CSA] [CROSSREF]
- Akcha , F. , Izuel , C. , Venier , P. , Budzinski , H. , Burgeot , T. and Narbonne , J. F. 2000 . Enzymatic Biomarker Measurement and Study of DNA Adduct Formation in Benzo[a] pyrene-Contaminated Mussels, Mytilus galloprovincialis . Aquatic Toxicology , 49 : 269 – 287 . [INFOTRIEVE] [CSA] [CROSSREF]
- Id Halla , M. , Bouhaimi , A. , Zekhnini , A. , Narbonne , J. F. , Mathieu , M. and Moukrim , A. 1997 . Etude du cycle de reproduction de deux espèces de moules Perna perna (Linné, 1758) et Mytilus galloprovincialis Lamarck, 1819 dans la baie d'Agadir (Sud du Maroc) . Haliotis , 26 : 51 – 62 . [CSA]
- Budzinski , H. , Papineau , A. , Baumard , P. and Garrigues , P. 1995 . Extraction Assistée par Chauffage Micro-onde Focalisées (MOF) à Pression Ambiante des Composés Organiques dans les Matrices Naturelles: Application à l'Analyse des Composés Aromatiques . Comptes Rendus de l'Académie des Sciences de Paris , 321 : 69 – 76 . [CSA]
- Behar , F. , Leblond , C. and Saint-Paul , C. 1989 . Analyse Quantitative des Effluents de Pyrolyse en Milieu ouvert et Fermé. Revue de l'Institut Français du Pétrole . Revue de l'Institut Français du Pétrole , 44 : 387 – 441 . [CSA]
- Baumard , P. , Budzinski , H. and Garrigues , P. 1997a . Analytical Procedure for the Analysis of PAHs in Biological Tissues by Gas Chromatography Coupled to Mass Spectrometry: Application to Mussels . Fresenius' Journal of Analytical Chemistry , 359 : 502 – 509 . [CSA] [CROSSREF]
- Baumard , P. and Budzinski , H. 1997b . Internal Standard Quantification Method and Gas Chromatograph-Mass Spectrometer (GC-MS): A Reliable Tool for Polycyclic Aromatic Hydrocarbon (PAH) Quantification in Natural Matrices . Analysis , 25 : 246 – 252 . [CSA]
- Quilliam , M. A. , Hardstaff , W. R. , Anacleto , J. F. , LaBlanc , M. D. , Stergiopoulos , V. , Dick , K. L. , Bowusert , M. T. , Curtis , J. M. , Embree , D. J. , Sim , P. G. and Boyd , R. K. 1994 . Preparation and Certification of Solutions of Predeuterated Polycyclic Aromatic Compounds Intended for Use as Surrogate Internal Standards . Fresenius Journal Analytical Chemistry , 350 : 109 – 118 . [CSA] [CROSSREF]
- Manodori , L. , Gambaro , A. , Piazza , R. , Ferrari , S. , Stortini , A. M. , Moret , I. and Capodaglio , G. 2006 . PCBs and PAHs in Sea-Surface Microlayer and Subsurface Water Samples of the Venice Lagoon (Italy) . Marine Pollution Bulletin , 52 : 184 – 192 . [INFOTRIEVE] [CSA] [CROSSREF]
- Budzinski , H. , Jones , I. , Bellocq , J. , Pierard , C. and Garrigues , P. 1997 . Evaluation of Sediment Contamination by Polycyclic Aromatic Hydrocarbons in the Gironde Estuary . Marine Chemistry , 58 : 85 – 97 . [CSA] [CROSSREF]
- Bradford , M. 1976 . A Rapid and Sensitive Assay of Protein Utilizing the Principle of Dye Binding . Analytical Biochemistry , 772 : 242 – 264 . [CSA]
- Beliaeff , B. , O'Connor , P. , Munschy , C. , Raffin , B. and Claisse , D. 2002 . Comparaison of Polycyclic Aromatic Hydrocarbon Levels in Mussels and Oysters in France and the United States . Environmental Toxicology and Chemistry , 21 : 1783 – 1787 . [INFOTRIEVE] [CSA] [CROSSREF]
- Piccardo , M. T. , Coradeghini , R. and Valerio , F. 2001 . Polycyclic Aromatic Hydrocarbon Pollution in Native and Caged Mussels . Marine Pollution Bulletin , 42 : 951 – 956 . [INFOTRIEVE] [CSA] [CROSSREF]
- Baumard , P. , Budzinski , H. , Garrigues , P. , Dizer , H. and Hansen , P. D. 1999 . Polycyclic Aromatic Hydrocarbons in Recent Sediments and Mussels (Mytilus edulis) from the Western Baltic Sea: Occurrence, Bioavailability and Seasonal Variations . Marine Environmental Research , 47 : 17 – 47 . [CSA] [CROSSREF]
- Casas , S. and Bacher , C. 2006 . Modelling Trace Metal (Hg and Pb) Bioaccumulation in the Mediterranean Mussel, Mytilus galloprovincialis, Applied to Environmental Monitoring . Journal of Sea Research , In press.[CSA]
- Bodin , N. , Burgeot , T. , Stanisière , J. Y. , Bocquené , G. , Ménard , D. , Minier , C. , Boutet , I. , Amat , A. , Cherel , Y. and Budzinski , H. 2004 . Seasonal Variations of a Battery of Biomarkers and Physiological Indices for the Mussel Mytilus galloprovincialis Transplanted into the Northwest Mediterranean Sea . Comparative Biochemistry and Physiology , 138 : 411 – 427 . Part C.[INFOTRIEVE] [CSA]
- Durand , F. , Peters , L. D. and Livingstone , D. R. 2002 . Effect of Intertidal Compared to Subtidal Exposure on the Uptake, Loss and Oxidative Toxicity of Water-Born Benzo[a]pyrene in the Mantle and Whole Tissues of the Mussel, Mytilus edulis L . Mar. Environ. Res , 54 : 271 – 274 . [INFOTRIEVE] [CSA] [CROSSREF]
- Widdows , J. , Bayne , B. L. , Livingstone , D. R. , Newell , R. I. E. and Donkin , P. 1979 . Physiological and Biochemical Responses of Bivalve Molluscs to Exposure to Air . Comparative Biochemistry and Physiology , 62A : 301 – 308 . [CSA]
- Ahsanullah , M. and Ying , W. 1993 . Tidal Rhythms and Accumulation of Cadmium from Water and Sediment by Soldier Crabs . Marine Pollution Bulletin , 26 : 20 – 23 . [CSA] [CROSSREF]
- Gharrett , J. A. and Rice , S. D. 1987 . Influence of Simulated Tidal Cycles on Aromatic Hydrocarbon Uptake and Elimination by the Shore Crab Hemigrapsus nudus . Marine. Biology , 95 : 365 – 370 . [CSA] [CROSSREF]
- De Zwaan , A. , Cortesi , P. and Cattani , O. 1995 . Resistance of Bivalves to Anoxia as a Response to Pollution-Induced Environmental Stress . Science of the Total Environment , 171 : 121 – 125 . [CSA] [CROSSREF]
- Livingstone , D. R. and Martinez , P. G. 1989 . Menadione-Stimulated Oxyradical Formation in Digestive Gland Microsomes of the Common Mussel, Mytilus edulis L . Aquatic Toxicology , 15 : 213 – 236 . [CSA] [CROSSREF]
- Michel , X. , Suteau , P. , Robertson , L. W. and Narbonne , J. F. 1993 . Effects of Benzo(a)pyrene, 3,3′, 4,4-tetrachlorobiphenyl and 2,2; 4,4; 5,5-hexachlorobiphenyl on the Xenobiotic-Metabolizing Enzymes in the Mussel (Mytilus galloprovincialis) . Aquatic Toxicology , 27 : 335 – 344 . [CSA] [CROSSREF]
- Michel , X. , Narbonne , J. F. , Mora , P. , Daubèze , M. , Ribera , D. , Lafaurie , M. , Budzinski , H. and Garrigues , P. 1998 . Utilisation de Biomarqueurs pour la Surveillance de la Qualité de l'Environnement , Edited by: Lagadic , L. , Caquet , T. , Amiard , J. C. and Ramad , F . 9 – 32 . Paris : Lavoisier TEC & DOC .
- Förlin , L. , Livingstone , D. R. , Magnusson , K. , Peters , L. D. , Solé , M. , Sjölin , A. and Granmo , A. 1996 . Molecular Investigations into Pollutant Impact on Roundnose Grenadier (C. rupestris) and Transplanted Common Mussel (M. edulis) in Skagerrak, the North Sea . Marine Environmental Research , 42 : 209 – 212 . [CSA] [CROSSREF]
- Narbonne , J. F. , Aarab , N. , Clérandeau , C. , Daubèze , M. , Narbonne , J. , Champeau , O. and Garrigues , P. 2005 . Scale of Classification Based on Biochemical Markers in Mussel: Application to Pollution Monitoring in Mediterranean Coasts and Temporal Trends . Biomarkers , 21 : 261 – 270 . [CSA]
- Nasci , C. , Da Ros , L. , Campesan , G. , Van Vleet , E. S. , Salizzato , M. , Sperni , L. and Pavoni , B. 1999 . Clam Transplantation and Stress-Related Biomarkers as Useful Tools for Assessing Water Quality in Coastal Environments . Marine Pollution Bulletin , 39 : 1 – 12 . [CSA] [CROSSREF]
- Porte , C. , Biosca , X. , Solé , M. and Albaigés , J. 2001 . The Integrated Use of Chemical Analysis, Cytochrome P450 and Stress Proteins in Mussels Assess Pollution along the Galician Coast (NW Sapin) . Environmental Pollution , 112 : 261 – 268 . [INFOTRIEVE] [CSA] [CROSSREF]
- Carajaville , M. P. , Bebianno , M. J. , Blasco , J. , Porte , C. , Sarasquete , C. and Viarengo , A. 2000 . The Use of Biomarkers to Assess the Impact of Pollution in Coastal Environments of the Iberian Peninsula: A Practical Approach . The Science of the Total Environment , 247 : 295 – 311 . [CSA] [CROSSREF]
- Neff , J. M. 2002 . “ Bioaccumulation of PAHs by Marine Organisms ” . In Bioaccumulation in Marine Organisms. Effects of Contaminants from Oil Well Produced Water , 277 – 293 . London, UK : Elsevier . (Chapter 15.5)